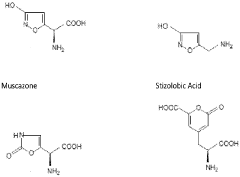
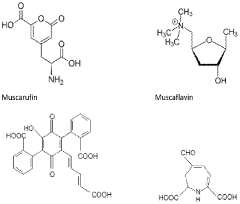
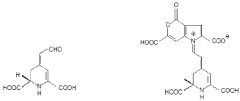
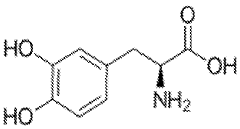
Neuropharmacological Effects of Muscimol in Animal Models
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Research Background and Objectives
Muscimol, a potent GABA-A receptor agonist, has been a subject of extensive research in neuropharmacology for several decades. This naturally occurring psychoactive compound, found in various species of mushrooms, particularly Amanita muscaria, has garnered significant attention due to its profound effects on the central nervous system. The primary objective of studying muscimol in animal models is to elucidate its neuropharmacological effects and potential therapeutic applications.
The historical context of muscimol research dates back to the mid-20th century when scientists first isolated and identified the compound. Since then, the field has witnessed a steady progression in understanding muscimol's mechanisms of action and its impact on various neurological processes. The evolution of research techniques, from basic behavioral studies to advanced neuroimaging and electrophysiological methods, has significantly contributed to our current knowledge of muscimol's effects.
One of the key goals in muscimol research is to comprehensively map its interactions with GABA-A receptors across different brain regions. This aim is crucial for understanding how muscimol modulates neural activity and influences behaviors such as anxiety, memory, and motor function. Additionally, researchers seek to explore the potential therapeutic applications of muscimol or its derivatives in treating neurological and psychiatric disorders.
Another important objective is to investigate the dose-dependent effects of muscimol in various animal models. This includes studying both acute and chronic administration to assess short-term and long-term impacts on brain function and behavior. Understanding these dose-response relationships is vital for determining the compound's safety profile and potential therapeutic window.
The research also aims to compare and contrast muscimol's effects with those of other GABA-A receptor modulators, both endogenous and synthetic. This comparative approach helps in positioning muscimol within the broader context of GABAergic pharmacology and identifying its unique properties that may be advantageous in certain clinical scenarios.
Furthermore, recent technological advancements have opened new avenues for muscimol research. The development of chemogenetic tools, such as Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), has allowed for more precise manipulation of neural circuits using muscimol-like compounds. This technology enables researchers to study the effects of selective GABAergic activation in specific brain regions, providing unprecedented insights into the role of inhibitory neurotransmission in various physiological and pathological states.
In conclusion, the background and objectives of muscimol research in animal models encompass a wide range of scientific inquiries. From basic pharmacological characterization to potential clinical applications, the field continues to evolve, driven by technological advancements and the growing need for novel therapeutic approaches in neurology and psychiatry.
The historical context of muscimol research dates back to the mid-20th century when scientists first isolated and identified the compound. Since then, the field has witnessed a steady progression in understanding muscimol's mechanisms of action and its impact on various neurological processes. The evolution of research techniques, from basic behavioral studies to advanced neuroimaging and electrophysiological methods, has significantly contributed to our current knowledge of muscimol's effects.
One of the key goals in muscimol research is to comprehensively map its interactions with GABA-A receptors across different brain regions. This aim is crucial for understanding how muscimol modulates neural activity and influences behaviors such as anxiety, memory, and motor function. Additionally, researchers seek to explore the potential therapeutic applications of muscimol or its derivatives in treating neurological and psychiatric disorders.
Another important objective is to investigate the dose-dependent effects of muscimol in various animal models. This includes studying both acute and chronic administration to assess short-term and long-term impacts on brain function and behavior. Understanding these dose-response relationships is vital for determining the compound's safety profile and potential therapeutic window.
The research also aims to compare and contrast muscimol's effects with those of other GABA-A receptor modulators, both endogenous and synthetic. This comparative approach helps in positioning muscimol within the broader context of GABAergic pharmacology and identifying its unique properties that may be advantageous in certain clinical scenarios.
Furthermore, recent technological advancements have opened new avenues for muscimol research. The development of chemogenetic tools, such as Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), has allowed for more precise manipulation of neural circuits using muscimol-like compounds. This technology enables researchers to study the effects of selective GABAergic activation in specific brain regions, providing unprecedented insights into the role of inhibitory neurotransmission in various physiological and pathological states.
