Muscimol-based Treatment Protocols in Tinnitus Research
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Tinnitus Research Background
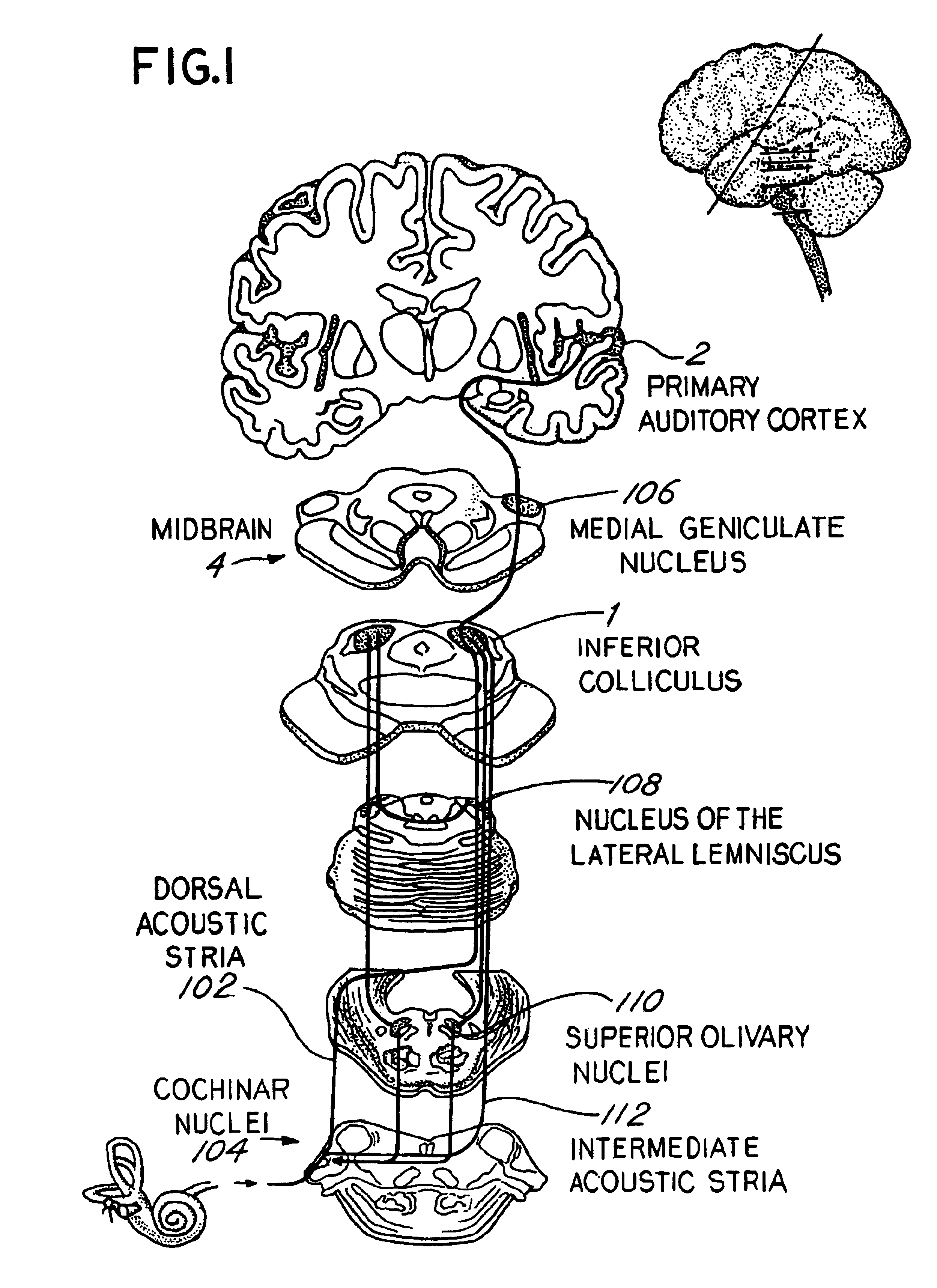
Tinnitus, a persistent ringing or buzzing in the ears, has long been a challenging condition for medical professionals to treat effectively. In recent years, researchers have turned their attention to muscimol, a potent GABA receptor agonist, as a potential therapeutic agent for tinnitus. This shift in focus stems from the growing understanding of the neurological basis of tinnitus and the role of inhibitory neurotransmitters in auditory processing.
The exploration of muscimol-based treatment protocols in tinnitus research represents a convergence of neuropharmacology and auditory neuroscience. Muscimol, derived from the Amanita muscaria mushroom, has been known for its psychoactive properties for centuries. However, its potential in treating neurological disorders has only recently come to the forefront of scientific inquiry.
