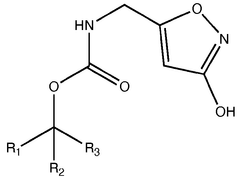
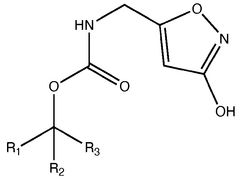
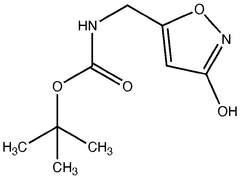
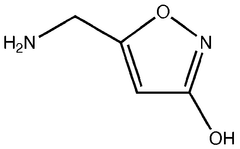
Muscimol Biosynthesis: Pathways and Enzymatic Mechanisms
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Biosynthesis Background and Objectives
Muscimol, a potent GABA receptor agonist, has garnered significant attention in the fields of neuroscience and pharmacology due to its unique psychoactive properties. The biosynthesis of muscimol represents a fascinating area of research, with implications spanning from ecological interactions to potential therapeutic applications. This technical exploration aims to elucidate the pathways and enzymatic mechanisms involved in muscimol production, providing a comprehensive overview of the current state of knowledge and identifying key areas for future investigation.
The journey of muscimol biosynthesis research began in the 1960s with the isolation and characterization of this compound from the Amanita muscaria mushroom, commonly known as the fly agaric. Since then, significant strides have been made in understanding the biochemical processes underlying its production. The primary objective of this investigation is to map out the complete biosynthetic pathway of muscimol, from its precursor molecules to the final product, and to elucidate the specific enzymatic reactions involved at each step.
One of the key challenges in this field has been the identification of the genes encoding the enzymes responsible for muscimol biosynthesis. Recent advancements in genomic and transcriptomic analyses have opened new avenues for exploring the genetic basis of this process. By leveraging these cutting-edge technologies, researchers aim to uncover the full complement of genes involved in the muscimol biosynthetic pathway, paving the way for potential biotechnological applications.
The evolution of muscimol biosynthesis presents an intriguing case study in chemical ecology. Understanding how and why certain fungi developed the ability to produce this compound can provide insights into the adaptive strategies of these organisms. Moreover, exploring the distribution of muscimol-producing species across different ecosystems may shed light on the environmental factors that influence the evolution of secondary metabolite production in fungi.
From a technological perspective, elucidating the muscimol biosynthetic pathway has the potential to enable the development of novel biocatalytic processes. These could be harnessed for the efficient and sustainable production of muscimol and related compounds, opening up new possibilities in the pharmaceutical and agrochemical industries. Additionally, understanding the enzymatic mechanisms involved may inspire the design of new synthetic routes for the production of muscimol analogues with tailored properties.
As we delve deeper into the intricacies of muscimol biosynthesis, we anticipate uncovering not only the specific biochemical reactions involved but also the regulatory mechanisms that control its production. This holistic approach will provide a more complete picture of how fungi orchestrate the synthesis of this complex molecule, potentially revealing new paradigms in fungal secondary metabolism.
The journey of muscimol biosynthesis research began in the 1960s with the isolation and characterization of this compound from the Amanita muscaria mushroom, commonly known as the fly agaric. Since then, significant strides have been made in understanding the biochemical processes underlying its production. The primary objective of this investigation is to map out the complete biosynthetic pathway of muscimol, from its precursor molecules to the final product, and to elucidate the specific enzymatic reactions involved at each step.
One of the key challenges in this field has been the identification of the genes encoding the enzymes responsible for muscimol biosynthesis. Recent advancements in genomic and transcriptomic analyses have opened new avenues for exploring the genetic basis of this process. By leveraging these cutting-edge technologies, researchers aim to uncover the full complement of genes involved in the muscimol biosynthetic pathway, paving the way for potential biotechnological applications.