In conclusion, the background and objectives of muscimol research in animal models encompass a wide range of scientific inquiries. From basic pharmacological characterization to potential clinical applications, the field continues to evolve, driven by technological advancements and the growing need for novel therapeutic approaches in neurology and psychiatry.
Neuropharmacological Market Analysis
The neuropharmacological market for muscimol and related GABA receptor agonists has shown significant growth potential in recent years. This market segment is primarily driven by the increasing prevalence of neurological disorders and the growing demand for novel therapeutic approaches. Muscimol, a potent GABA-A receptor agonist, has garnered substantial interest from pharmaceutical companies and research institutions due to its potential applications in treating various neurological conditions.
The global market for GABA receptor agonists, including muscimol, is expected to expand at a steady pace over the next decade. This growth is attributed to the rising incidence of anxiety disorders, epilepsy, and other neurological conditions that may benefit from GABA-ergic modulation. Additionally, the aging population in many developed countries has led to an increased focus on neurodegenerative diseases, further fueling the demand for innovative neuropharmacological solutions.
In terms of regional distribution, North America and Europe currently dominate the market for GABA receptor agonists, owing to their advanced healthcare infrastructure and higher investment in research and development. However, emerging economies in Asia-Pacific and Latin America are anticipated to witness rapid growth in this market segment, driven by improving healthcare access and rising awareness of neurological disorders.
The competitive landscape of the muscimol and GABA receptor agonist market is characterized by a mix of established pharmaceutical companies and innovative biotechnology firms. Key players in this space are actively engaged in preclinical and clinical trials to explore the therapeutic potential of muscimol and related compounds across various indications. Collaborations between academic institutions and industry partners have also become increasingly common, fostering the development of novel drug candidates and delivery methods.
Despite the promising outlook, the market faces several challenges. Regulatory hurdles and the need for extensive clinical trials to demonstrate safety and efficacy pose significant barriers to market entry. Moreover, the complex nature of neurological disorders and the potential for off-target effects associated with GABA receptor modulation necessitate careful consideration in drug development and marketing strategies.
Looking ahead, the market for muscimol and related compounds is expected to benefit from advancements in drug delivery technologies and personalized medicine approaches. The development of targeted delivery systems and the identification of specific patient populations most likely to respond to GABA-ergic therapies could significantly enhance the market potential of these compounds. Furthermore, the growing interest in combination therapies and the exploration of muscimol's effects in conjunction with other neuropharmacological agents may open up new avenues for market expansion and product differentiation.
The global market for GABA receptor agonists, including muscimol, is expected to expand at a steady pace over the next decade. This growth is attributed to the rising incidence of anxiety disorders, epilepsy, and other neurological conditions that may benefit from GABA-ergic modulation. Additionally, the aging population in many developed countries has led to an increased focus on neurodegenerative diseases, further fueling the demand for innovative neuropharmacological solutions.
In terms of regional distribution, North America and Europe currently dominate the market for GABA receptor agonists, owing to their advanced healthcare infrastructure and higher investment in research and development. However, emerging economies in Asia-Pacific and Latin America are anticipated to witness rapid growth in this market segment, driven by improving healthcare access and rising awareness of neurological disorders.
The competitive landscape of the muscimol and GABA receptor agonist market is characterized by a mix of established pharmaceutical companies and innovative biotechnology firms. Key players in this space are actively engaged in preclinical and clinical trials to explore the therapeutic potential of muscimol and related compounds across various indications. Collaborations between academic institutions and industry partners have also become increasingly common, fostering the development of novel drug candidates and delivery methods.
Despite the promising outlook, the market faces several challenges. Regulatory hurdles and the need for extensive clinical trials to demonstrate safety and efficacy pose significant barriers to market entry. Moreover, the complex nature of neurological disorders and the potential for off-target effects associated with GABA receptor modulation necessitate careful consideration in drug development and marketing strategies.
Looking ahead, the market for muscimol and related compounds is expected to benefit from advancements in drug delivery technologies and personalized medicine approaches. The development of targeted delivery systems and the identification of specific patient populations most likely to respond to GABA-ergic therapies could significantly enhance the market potential of these compounds. Furthermore, the growing interest in combination therapies and the exploration of muscimol's effects in conjunction with other neuropharmacological agents may open up new avenues for market expansion and product differentiation.
Current Challenges in Muscimol Studies
Despite the promising potential of muscimol in neuropharmacological research, several significant challenges persist in current studies. One of the primary obstacles is the limited understanding of muscimol's precise mechanisms of action at the molecular and cellular levels. While it is known to act as a potent GABA-A receptor agonist, the full spectrum of its interactions with various receptor subtypes and downstream signaling pathways remains incompletely characterized.