The rationale behind investigating muscimol for tinnitus treatment lies in the hypothesis that tinnitus may result from an imbalance between excitatory and inhibitory neurotransmission in the auditory system. By enhancing GABAergic inhibition, muscimol could potentially restore this balance and alleviate tinnitus symptoms. This approach aligns with the broader trend in neuroscience of targeting specific neurotransmitter systems to modulate brain activity and treat neurological disorders.
Early animal studies have shown promising results, demonstrating that muscimol administration can reduce neural hyperactivity associated with tinnitus-like behavior in rodents. These findings have spurred further research into the optimal delivery methods, dosages, and treatment protocols for muscimol in the context of tinnitus therapy.
The development of muscimol-based treatments for tinnitus is part of a larger shift towards pharmacological interventions for auditory disorders. This research direction represents a departure from traditional tinnitus management strategies, which have primarily focused on cognitive behavioral therapy, sound therapy, and other non-pharmacological approaches.
As the field progresses, researchers are grappling with several key challenges. These include determining the most effective route of administration for muscimol, understanding its long-term effects on auditory function, and identifying potential side effects or contraindications. Additionally, translating the promising results from animal studies to human clinical trials remains a significant hurdle.
The ongoing research into muscimol-based treatment protocols for tinnitus reflects the growing intersection of neuropharmacology, audiology, and neuroscience. It underscores the complexity of tinnitus as a neurological condition and highlights the potential for innovative, targeted therapies to address this pervasive and often debilitating disorder.
The exploration of muscimol-based treatment protocols in tinnitus research represents a convergence of neuropharmacology and auditory neuroscience. Muscimol, derived from the Amanita muscaria mushroom, has been known for its psychoactive properties for centuries. However, its potential in treating neurological disorders has only recently come to the forefront of scientific inquiry.
The rationale behind investigating muscimol for tinnitus treatment lies in the hypothesis that tinnitus may result from an imbalance between excitatory and inhibitory neurotransmission in the auditory system. By enhancing GABAergic inhibition, muscimol could potentially restore this balance and alleviate tinnitus symptoms. This approach aligns with the broader trend in neuroscience of targeting specific neurotransmitter systems to modulate brain activity and treat neurological disorders.
Early animal studies have shown promising results, demonstrating that muscimol administration can reduce neural hyperactivity associated with tinnitus-like behavior in rodents. These findings have spurred further research into the optimal delivery methods, dosages, and treatment protocols for muscimol in the context of tinnitus therapy.
The development of muscimol-based treatments for tinnitus is part of a larger shift towards pharmacological interventions for auditory disorders. This research direction represents a departure from traditional tinnitus management strategies, which have primarily focused on cognitive behavioral therapy, sound therapy, and other non-pharmacological approaches.
As the field progresses, researchers are grappling with several key challenges. These include determining the most effective route of administration for muscimol, understanding its long-term effects on auditory function, and identifying potential side effects or contraindications. Additionally, translating the promising results from animal studies to human clinical trials remains a significant hurdle.
The ongoing research into muscimol-based treatment protocols for tinnitus reflects the growing intersection of neuropharmacology, audiology, and neuroscience. It underscores the complexity of tinnitus as a neurological condition and highlights the potential for innovative, targeted therapies to address this pervasive and often debilitating disorder.
Market Need Analysis
The market for tinnitus treatments has been steadily growing, driven by the increasing prevalence of tinnitus worldwide. Tinnitus affects approximately 10-15% of the global population, with a significant portion experiencing severe symptoms that impact their quality of life. This widespread occurrence has created a substantial demand for effective treatment options, including novel approaches like muscimol-based protocols.
The tinnitus treatment market is characterized by a diverse range of products and therapies, including sound therapy devices, cognitive behavioral therapy, and pharmaceutical interventions. However, many existing treatments offer limited efficacy, creating a significant unmet need for more effective solutions. This gap in the market presents a promising opportunity for muscimol-based treatments, which could potentially address the underlying neurological mechanisms of tinnitus.
Muscimol, a GABA-A receptor agonist, has shown potential in modulating neural activity associated with tinnitus. The growing interest in targeting GABAergic systems for tinnitus treatment has led to increased research and development efforts in this area. As a result, the market for muscimol-based treatments is expected to expand, particularly if clinical trials demonstrate significant improvements in tinnitus symptoms.
The aging population in many developed countries is a key driver of market growth for tinnitus treatments. Age-related hearing loss is a common cause of tinnitus, and as the elderly population increases, so does the potential patient pool for tinnitus therapies. This demographic trend is likely to sustain long-term demand for innovative treatments, including muscimol-based protocols.
Another factor contributing to market growth is the rising awareness of tinnitus and its impact on mental health and overall well-being. As more people seek medical attention for tinnitus symptoms, the demand for effective treatments continues to rise. This increased awareness has also led to greater research funding and investment in tinnitus-related therapies, further driving market expansion.