The evolution of muscimol biosynthesis presents an intriguing case study in chemical ecology. Understanding how and why certain fungi developed the ability to produce this compound can provide insights into the adaptive strategies of these organisms. Moreover, exploring the distribution of muscimol-producing species across different ecosystems may shed light on the environmental factors that influence the evolution of secondary metabolite production in fungi.
From a technological perspective, elucidating the muscimol biosynthetic pathway has the potential to enable the development of novel biocatalytic processes. These could be harnessed for the efficient and sustainable production of muscimol and related compounds, opening up new possibilities in the pharmaceutical and agrochemical industries. Additionally, understanding the enzymatic mechanisms involved may inspire the design of new synthetic routes for the production of muscimol analogues with tailored properties.
As we delve deeper into the intricacies of muscimol biosynthesis, we anticipate uncovering not only the specific biochemical reactions involved but also the regulatory mechanisms that control its production. This holistic approach will provide a more complete picture of how fungi orchestrate the synthesis of this complex molecule, potentially revealing new paradigms in fungal secondary metabolism.
Market Analysis for Muscimol-based Products
The market for muscimol-based products is experiencing significant growth potential, driven by increasing research into its therapeutic applications and the rising demand for novel psychoactive compounds. Muscimol, a potent GABA receptor agonist found naturally in Amanita mushrooms, has garnered attention for its potential use in treating various neurological and psychiatric disorders.
The pharmaceutical sector represents the primary market for muscimol-based products, with ongoing clinical trials exploring its efficacy in treating epilepsy, anxiety disorders, and sleep disturbances. The global market for antiepileptic drugs, a potential application area for muscimol, is projected to expand substantially in the coming years, indicating a promising opportunity for muscimol-based therapies.
In the field of neurology, muscimol's ability to modulate GABA receptors has sparked interest in its potential to treat conditions such as Parkinson's disease and multiple sclerosis. As the global burden of neurological disorders continues to rise, the demand for innovative treatments is expected to drive further research and development of muscimol-derived pharmaceuticals.
The nutraceutical and functional food industries are also showing interest in muscimol-based products, particularly in the context of stress relief and cognitive enhancement. With the growing consumer trend towards natural and plant-based remedies, muscimol extracted from mushrooms could find applications in dietary supplements and functional beverages.
However, the market for muscimol-based products faces several challenges. Regulatory hurdles, particularly due to muscimol's psychoactive properties, may impede rapid market entry in many jurisdictions. Additionally, the limited natural availability of muscimol-producing mushrooms necessitates the development of efficient biosynthetic pathways for large-scale production.
The potential for muscimol in the veterinary market should not be overlooked. Its anxiolytic properties could be beneficial in treating anxiety-related behaviors in companion animals, a growing concern among pet owners. This represents an additional avenue for market expansion.
As research into muscimol biosynthesis advances, the ability to produce the compound through engineered microorganisms or plant cell cultures could significantly reduce production costs and increase supply stability. This development would likely accelerate market growth and open up new application areas.
In conclusion, the market for muscimol-based products shows promise across multiple sectors, with the pharmaceutical industry leading the way. As biosynthetic production methods improve and regulatory pathways become clearer, the market is poised for substantial growth in the coming years.
The pharmaceutical sector represents the primary market for muscimol-based products, with ongoing clinical trials exploring its efficacy in treating epilepsy, anxiety disorders, and sleep disturbances. The global market for antiepileptic drugs, a potential application area for muscimol, is projected to expand substantially in the coming years, indicating a promising opportunity for muscimol-based therapies.
In the field of neurology, muscimol's ability to modulate GABA receptors has sparked interest in its potential to treat conditions such as Parkinson's disease and multiple sclerosis. As the global burden of neurological disorders continues to rise, the demand for innovative treatments is expected to drive further research and development of muscimol-derived pharmaceuticals.
The nutraceutical and functional food industries are also showing interest in muscimol-based products, particularly in the context of stress relief and cognitive enhancement. With the growing consumer trend towards natural and plant-based remedies, muscimol extracted from mushrooms could find applications in dietary supplements and functional beverages.