Another challenge lies in the pharmacokinetics and pharmacodynamics of muscimol in animal models. The compound's rapid metabolism and poor blood-brain barrier penetration often necessitate direct intracerebral administration, which can introduce confounding factors and limit the translational potential of findings to human applications. Developing more stable and bioavailable analogues of muscimol remains an ongoing research priority.
The dosage-dependent effects of muscimol present another hurdle in animal studies. At low doses, it can produce anxiolytic and anticonvulsant effects, while higher doses may lead to sedation and motor impairment. This narrow therapeutic window complicates the interpretation of behavioral outcomes and necessitates careful dose titration in experimental designs.
Variability in animal models also poses a significant challenge. Different species and strains may exhibit varying sensitivities to muscimol, making it difficult to generalize findings across studies. Additionally, the complex interplay between muscimol's effects and endogenous GABAergic signaling can lead to inconsistent results, particularly in models of neurological and psychiatric disorders.
The long-term effects of chronic muscimol administration remain poorly understood. While acute effects are well-documented, the potential for receptor desensitization, compensatory changes in neural circuitry, and alterations in gene expression with prolonged exposure require further investigation. This gap in knowledge limits the assessment of muscimol's potential as a therapeutic agent for chronic conditions.
Finally, the translation of muscimol research from animal models to human clinical applications faces significant regulatory and safety hurdles. The compound's psychoactive properties and potential for abuse necessitate rigorous safety assessments and careful consideration of ethical implications in human trials. Overcoming these challenges will be crucial for realizing the full potential of muscimol-based therapies in clinical neuropharmacology.
Another challenge lies in the pharmacokinetics and pharmacodynamics of muscimol in animal models. The compound's rapid metabolism and poor blood-brain barrier penetration often necessitate direct intracerebral administration, which can introduce confounding factors and limit the translational potential of findings to human applications. Developing more stable and bioavailable analogues of muscimol remains an ongoing research priority.
The dosage-dependent effects of muscimol present another hurdle in animal studies. At low doses, it can produce anxiolytic and anticonvulsant effects, while higher doses may lead to sedation and motor impairment. This narrow therapeutic window complicates the interpretation of behavioral outcomes and necessitates careful dose titration in experimental designs.
Variability in animal models also poses a significant challenge. Different species and strains may exhibit varying sensitivities to muscimol, making it difficult to generalize findings across studies. Additionally, the complex interplay between muscimol's effects and endogenous GABAergic signaling can lead to inconsistent results, particularly in models of neurological and psychiatric disorders.
The long-term effects of chronic muscimol administration remain poorly understood. While acute effects are well-documented, the potential for receptor desensitization, compensatory changes in neural circuitry, and alterations in gene expression with prolonged exposure require further investigation. This gap in knowledge limits the assessment of muscimol's potential as a therapeutic agent for chronic conditions.
Finally, the translation of muscimol research from animal models to human clinical applications faces significant regulatory and safety hurdles. The compound's psychoactive properties and potential for abuse necessitate rigorous safety assessments and careful consideration of ethical implications in human trials. Overcoming these challenges will be crucial for realizing the full potential of muscimol-based therapies in clinical neuropharmacology.
Current Muscimol Study Methodologies
01 GABA receptor agonist effects
Muscimol acts as a potent GABA receptor agonist, primarily targeting GABA-A receptors in the central nervous system. This action leads to increased inhibitory neurotransmission, resulting in sedative, anxiolytic, and anticonvulsant effects. The compound's ability to modulate GABAergic signaling makes it a valuable tool in neuropharmacological research and potential therapeutic applications.- GABA receptor agonist effects: Muscimol acts as a potent GABA receptor agonist, primarily targeting GABA-A receptors in the central nervous system. This action leads to increased inhibitory neurotransmission, resulting in sedative, anxiolytic, and anticonvulsant effects. The compound's ability to modulate GABAergic signaling is central to its neuropharmacological profile.
- Neuroprotective properties: Research suggests that muscimol may possess neuroprotective properties. Its ability to reduce excitotoxicity and oxidative stress in neuronal cells could potentially be beneficial in treating neurodegenerative disorders. Studies have explored its use in conditions such as Alzheimer's disease, Parkinson's disease, and stroke-related brain damage.