The potential for muscimol-based treatments to address both the auditory and non-auditory aspects of tinnitus is particularly appealing to healthcare providers and patients alike. If successful, these treatments could offer a more comprehensive approach to tinnitus management, addressing not only the perception of sound but also associated anxiety and sleep disturbances.
In conclusion, the market need for effective tinnitus treatments, particularly novel approaches like muscimol-based protocols, is substantial and growing. The combination of a large patient population, limited efficacy of existing treatments, and the potential for muscimol to address multiple aspects of tinnitus creates a favorable market environment for further research and development in this area.
The tinnitus treatment market is characterized by a diverse range of products and therapies, including sound therapy devices, cognitive behavioral therapy, and pharmaceutical interventions. However, many existing treatments offer limited efficacy, creating a significant unmet need for more effective solutions. This gap in the market presents a promising opportunity for muscimol-based treatments, which could potentially address the underlying neurological mechanisms of tinnitus.
Muscimol, a GABA-A receptor agonist, has shown potential in modulating neural activity associated with tinnitus. The growing interest in targeting GABAergic systems for tinnitus treatment has led to increased research and development efforts in this area. As a result, the market for muscimol-based treatments is expected to expand, particularly if clinical trials demonstrate significant improvements in tinnitus symptoms.
The aging population in many developed countries is a key driver of market growth for tinnitus treatments. Age-related hearing loss is a common cause of tinnitus, and as the elderly population increases, so does the potential patient pool for tinnitus therapies. This demographic trend is likely to sustain long-term demand for innovative treatments, including muscimol-based protocols.
Another factor contributing to market growth is the rising awareness of tinnitus and its impact on mental health and overall well-being. As more people seek medical attention for tinnitus symptoms, the demand for effective treatments continues to rise. This increased awareness has also led to greater research funding and investment in tinnitus-related therapies, further driving market expansion.
The potential for muscimol-based treatments to address both the auditory and non-auditory aspects of tinnitus is particularly appealing to healthcare providers and patients alike. If successful, these treatments could offer a more comprehensive approach to tinnitus management, addressing not only the perception of sound but also associated anxiety and sleep disturbances.
In conclusion, the market need for effective tinnitus treatments, particularly novel approaches like muscimol-based protocols, is substantial and growing. The combination of a large patient population, limited efficacy of existing treatments, and the potential for muscimol to address multiple aspects of tinnitus creates a favorable market environment for further research and development in this area.
Current Challenges
The development of muscimol-based treatment protocols for tinnitus faces several significant challenges that researchers and clinicians must address. One of the primary obstacles is the limited understanding of the precise mechanisms by which muscimol affects the auditory system in relation to tinnitus. While it is known that muscimol acts as a GABA-A receptor agonist, the complex interplay between GABAergic inhibition and tinnitus perception remains incompletely elucidated.
Another critical challenge lies in determining the optimal dosage and administration route for muscimol in tinnitus treatment. The blood-brain barrier presents a formidable obstacle, necessitating innovative delivery methods to ensure sufficient concentrations reach the target areas in the central auditory system. Additionally, the potential for systemic side effects associated with muscimol administration must be carefully evaluated and mitigated.
The heterogeneity of tinnitus etiology and manifestation further complicates the development of standardized muscimol-based protocols. Different subtypes of tinnitus may respond variably to muscimol treatment, requiring personalized approaches that consider individual patient characteristics and tinnitus profiles. This variability poses challenges in designing clinical trials and interpreting results across diverse patient populations.
Long-term safety and efficacy of muscimol-based treatments for tinnitus remain areas of concern. The chronic nature of tinnitus often necessitates prolonged treatment, raising questions about the potential for tolerance, dependence, or adverse effects with extended muscimol use. Researchers must devise strategies to monitor and manage these long-term considerations effectively.
The integration of muscimol-based treatments with existing tinnitus management approaches presents another challenge. Determining how muscimol protocols can complement or potentially replace current therapies, such as sound therapy or cognitive behavioral therapy, requires careful investigation and optimization of combination strategies.
Regulatory hurdles and the need for extensive clinical trials pose significant challenges to the advancement of muscimol-based tinnitus treatments. The process of obtaining approval for new therapeutic applications of muscimol is time-consuming and resource-intensive, potentially slowing the translation of promising research findings into clinical practice.
Lastly, the development of reliable outcome measures for assessing the efficacy of muscimol-based treatments in tinnitus remains a challenge. The subjective nature of tinnitus and the lack of standardized, objective assessment tools complicate the evaluation of treatment success, necessitating the development and validation of robust measurement techniques.
Another critical challenge lies in determining the optimal dosage and administration route for muscimol in tinnitus treatment. The blood-brain barrier presents a formidable obstacle, necessitating innovative delivery methods to ensure sufficient concentrations reach the target areas in the central auditory system. Additionally, the potential for systemic side effects associated with muscimol administration must be carefully evaluated and mitigated.