However, the market for muscimol-based products faces several challenges. Regulatory hurdles, particularly due to muscimol's psychoactive properties, may impede rapid market entry in many jurisdictions. Additionally, the limited natural availability of muscimol-producing mushrooms necessitates the development of efficient biosynthetic pathways for large-scale production.
The potential for muscimol in the veterinary market should not be overlooked. Its anxiolytic properties could be beneficial in treating anxiety-related behaviors in companion animals, a growing concern among pet owners. This represents an additional avenue for market expansion.
As research into muscimol biosynthesis advances, the ability to produce the compound through engineered microorganisms or plant cell cultures could significantly reduce production costs and increase supply stability. This development would likely accelerate market growth and open up new application areas.
In conclusion, the market for muscimol-based products shows promise across multiple sectors, with the pharmaceutical industry leading the way. As biosynthetic production methods improve and regulatory pathways become clearer, the market is poised for substantial growth in the coming years.
Current Challenges in Muscimol Production
Despite the growing interest in muscimol as a potential therapeutic agent, its production faces several significant challenges. The primary obstacle lies in the complexity of the biosynthetic pathway, which is not yet fully elucidated. Current understanding suggests that muscimol is derived from glutamate, but the exact sequence of enzymatic reactions and intermediate compounds remains unclear. This knowledge gap hinders the development of efficient production methods.
Another major challenge is the low yield of muscimol in natural sources. The Amanita mushroom species, particularly Amanita muscaria, are the primary natural producers of muscimol. However, the concentration of muscimol in these mushrooms is relatively low, making large-scale extraction economically unfeasible. Additionally, the cultivation of Amanita species is challenging due to their mycorrhizal nature, requiring specific symbiotic relationships with tree roots.
The lack of a robust heterologous expression system for muscimol production presents a significant hurdle. While synthetic biology approaches have been successful for many other natural products, the complex and poorly understood biosynthetic pathway of muscimol has made it difficult to engineer microorganisms for its production. This limitation severely restricts the ability to scale up production and meet potential commercial demands.
Safety concerns also pose challenges in muscimol production. Amanita mushrooms contain other toxic compounds, such as ibotenic acid, which can be difficult to separate from muscimol during extraction processes. Ensuring the purity and safety of muscimol products is crucial, especially if intended for therapeutic use, and requires the development of sophisticated purification techniques.
Regulatory hurdles further complicate muscimol production. As a psychoactive compound, muscimol is subject to strict regulations in many countries. This regulatory environment can make research and development challenging, limiting the ability to conduct large-scale studies or clinical trials necessary for advancing muscimol-based therapies.
Lastly, the stability of muscimol during storage and formulation presents technical challenges. Muscimol is known to be sensitive to pH changes and can degrade under certain conditions. Developing stable formulations that maintain the compound's efficacy over time is essential for any potential commercial applications, particularly in the pharmaceutical industry.
Another major challenge is the low yield of muscimol in natural sources. The Amanita mushroom species, particularly Amanita muscaria, are the primary natural producers of muscimol. However, the concentration of muscimol in these mushrooms is relatively low, making large-scale extraction economically unfeasible. Additionally, the cultivation of Amanita species is challenging due to their mycorrhizal nature, requiring specific symbiotic relationships with tree roots.
The lack of a robust heterologous expression system for muscimol production presents a significant hurdle. While synthetic biology approaches have been successful for many other natural products, the complex and poorly understood biosynthetic pathway of muscimol has made it difficult to engineer microorganisms for its production. This limitation severely restricts the ability to scale up production and meet potential commercial demands.
Safety concerns also pose challenges in muscimol production. Amanita mushrooms contain other toxic compounds, such as ibotenic acid, which can be difficult to separate from muscimol during extraction processes. Ensuring the purity and safety of muscimol products is crucial, especially if intended for therapeutic use, and requires the development of sophisticated purification techniques.