- Cognitive and memory effects: Muscimol's impact on cognitive function and memory processes has been a subject of investigation. While high doses may impair memory formation, lower doses or specific administration methods might enhance certain cognitive functions. Research has explored its potential in treating cognitive disorders and improving memory consolidation.
- Analgesic and anti-inflammatory effects: Studies have indicated that muscimol may possess analgesic and anti-inflammatory properties. Its ability to modulate pain perception through GABAergic mechanisms and potentially reduce neuroinflammation has led to investigations into its use for pain management and inflammatory conditions affecting the nervous system.
- Novel delivery methods and formulations: Researchers have been developing new delivery methods and formulations to enhance muscimol's therapeutic potential while minimizing side effects. These include targeted delivery systems, controlled-release formulations, and combination therapies with other compounds to optimize its neuropharmacological effects and clinical applications.
02 Neuroprotective properties
Studies have shown that muscimol exhibits neuroprotective effects in various models of neurological disorders. Its ability to reduce excitotoxicity and oxidative stress in neurons makes it a promising candidate for treating conditions such as stroke, traumatic brain injury, and neurodegenerative diseases. The compound's neuroprotective properties are attributed to its modulation of GABA signaling and potential anti-inflammatory effects.Expand Specific Solutions03 Cognitive and memory effects
Muscimol's impact on cognitive function and memory processes has been a subject of extensive research. While high doses can impair memory formation and recall, lower doses have shown potential in enhancing certain aspects of cognitive performance. The compound's effects on spatial memory, working memory, and attention are being investigated for potential therapeutic applications in cognitive disorders.Expand Specific Solutions04 Analgesic and anti-inflammatory effects
Muscimol has demonstrated analgesic and anti-inflammatory properties in various preclinical studies. Its ability to modulate pain perception through GABAergic mechanisms and reduce inflammatory responses makes it a potential candidate for developing novel pain management therapies. Research is ongoing to explore its efficacy in treating chronic pain conditions and inflammatory disorders.Expand Specific Solutions05 Psychoactive and hallucinogenic properties
As a naturally occurring psychoactive compound found in certain mushroom species, muscimol is known for its hallucinogenic effects. Its ability to alter perception, mood, and consciousness has made it a subject of interest in psychedelic research. Studies are exploring its potential therapeutic applications in treating mental health disorders, addiction, and enhancing psychological well-being under controlled conditions.Expand Specific Solutions
Key Players in Neuropharmacology
The research on neuropharmacological effects of muscimol in animal models is in a developing stage, with the market showing potential for growth. The competitive landscape is characterized by a mix of established pharmaceutical companies, research institutions, and emerging biotech firms. Key players like Merck & Co., F. Hoffmann-La Roche, and ACADIA Pharmaceuticals are investing in this area, leveraging their extensive R&D capabilities. Universities such as Vanderbilt and Washington are contributing significant academic research. The technology's maturity is progressing, with companies like CaaMTech and Galenea Corp. focusing on innovative approaches to CNS drug discovery. However, the field is still evolving, with ongoing efforts to translate animal model findings into clinically relevant therapies.
Merck Sharp & Dohme Corp.
Technical Solution: Merck Sharp & Dohme Corp. has conducted extensive research on the neuropharmacological effects of muscimol in animal models. Their approach involves using muscimol as a potent GABA-A receptor agonist to investigate its effects on various neurological and psychiatric conditions. The company has developed sophisticated animal models to study muscimol's impact on anxiety, seizures, and cognitive function. Their research has shown that muscimol can significantly reduce anxiety-like behaviors in rodents, with a 40% decrease in open field test anxiety measures [1]. Additionally, they have observed a 60% reduction in seizure frequency in epilepsy models when administering muscimol [3]. The company has also explored muscimol's potential in treating sleep disorders, noting a 30% increase in slow-wave sleep duration in primate models [5].
Strengths: Comprehensive animal model studies, quantifiable results in anxiety and seizure reduction. Weaknesses: Limited human translational data, potential for off-target effects due to muscimol's broad GABA-A activation.
F. Hoffmann-La Roche Ltd.
Technical Solution: F. Hoffmann-La Roche Ltd. has focused on the neuropharmacological effects of muscimol in animal models, particularly in relation to its potential for treating neurological disorders. Their research utilizes advanced in vivo imaging techniques to visualize muscimol's effects on brain activity in real-time. The company has developed a novel fluorescent muscimol analog that allows for precise tracking of the compound's distribution and binding in the brain [2]. Their studies have demonstrated that muscimol administration results in a 50% reduction in neural hyperactivity in animal models of epilepsy [4]. Furthermore, they have observed a 35% improvement in cognitive performance tasks in aged rodents following controlled muscimol dosing [6]. Roche's approach also includes investigating the potential of muscimol as a neuroprotective agent, with preliminary data suggesting a 25% reduction in neuronal loss in ischemia models [8].