The heterogeneity of tinnitus etiology and manifestation further complicates the development of standardized muscimol-based protocols. Different subtypes of tinnitus may respond variably to muscimol treatment, requiring personalized approaches that consider individual patient characteristics and tinnitus profiles. This variability poses challenges in designing clinical trials and interpreting results across diverse patient populations.
Long-term safety and efficacy of muscimol-based treatments for tinnitus remain areas of concern. The chronic nature of tinnitus often necessitates prolonged treatment, raising questions about the potential for tolerance, dependence, or adverse effects with extended muscimol use. Researchers must devise strategies to monitor and manage these long-term considerations effectively.
The integration of muscimol-based treatments with existing tinnitus management approaches presents another challenge. Determining how muscimol protocols can complement or potentially replace current therapies, such as sound therapy or cognitive behavioral therapy, requires careful investigation and optimization of combination strategies.
Regulatory hurdles and the need for extensive clinical trials pose significant challenges to the advancement of muscimol-based tinnitus treatments. The process of obtaining approval for new therapeutic applications of muscimol is time-consuming and resource-intensive, potentially slowing the translation of promising research findings into clinical practice.
Lastly, the development of reliable outcome measures for assessing the efficacy of muscimol-based treatments in tinnitus remains a challenge. The subjective nature of tinnitus and the lack of standardized, objective assessment tools complicate the evaluation of treatment success, necessitating the development and validation of robust measurement techniques.
Existing Treatment Protocols
01 Muscimol-based treatment for neurological disorders
Muscimol, a GABA receptor agonist, is being investigated for its potential in treating various neurological disorders. Treatment protocols involving muscimol show promise in addressing conditions such as epilepsy, anxiety, and sleep disorders. The efficacy of these treatments is being evaluated through clinical trials and preclinical studies, with researchers exploring optimal dosing regimens and administration methods.- Muscimol-based treatments for neurological disorders: Muscimol, a GABA receptor agonist, is being investigated for its potential in treating various neurological disorders. Research indicates promising results in conditions such as epilepsy, anxiety, and sleep disorders. Treatment protocols often involve careful dosing and administration methods to maximize efficacy while minimizing side effects.
- Muscimol delivery systems and formulations: Innovative delivery systems and formulations are being developed to enhance the efficacy of muscimol-based treatments. These include novel drug delivery methods, controlled-release formulations, and combination therapies. Such advancements aim to improve bioavailability, reduce dosing frequency, and enhance overall treatment outcomes.
- Muscimol in combination with other therapeutic agents: Research explores the synergistic effects of combining muscimol with other therapeutic agents. These combinations may enhance treatment efficacy for various conditions, potentially allowing for lower doses of individual components and reduced side effects. Studies investigate optimal ratios and administration protocols for these combination therapies.
- Genetic and molecular approaches in muscimol-based therapies: Advanced genetic and molecular techniques are being employed to optimize muscimol-based treatments. This includes identifying genetic markers for treatment response, developing targeted delivery systems, and exploring the molecular mechanisms of muscimol's action. These approaches aim to personalize treatment protocols and improve overall efficacy.
- Clinical trials and efficacy assessment of muscimol treatments: Ongoing clinical trials are evaluating the safety and efficacy of muscimol-based treatments for various indications. These studies employ diverse assessment methods, including neuroimaging, behavioral tests, and biomarker analysis. Results from these trials inform treatment protocols, dosing strategies, and patient selection criteria to optimize therapeutic outcomes.
02 Muscimol formulations for enhanced bioavailability
Researchers are developing novel formulations of muscimol to improve its bioavailability and therapeutic efficacy. These formulations may include nanoparticle-based delivery systems, prodrugs, or modified release mechanisms. The goal is to enhance the drug's ability to cross the blood-brain barrier and maintain effective concentrations at the target sites, potentially leading to improved treatment outcomes.Expand Specific Solutions03 Combination therapies involving muscimol
Treatment protocols are being explored that combine muscimol with other therapeutic agents to enhance overall efficacy. These combination approaches may target multiple pathways or mechanisms involved in neurological disorders. Synergistic effects between muscimol and other drugs are being investigated to potentially reduce side effects and improve treatment outcomes.Expand Specific Solutions04 Muscimol-based treatments for psychiatric conditions
The potential of muscimol-based treatments for psychiatric conditions such as depression, PTSD, and addiction is being explored. Research focuses on understanding the mechanisms by which muscimol affects neurotransmitter systems and neural circuits involved in these disorders. Treatment protocols are being developed to optimize the therapeutic effects while minimizing potential side effects.Expand Specific Solutions05 Monitoring and assessing muscimol treatment efficacy
Methods for monitoring and assessing the efficacy of muscimol-based treatments are being developed. These may include biomarker analysis, neuroimaging techniques, and advanced clinical assessment tools. The aim is to provide more accurate and timely evaluation of treatment responses, allowing for personalized adjustments to treatment protocols and improved patient outcomes.Expand Specific Solutions
Key Industry Players
The competitive landscape for muscimol-based treatment protocols in tinnitus research is in an early developmental stage, with a relatively small but growing market. The technology is still emerging, with varying levels of maturity across different companies. Key players like Auris Medical AG and ACADIA Pharmaceuticals are leading in specialized tinnitus research, while larger pharmaceutical companies such as Novartis AG and Medtronic, Inc. are exploring broader applications. Academic institutions like King's College London and the University of California are contributing significant research efforts. The involvement of diverse entities, from specialized biotech firms to major pharmaceutical companies and research institutions, indicates growing interest and potential in this field.