Regulatory hurdles further complicate muscimol production. As a psychoactive compound, muscimol is subject to strict regulations in many countries. This regulatory environment can make research and development challenging, limiting the ability to conduct large-scale studies or clinical trials necessary for advancing muscimol-based therapies.
Lastly, the stability of muscimol during storage and formulation presents technical challenges. Muscimol is known to be sensitive to pH changes and can degrade under certain conditions. Developing stable formulations that maintain the compound's efficacy over time is essential for any potential commercial applications, particularly in the pharmaceutical industry.
Existing Muscimol Biosynthesis Methods
01 Pharmaceutical compositions containing muscimol
Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include different formulations and delivery methods to enhance the efficacy and bioavailability of muscimol. The compositions can be designed for treating neurological disorders, anxiety, or other conditions affected by GABA receptor modulation.- Pharmaceutical compositions containing muscimol: Muscimol is used in various pharmaceutical compositions for treating neurological and psychiatric disorders. These formulations may include specific dosage forms, delivery methods, and combinations with other active ingredients to enhance therapeutic effects or reduce side effects.
- Muscimol derivatives and analogs: Research focuses on developing muscimol derivatives and analogs with improved pharmacological properties. These modified compounds aim to enhance efficacy, reduce side effects, or alter the pharmacokinetic profile of muscimol for specific therapeutic applications.
- Muscimol in neurostimulation therapies: Muscimol is explored in combination with neurostimulation techniques for treating various neurological conditions. This approach may involve targeted delivery of muscimol to specific brain regions in conjunction with electrical or magnetic stimulation to modulate neural activity.
- Muscimol for treating addiction and substance abuse: Research investigates the potential of muscimol in treating addiction and substance abuse disorders. Studies explore its effects on neurotransmitter systems involved in addiction pathways and its ability to reduce cravings or withdrawal symptoms.
- Muscimol in combination with other GABAergic compounds: Investigations focus on combining muscimol with other GABAergic compounds to enhance therapeutic effects or reduce side effects. These combinations may target multiple GABA receptor subtypes or modulate GABAergic signaling through different mechanisms.
02 Muscimol analogs and derivatives
Research focuses on developing and synthesizing muscimol analogs and derivatives. These modified compounds aim to improve upon the properties of muscimol, such as increased potency, selectivity, or reduced side effects. The analogs may be designed to target specific GABA receptor subtypes or to have enhanced pharmacokinetic profiles.Expand Specific Solutions03 Use of muscimol in neurostimulation therapies
Muscimol is explored in combination with neurostimulation techniques for treating neurological and psychiatric disorders. This approach may involve the use of muscimol to enhance the effects of electrical or magnetic stimulation of the brain, potentially improving outcomes in conditions such as depression or epilepsy.Expand Specific Solutions04 Muscimol in combination therapies
Muscimol is investigated as part of combination therapies with other active compounds. These combinations may target multiple pathways or receptors simultaneously, potentially leading to synergistic effects in treating various disorders. The combinations could include other GABA modulators, antidepressants, or anxiolytics.Expand Specific Solutions05 Novel delivery systems for muscimol
Innovative delivery systems are being developed to improve the administration and efficacy of muscimol. These may include transdermal patches, nanoparticle formulations, or controlled-release systems. The goal is to enhance the bioavailability of muscimol, reduce dosing frequency, or target specific areas of the body or brain.Expand Specific Solutions
Key Players in Muscimol Research and Production
The muscimol biosynthesis market is in its early development stage, characterized by limited commercial applications and ongoing research efforts. The market size remains relatively small, primarily driven by academic and pharmaceutical research interests. Technologically, muscimol biosynthesis is still evolving, with various approaches being explored by research institutions and biotechnology companies. Key players like Genomatica, BASF Plant Science, and GreenLight Biosciences are leveraging their expertise in bioengineering and synthetic biology to advance the field. Academic institutions such as MIT, Zhejiang University, and the University of Manchester are contributing significant research to elucidate biosynthetic pathways and enzymatic mechanisms. While the technology shows promise, it has not yet reached full commercial maturity, indicating potential for future growth and innovation in this niche sector.