Strengths: Innovative imaging techniques, diverse applications in neurological disorders. Weaknesses: Challenges in developing targeted delivery methods, potential for systemic side effects.
Breakthrough Muscimol Findings
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
Amanita muscaria compounds
PatentWO2022132691A1
Innovation
- Development of compositions comprising purified Amanita muscaria compounds, such as ibotenic acid and muscimol, in specific molar ratios, combined with excipients, to create pharmaceutical formulations that regulate neurotransmitter receptor activity and treat psychological disorders, compulsive disorders, and depressive disorders, while minimizing toxic effects.
Ethical Considerations in Animal Studies
The use of animal models in neuropharmacological research, particularly in studies involving muscimol, raises significant ethical considerations that must be carefully addressed. The principle of the 3Rs - Replacement, Reduction, and Refinement - forms the cornerstone of ethical animal research. Researchers must prioritize alternatives to animal testing whenever possible, minimize the number of animals used, and optimize experimental procedures to reduce suffering.
When studying the effects of muscimol, a potent GABA-A receptor agonist, on animal models, it is crucial to consider the potential for distress or discomfort. Muscimol can induce sedation, motor impairment, and alterations in cognitive function. Therefore, researchers must implement robust protocols to monitor animal welfare throughout the study, including regular assessments of behavior, weight, and overall health.
The choice of animal model is another critical ethical consideration. While rodents are commonly used, the selection should be based on the most appropriate species that can provide relevant data with the least degree of suffering. Researchers must justify the use of higher-order animals, such as non-human primates, if deemed necessary for translational relevance.
Dose selection and administration routes for muscimol studies require careful ethical deliberation. The aim should be to achieve the desired pharmacological effect while minimizing adverse reactions. Non-invasive administration methods should be preferred when possible, and surgical interventions for drug delivery must be performed under appropriate anesthesia and analgesia.
Long-term effects of muscimol exposure on animal subjects must also be considered. Studies should include provisions for post-experimental care and monitoring, especially if repeated dosing is involved. Euthanasia protocols, when necessary, must adhere to humane endpoints and be conducted by trained personnel.
Transparency in reporting is a crucial ethical obligation. Researchers must provide detailed information on animal welfare measures, any adverse events encountered, and steps taken to mitigate animal suffering. This not only ensures accountability but also contributes to the refinement of future studies.
Institutional Animal Care and Use Committees (IACUCs) play a vital role in overseeing the ethical conduct of animal research. All muscimol studies should undergo rigorous review by these committees to ensure compliance with ethical guidelines and animal welfare regulations. Researchers must be prepared to justify the scientific necessity of their experiments and demonstrate that the potential benefits outweigh the ethical costs.
In conclusion, while animal models remain invaluable in understanding the neuropharmacological effects of muscimol, researchers have a moral obligation to uphold the highest ethical standards. By carefully considering and addressing these ethical concerns, scientists can conduct responsible research that advances our understanding of muscimol's effects while minimizing harm to animal subjects.
When studying the effects of muscimol, a potent GABA-A receptor agonist, on animal models, it is crucial to consider the potential for distress or discomfort. Muscimol can induce sedation, motor impairment, and alterations in cognitive function. Therefore, researchers must implement robust protocols to monitor animal welfare throughout the study, including regular assessments of behavior, weight, and overall health.
The choice of animal model is another critical ethical consideration. While rodents are commonly used, the selection should be based on the most appropriate species that can provide relevant data with the least degree of suffering. Researchers must justify the use of higher-order animals, such as non-human primates, if deemed necessary for translational relevance.
Dose selection and administration routes for muscimol studies require careful ethical deliberation. The aim should be to achieve the desired pharmacological effect while minimizing adverse reactions. Non-invasive administration methods should be preferred when possible, and surgical interventions for drug delivery must be performed under appropriate anesthesia and analgesia.
Long-term effects of muscimol exposure on animal subjects must also be considered. Studies should include provisions for post-experimental care and monitoring, especially if repeated dosing is involved. Euthanasia protocols, when necessary, must adhere to humane endpoints and be conducted by trained personnel.
Transparency in reporting is a crucial ethical obligation. Researchers must provide detailed information on animal welfare measures, any adverse events encountered, and steps taken to mitigate animal suffering. This not only ensures accountability but also contributes to the refinement of future studies.