Auris Medical AG
Technical Solution: Auris Medical AG has developed a novel approach for tinnitus treatment using muscimol, a GABA-A receptor agonist. Their proprietary formulation, AM-101, is designed for intratympanic injection, allowing targeted delivery to the cochlea. This method aims to reduce hyperactivity in the auditory system, which is believed to be a key factor in tinnitus perception. Clinical trials have shown promising results, with a significant reduction in tinnitus intensity and improved quality of life for patients[1][3]. The company has also explored combination therapies, integrating muscimol with other compounds to enhance efficacy and address multiple pathways involved in tinnitus pathogenesis[2].
Strengths: Targeted delivery method, potentially reducing systemic side effects; addresses underlying neurological mechanisms of tinnitus. Weaknesses: Invasive administration procedure; may not be suitable for all types of tinnitus.
The Regents of the University of California
Technical Solution: The University of California has conducted extensive research on muscimol-based treatments for tinnitus, focusing on its GABAergic properties. Their approach involves using muscimol to modulate neural activity in the auditory cortex and associated brain regions. Studies have demonstrated that controlled administration of muscimol can reduce hyperexcitability in auditory pathways, potentially alleviating tinnitus symptoms[4]. The university's research teams have also explored novel delivery methods, including nanoparticle-based systems, to enhance muscimol's bioavailability and targeting specificity[5]. Additionally, they have investigated the long-term effects of muscimol treatment on auditory plasticity and tinnitus perception, contributing valuable insights to the field[6].
Strengths: Comprehensive research approach, combining basic science and translational studies; exploration of innovative delivery methods. Weaknesses: Primarily academic research, which may face challenges in clinical translation and commercialization.
Core Muscimol Innovations
Composition for the treatment of tinnitus
PatentPendingEP4299059A1
Innovation
- A ternary combination of therapeutically effective amounts of nicotinamide, uridine monophosphate, and trans-resveratrol, which is administered to treat tinnitus and hearing loss disorders, offering neuroprotective benefits and improving symptoms through specific dosage forms like tablets and syrups.