BASF Plant Science LLC
Technical Solution: BASF Plant Science LLC has been exploring the potential of muscimol biosynthesis in plants. Their approach involves metabolic engineering of plant systems to produce muscimol and related compounds. They have focused on introducing fungal biosynthetic genes into plant hosts, aiming to create a sustainable and scalable production platform. Their research includes optimizing gene expression, metabolic flux analysis, and developing plant-based bioreactors for muscimol production[3]. BASF has also investigated the use of synthetic biology tools to enhance muscimol yields in engineered plant systems.
Strengths: Strong expertise in plant biotechnology and metabolic engineering. Extensive resources for scaling up production. Weaknesses: Potential regulatory challenges associated with genetically modified plants.
Inbiose NV
Technical Solution: Inbiose NV has developed a proprietary fermentation-based platform for the production of complex carbohydrates and related compounds, which they have adapted for muscimol biosynthesis. Their approach involves engineering microbial strains, particularly yeast and bacteria, to express the necessary enzymes for muscimol production. They have focused on optimizing fermentation conditions and developing efficient downstream processing methods to isolate and purify muscimol[4]. Inbiose's technology allows for the production of muscimol and its precursors using renewable feedstocks, potentially offering a more sustainable alternative to traditional extraction methods.
Strengths: Expertise in industrial fermentation and process optimization. Flexible platform adaptable to various compounds. Weaknesses: May face challenges in achieving high yields compared to plant-based systems.
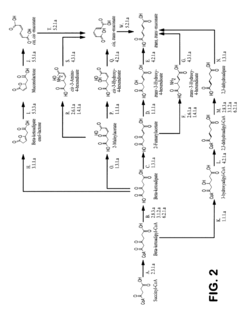
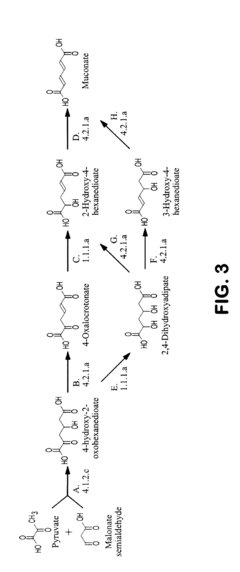
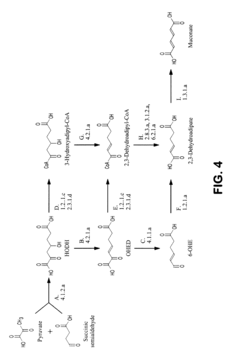
Critical Enzymes in Muscimol Biosynthesis
Pharmaceutical intermediates and methods for preparing the same in the synthesis of muscimol and congeners and derivatives thereof
PatentWO2025128106A1
Innovation
- A novel method for preparing muscimol mono-BOC and muscimol hydrochloride that avoids the use of ion exchange chromatography by modifying the original synthetic route to include a flow reactor for the cyclization step and using BOC anhydride to purify the muscimol, thereby stabilizing the product and improving yields.
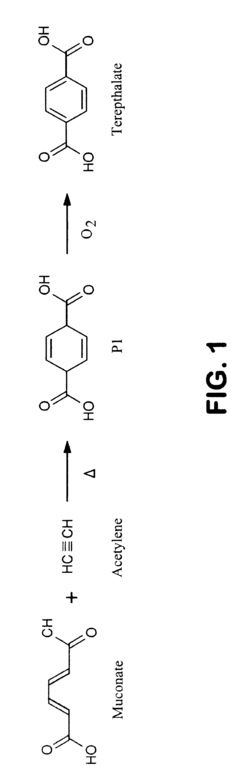
Semi-synthetic terephthalic acid via microorganisms that produce muconic acid
PatentInactiveUS20180312883A1
Innovation
- Development of a non-naturally occurring microbial organism with a muconate pathway that includes specific enzymes such as beta-ketothiolase, beta-ketoadipyl-CoA hydrolase, and muconate cis/trans isomerase to produce trans,trans and cis,trans muconate from simple carbohydrate feedstocks, enabling conversion to PET through a two-step process.