Institutional Animal Care and Use Committees (IACUCs) play a vital role in overseeing the ethical conduct of animal research. All muscimol studies should undergo rigorous review by these committees to ensure compliance with ethical guidelines and animal welfare regulations. Researchers must be prepared to justify the scientific necessity of their experiments and demonstrate that the potential benefits outweigh the ethical costs.
In conclusion, while animal models remain invaluable in understanding the neuropharmacological effects of muscimol, researchers have a moral obligation to uphold the highest ethical standards. By carefully considering and addressing these ethical concerns, scientists can conduct responsible research that advances our understanding of muscimol's effects while minimizing harm to animal subjects.
Regulatory Framework for Neuropharmacology
The regulatory framework for neuropharmacology plays a crucial role in ensuring the safety, efficacy, and ethical use of neuropharmacological agents such as muscimol. In the context of animal models, this framework encompasses various aspects of research, development, and clinical application.
At the international level, organizations like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provide guidelines for the development and testing of new drugs, including those targeting the nervous system. These guidelines outline the necessary preclinical studies, including animal models, required to demonstrate safety and efficacy before progressing to human trials.
National regulatory bodies, such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe, enforce strict regulations governing the use of animal models in neuropharmacological research. These regulations typically include requirements for ethical treatment of animals, standardization of experimental protocols, and rigorous documentation of results.
Specific to muscimol and similar GABA receptor agonists, regulatory frameworks often mandate comprehensive toxicology studies in multiple animal species. These studies must assess both acute and chronic effects, as well as potential for addiction or abuse. Given muscimol's psychoactive properties, additional scrutiny may be applied to its potential for off-label use or misuse.
Regulatory bodies also require thorough investigation of pharmacokinetics and pharmacodynamics in animal models before human trials can begin. This includes detailed analysis of muscimol's distribution, metabolism, and excretion, as well as its effects on various neurotransmitter systems and brain regions.
In recent years, there has been an increased focus on reducing animal testing in drug development. Regulatory frameworks are evolving to incorporate alternative methods, such as in vitro assays and computer modeling, where possible. However, for complex neuropharmacological agents like muscimol, animal models remain an essential component of the regulatory process.
The regulatory landscape also addresses the ethical considerations of using psychoactive substances in research. This includes guidelines for the humane treatment of animals, minimizing distress, and ensuring that the potential benefits of the research outweigh any harm to the animals involved.
As neuropharmacology continues to advance, regulatory frameworks are adapting to keep pace with new technologies and methodologies. This includes the development of more sophisticated animal models that better mimic human neurological conditions, as well as the integration of biomarkers and imaging techniques to enhance the predictive value of preclinical studies.
At the international level, organizations like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provide guidelines for the development and testing of new drugs, including those targeting the nervous system. These guidelines outline the necessary preclinical studies, including animal models, required to demonstrate safety and efficacy before progressing to human trials.
National regulatory bodies, such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe, enforce strict regulations governing the use of animal models in neuropharmacological research. These regulations typically include requirements for ethical treatment of animals, standardization of experimental protocols, and rigorous documentation of results.
Specific to muscimol and similar GABA receptor agonists, regulatory frameworks often mandate comprehensive toxicology studies in multiple animal species. These studies must assess both acute and chronic effects, as well as potential for addiction or abuse. Given muscimol's psychoactive properties, additional scrutiny may be applied to its potential for off-label use or misuse.
Regulatory bodies also require thorough investigation of pharmacokinetics and pharmacodynamics in animal models before human trials can begin. This includes detailed analysis of muscimol's distribution, metabolism, and excretion, as well as its effects on various neurotransmitter systems and brain regions.
In recent years, there has been an increased focus on reducing animal testing in drug development. Regulatory frameworks are evolving to incorporate alternative methods, such as in vitro assays and computer modeling, where possible. However, for complex neuropharmacological agents like muscimol, animal models remain an essential component of the regulatory process.
The regulatory landscape also addresses the ethical considerations of using psychoactive substances in research. This includes guidelines for the humane treatment of animals, minimizing distress, and ensuring that the potential benefits of the research outweigh any harm to the animals involved.
As neuropharmacology continues to advance, regulatory frameworks are adapting to keep pace with new technologies and methodologies. This includes the development of more sophisticated animal models that better mimic human neurological conditions, as well as the integration of biomarkers and imaging techniques to enhance the predictive value of preclinical studies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!