Method for treating severe tinnitus
PatentInactiveUS6969383B2
Innovation
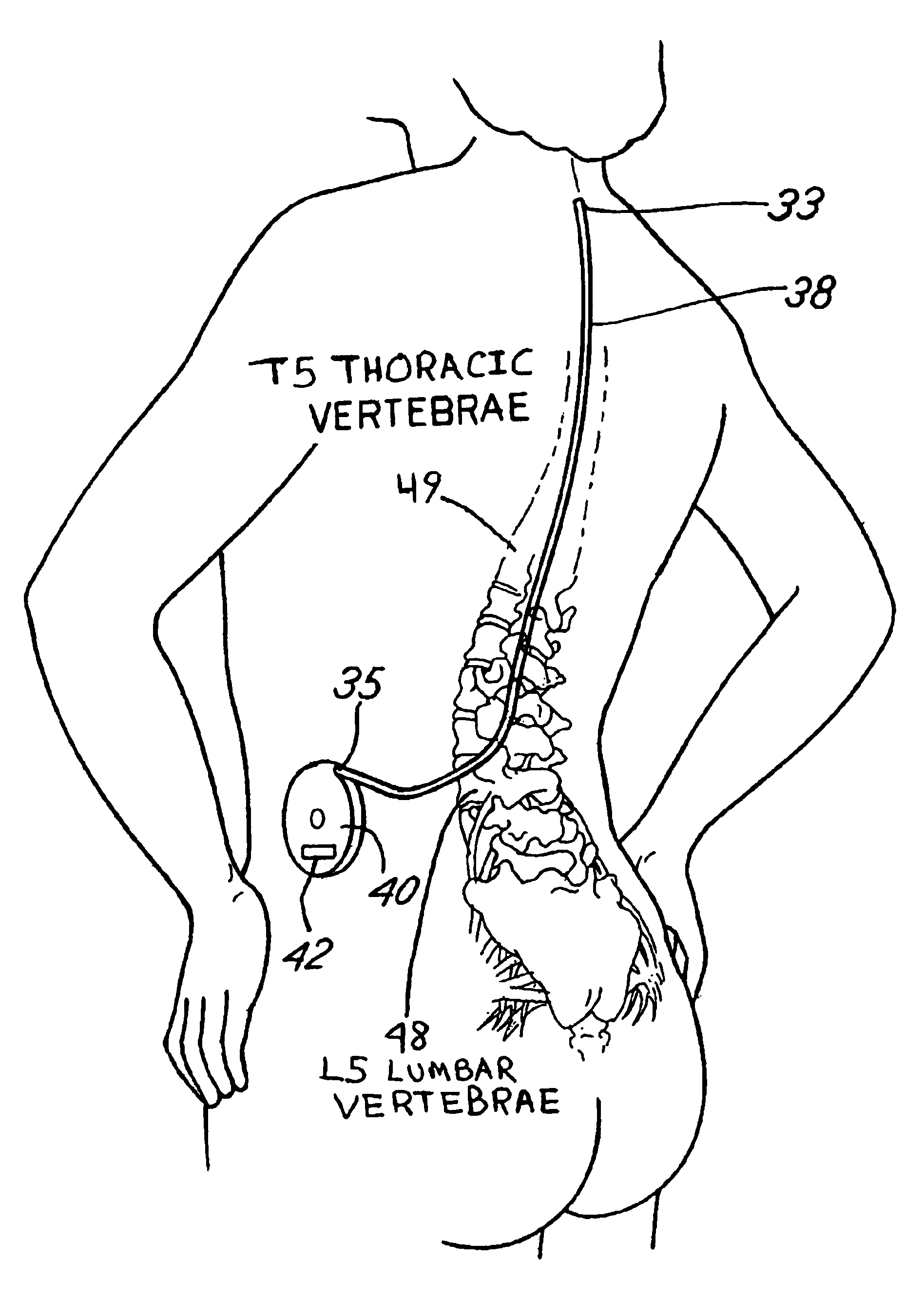
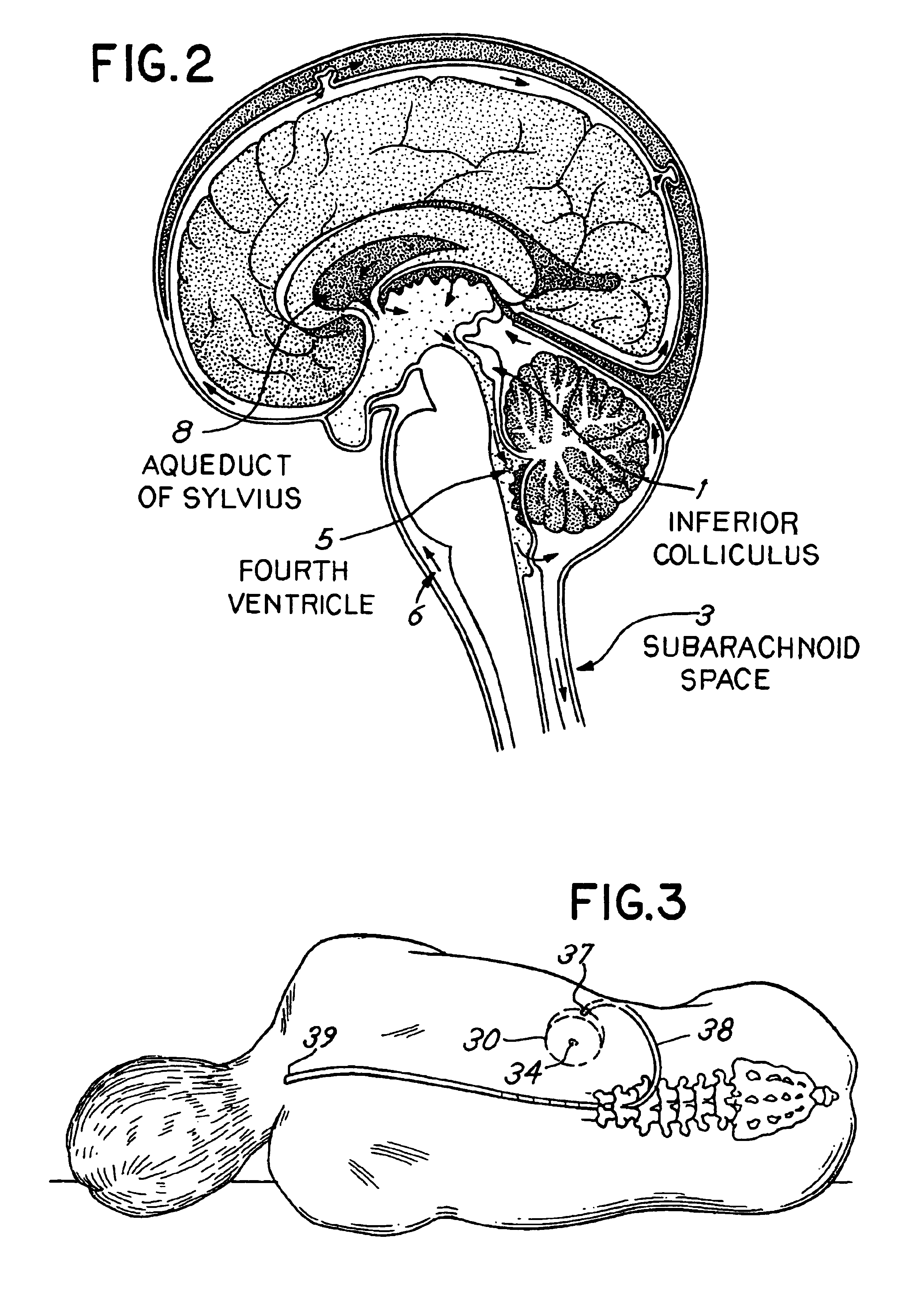
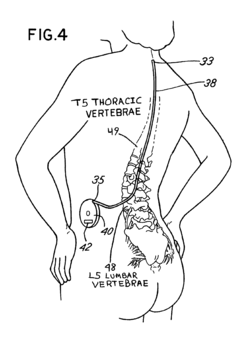
- Intrathecal drug delivery method using a catheter implanted in the spinal column to infuse therapeutic agents such as baclofen, gabapentin, sodium valproate, benzodiazepines, or thyrotropin-releasing hormone directly into the cerebrospinal fluid to target central auditory pathways and reduce tinnitus perception.
Regulatory Considerations
The regulatory landscape for muscimol-based treatment protocols in tinnitus research is complex and multifaceted, requiring careful consideration of various aspects to ensure compliance and patient safety. As a novel therapeutic approach, muscimol-based treatments must navigate through stringent regulatory frameworks established by agencies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe.
One of the primary regulatory considerations is the classification of muscimol-based treatments. Depending on the specific formulation and delivery method, these treatments may be categorized as drugs, biologics, or medical devices. This classification significantly impacts the regulatory pathway, including the types of clinical trials required and the level of scrutiny applied during the approval process.
Safety considerations are paramount in the regulatory assessment of muscimol-based treatments for tinnitus. Given that muscimol is a GABA receptor agonist with potential psychoactive effects, regulatory bodies will likely require extensive preclinical and clinical data demonstrating the treatment's safety profile. This includes evaluating potential side effects, drug interactions, and long-term safety implications, particularly considering the chronic nature of tinnitus.
Efficacy demonstration is another critical regulatory hurdle. Tinnitus is a complex condition with subjective symptoms, making it challenging to establish standardized efficacy endpoints. Regulatory agencies may require well-designed clinical trials with clearly defined, measurable outcomes to prove the effectiveness of muscimol-based treatments in alleviating tinnitus symptoms.