Safety and Regulatory Considerations for Muscimol
The biosynthesis and use of muscimol, a potent GABA receptor agonist found in certain mushroom species, necessitates careful consideration of safety and regulatory aspects. Given its psychoactive properties and potential for misuse, muscimol is subject to strict regulatory oversight in many jurisdictions.
From a safety perspective, muscimol poses significant risks due to its powerful effects on the central nervous system. Acute toxicity can result in symptoms such as confusion, delirium, and loss of muscle control. Chronic exposure may lead to long-term neurological effects, though these are not fully characterized. Proper handling and containment protocols are essential for researchers and manufacturers working with muscimol to prevent accidental exposure.
Regulatory bodies, such as the FDA in the United States and the EMA in Europe, classify muscimol as a controlled substance. Its production, distribution, and use are tightly regulated, requiring special licenses and permits. Research involving muscimol typically requires institutional review board approval and adherence to strict protocols for human or animal studies.
In the pharmaceutical context, any potential therapeutic applications of muscimol or its derivatives would need to undergo rigorous clinical trials to establish safety and efficacy profiles. The regulatory pathway for approval would likely be complex, given the compound's psychoactive nature and potential for abuse.
Environmental considerations are also crucial. The biosynthesis of muscimol, whether through natural extraction or synthetic means, must adhere to environmental protection regulations to prevent contamination of water sources or ecosystems. Proper waste management and disposal procedures are essential to mitigate ecological risks.
Labeling and packaging requirements for muscimol-containing products are stringent, necessitating clear warnings about potential risks and contraindications. Chain of custody documentation is critical to ensure the compound is not diverted for illicit use.
As research into muscimol's biosynthesis and potential applications progresses, regulatory frameworks may evolve. Ongoing dialogue between researchers, industry stakeholders, and regulatory agencies is crucial to ensure that safety standards keep pace with scientific advancements while facilitating responsible research and development in this field.
From a safety perspective, muscimol poses significant risks due to its powerful effects on the central nervous system. Acute toxicity can result in symptoms such as confusion, delirium, and loss of muscle control. Chronic exposure may lead to long-term neurological effects, though these are not fully characterized. Proper handling and containment protocols are essential for researchers and manufacturers working with muscimol to prevent accidental exposure.
Regulatory bodies, such as the FDA in the United States and the EMA in Europe, classify muscimol as a controlled substance. Its production, distribution, and use are tightly regulated, requiring special licenses and permits. Research involving muscimol typically requires institutional review board approval and adherence to strict protocols for human or animal studies.
In the pharmaceutical context, any potential therapeutic applications of muscimol or its derivatives would need to undergo rigorous clinical trials to establish safety and efficacy profiles. The regulatory pathway for approval would likely be complex, given the compound's psychoactive nature and potential for abuse.
Environmental considerations are also crucial. The biosynthesis of muscimol, whether through natural extraction or synthetic means, must adhere to environmental protection regulations to prevent contamination of water sources or ecosystems. Proper waste management and disposal procedures are essential to mitigate ecological risks.
Labeling and packaging requirements for muscimol-containing products are stringent, necessitating clear warnings about potential risks and contraindications. Chain of custody documentation is critical to ensure the compound is not diverted for illicit use.
As research into muscimol's biosynthesis and potential applications progresses, regulatory frameworks may evolve. Ongoing dialogue between researchers, industry stakeholders, and regulatory agencies is crucial to ensure that safety standards keep pace with scientific advancements while facilitating responsible research and development in this field.
Environmental Impact of Muscimol Biosynthesis
The environmental impact of muscimol biosynthesis is a critical aspect to consider in the study of this compound's production and utilization. Muscimol, a psychoactive compound found in certain mushroom species, has gained attention for its potential therapeutic applications. However, its biosynthesis and subsequent environmental effects warrant careful examination.
The production of muscimol in natural ecosystems primarily occurs in Amanita mushroom species, which play crucial roles in forest ecosystems. These fungi form symbiotic relationships with trees through mycorrhizal associations, facilitating nutrient exchange and supporting overall forest health. The biosynthesis of muscimol within these organisms may influence their ecological interactions and the broader ecosystem dynamics.
One potential environmental concern is the impact of muscimol on soil microorganisms and other plants. As muscimol is released into the soil through fungal decomposition or root exudates, it may affect the growth and behavior of surrounding organisms. Some studies suggest that muscimol can act as an allelochemical, potentially inhibiting the growth of competing plants or altering microbial communities in the rhizosphere.
The presence of muscimol in the environment may also affect insect populations and their interactions with plants. Some insects may be deterred by the compound, while others might be attracted to it, potentially altering pollination patterns or herbivory rates in affected ecosystems. This could lead to cascading effects on plant reproduction and community composition.
Furthermore, the bioaccumulation of muscimol in the food chain is an area of concern. As animals consume mushrooms containing muscimol, the compound may accumulate in their tissues, potentially affecting predators higher up the food chain. This bioaccumulation could have unforeseen consequences on wildlife behavior and population dynamics.
From a broader perspective, the increasing interest in muscimol for pharmaceutical purposes may lead to increased cultivation of Amanita species or the development of synthetic production methods. This could result in habitat alterations or the introduction of non-native species, potentially disrupting local ecosystems. Careful management and regulation of muscimol production would be necessary to mitigate these risks.
In conclusion, while the biosynthesis of muscimol is a fascinating area of study with potential benefits, its environmental impact must be carefully considered. Further research is needed to fully understand the ecological consequences of muscimol production and to develop sustainable practices for its utilization in various applications.
The production of muscimol in natural ecosystems primarily occurs in Amanita mushroom species, which play crucial roles in forest ecosystems. These fungi form symbiotic relationships with trees through mycorrhizal associations, facilitating nutrient exchange and supporting overall forest health. The biosynthesis of muscimol within these organisms may influence their ecological interactions and the broader ecosystem dynamics.
One potential environmental concern is the impact of muscimol on soil microorganisms and other plants. As muscimol is released into the soil through fungal decomposition or root exudates, it may affect the growth and behavior of surrounding organisms. Some studies suggest that muscimol can act as an allelochemical, potentially inhibiting the growth of competing plants or altering microbial communities in the rhizosphere.
The presence of muscimol in the environment may also affect insect populations and their interactions with plants. Some insects may be deterred by the compound, while others might be attracted to it, potentially altering pollination patterns or herbivory rates in affected ecosystems. This could lead to cascading effects on plant reproduction and community composition.
Furthermore, the bioaccumulation of muscimol in the food chain is an area of concern. As animals consume mushrooms containing muscimol, the compound may accumulate in their tissues, potentially affecting predators higher up the food chain. This bioaccumulation could have unforeseen consequences on wildlife behavior and population dynamics.
From a broader perspective, the increasing interest in muscimol for pharmaceutical purposes may lead to increased cultivation of Amanita species or the development of synthetic production methods. This could result in habitat alterations or the introduction of non-native species, potentially disrupting local ecosystems. Careful management and regulation of muscimol production would be necessary to mitigate these risks.
In conclusion, while the biosynthesis of muscimol is a fascinating area of study with potential benefits, its environmental impact must be carefully considered. Further research is needed to fully understand the ecological consequences of muscimol production and to develop sustainable practices for its utilization in various applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!