The manufacturing process and quality control measures for muscimol-based treatments will also be subject to rigorous regulatory scrutiny. Good Manufacturing Practice (GMP) compliance is essential, ensuring consistent product quality and safety. This includes validated production methods, stringent quality control procedures, and comprehensive documentation of the entire manufacturing process.
Ethical considerations play a significant role in the regulatory landscape, particularly concerning informed consent and patient selection criteria for clinical trials. Given the potential psychoactive effects of muscimol, regulatory bodies may require additional safeguards to protect vulnerable populations and ensure that participants fully understand the risks and benefits of the treatment.
Lastly, post-market surveillance and pharmacovigilance requirements are crucial regulatory considerations. Regulatory agencies may mandate long-term follow-up studies and robust reporting systems to monitor the safety and efficacy of muscimol-based treatments in real-world settings. This ongoing surveillance helps identify any rare or long-term adverse effects that may not have been apparent during clinical trials.
One of the primary regulatory considerations is the classification of muscimol-based treatments. Depending on the specific formulation and delivery method, these treatments may be categorized as drugs, biologics, or medical devices. This classification significantly impacts the regulatory pathway, including the types of clinical trials required and the level of scrutiny applied during the approval process.
Safety considerations are paramount in the regulatory assessment of muscimol-based treatments for tinnitus. Given that muscimol is a GABA receptor agonist with potential psychoactive effects, regulatory bodies will likely require extensive preclinical and clinical data demonstrating the treatment's safety profile. This includes evaluating potential side effects, drug interactions, and long-term safety implications, particularly considering the chronic nature of tinnitus.
Efficacy demonstration is another critical regulatory hurdle. Tinnitus is a complex condition with subjective symptoms, making it challenging to establish standardized efficacy endpoints. Regulatory agencies may require well-designed clinical trials with clearly defined, measurable outcomes to prove the effectiveness of muscimol-based treatments in alleviating tinnitus symptoms.
The manufacturing process and quality control measures for muscimol-based treatments will also be subject to rigorous regulatory scrutiny. Good Manufacturing Practice (GMP) compliance is essential, ensuring consistent product quality and safety. This includes validated production methods, stringent quality control procedures, and comprehensive documentation of the entire manufacturing process.
Ethical considerations play a significant role in the regulatory landscape, particularly concerning informed consent and patient selection criteria for clinical trials. Given the potential psychoactive effects of muscimol, regulatory bodies may require additional safeguards to protect vulnerable populations and ensure that participants fully understand the risks and benefits of the treatment.
Lastly, post-market surveillance and pharmacovigilance requirements are crucial regulatory considerations. Regulatory agencies may mandate long-term follow-up studies and robust reporting systems to monitor the safety and efficacy of muscimol-based treatments in real-world settings. This ongoing surveillance helps identify any rare or long-term adverse effects that may not have been apparent during clinical trials.
Safety and Side Effects
The safety profile and potential side effects of muscimol-based treatment protocols in tinnitus research are critical considerations for both researchers and patients. Muscimol, a potent GABA-A receptor agonist, has shown promise in modulating neural activity associated with tinnitus. However, its use requires careful evaluation of potential risks and adverse effects.
One of the primary safety concerns with muscimol is its potent sedative effect. Patients undergoing muscimol-based treatments may experience drowsiness, dizziness, and impaired cognitive function. These effects can significantly impact daily activities and potentially pose safety risks, particularly if patients operate machinery or drive vehicles. Researchers must carefully titrate dosages and monitor patients closely to mitigate these risks.
Another important consideration is the potential for muscimol to induce temporary memory impairment. Studies have shown that muscimol can interfere with memory consolidation and retrieval processes. While these effects are generally reversible, they underscore the need for careful patient selection and comprehensive informed consent procedures in clinical trials.
Muscimol's action on GABA-A receptors may also lead to changes in mood and behavior. Some patients may experience anxiety, irritability, or mood swings during treatment. Long-term use of muscimol-based therapies could potentially alter GABA signaling in the brain, necessitating careful monitoring for any persistent changes in mood or cognitive function.
Physiological side effects of muscimol treatment may include changes in blood pressure, heart rate, and respiratory function. These effects are typically dose-dependent and require close medical supervision, especially in patients with pre-existing cardiovascular or respiratory conditions. Researchers must establish clear protocols for monitoring vital signs and adjusting dosages as needed.
The potential for drug interactions is another crucial safety consideration. Muscimol may interact with other medications that affect GABA signaling, such as benzodiazepines or barbiturates. These interactions could potentiate sedative effects or lead to unexpected adverse reactions. Comprehensive medication reviews and exclusion criteria are essential components of muscimol-based treatment protocols.
Long-term safety data for muscimol use in tinnitus treatment remains limited. While acute toxicity is generally low, the effects of prolonged exposure to muscimol on brain function and overall health require further investigation. Longitudinal studies are needed to assess any potential cumulative effects or long-term alterations in neural plasticity.
One of the primary safety concerns with muscimol is its potent sedative effect. Patients undergoing muscimol-based treatments may experience drowsiness, dizziness, and impaired cognitive function. These effects can significantly impact daily activities and potentially pose safety risks, particularly if patients operate machinery or drive vehicles. Researchers must carefully titrate dosages and monitor patients closely to mitigate these risks.
Another important consideration is the potential for muscimol to induce temporary memory impairment. Studies have shown that muscimol can interfere with memory consolidation and retrieval processes. While these effects are generally reversible, they underscore the need for careful patient selection and comprehensive informed consent procedures in clinical trials.
Muscimol's action on GABA-A receptors may also lead to changes in mood and behavior. Some patients may experience anxiety, irritability, or mood swings during treatment. Long-term use of muscimol-based therapies could potentially alter GABA signaling in the brain, necessitating careful monitoring for any persistent changes in mood or cognitive function.
Physiological side effects of muscimol treatment may include changes in blood pressure, heart rate, and respiratory function. These effects are typically dose-dependent and require close medical supervision, especially in patients with pre-existing cardiovascular or respiratory conditions. Researchers must establish clear protocols for monitoring vital signs and adjusting dosages as needed.
The potential for drug interactions is another crucial safety consideration. Muscimol may interact with other medications that affect GABA signaling, such as benzodiazepines or barbiturates. These interactions could potentiate sedative effects or lead to unexpected adverse reactions. Comprehensive medication reviews and exclusion criteria are essential components of muscimol-based treatment protocols.
Long-term safety data for muscimol use in tinnitus treatment remains limited. While acute toxicity is generally low, the effects of prolonged exposure to muscimol on brain function and overall health require further investigation. Longitudinal studies are needed to assess any potential cumulative effects or long-term alterations in neural plasticity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!