Mechanism of Action of Muscimol in CNS Disorders
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol CNS Mechanism Overview
Muscimol, a potent GABA-A receptor agonist, has garnered significant attention in the field of neuroscience for its potential therapeutic applications in various central nervous system (CNS) disorders. This naturally occurring psychoactive compound, found in certain species of mushrooms, exerts its effects by mimicking the action of gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the mammalian brain.
The mechanism of action of muscimol primarily involves its high-affinity binding to GABA-A receptors, which are ligand-gated ion channels widely distributed throughout the CNS. Upon binding, muscimol induces a conformational change in the receptor, leading to the opening of chloride ion channels. This influx of chloride ions results in hyperpolarization of the neuronal membrane, effectively reducing neuronal excitability and dampening overall neural activity.
The potency of muscimol stems from its ability to activate GABA-A receptors more efficiently than GABA itself, producing a more pronounced inhibitory effect. This enhanced GABAergic signaling has been associated with various physiological and behavioral effects, including sedation, anxiolysis, and anticonvulsant properties. These effects form the basis for muscimol's potential therapeutic applications in CNS disorders characterized by excessive neuronal excitation or impaired inhibitory control.
In the context of CNS disorders, muscimol's mechanism of action offers promising avenues for treatment. For instance, in epilepsy, where hyperexcitability of neuronal networks leads to seizures, muscimol's potent inhibitory effects could potentially suppress abnormal electrical activity. Similarly, in anxiety disorders, the anxiolytic properties of muscimol, mediated through enhanced GABAergic transmission, may help alleviate symptoms by modulating the brain's fear and stress responses.
Furthermore, muscimol's action on GABA-A receptors has implications for sleep disorders and certain neurodegenerative conditions. By promoting inhibitory neurotransmission, muscimol may facilitate sleep onset and maintenance, addressing insomnia and related sleep disturbances. In neurodegenerative diseases like Parkinson's, where an imbalance between excitatory and inhibitory signaling contributes to motor symptoms, muscimol's GABA-mimetic effects could potentially help restore this balance and alleviate motor deficits.
However, it is crucial to note that while muscimol's mechanism of action offers therapeutic potential, its use also presents challenges. The widespread distribution of GABA-A receptors throughout the CNS means that muscimol's effects are not localized, potentially leading to off-target effects. Additionally, the risk of developing tolerance and dependence with prolonged use necessitates careful consideration in its therapeutic application.
The mechanism of action of muscimol primarily involves its high-affinity binding to GABA-A receptors, which are ligand-gated ion channels widely distributed throughout the CNS. Upon binding, muscimol induces a conformational change in the receptor, leading to the opening of chloride ion channels. This influx of chloride ions results in hyperpolarization of the neuronal membrane, effectively reducing neuronal excitability and dampening overall neural activity.
The potency of muscimol stems from its ability to activate GABA-A receptors more efficiently than GABA itself, producing a more pronounced inhibitory effect. This enhanced GABAergic signaling has been associated with various physiological and behavioral effects, including sedation, anxiolysis, and anticonvulsant properties. These effects form the basis for muscimol's potential therapeutic applications in CNS disorders characterized by excessive neuronal excitation or impaired inhibitory control.
In the context of CNS disorders, muscimol's mechanism of action offers promising avenues for treatment. For instance, in epilepsy, where hyperexcitability of neuronal networks leads to seizures, muscimol's potent inhibitory effects could potentially suppress abnormal electrical activity. Similarly, in anxiety disorders, the anxiolytic properties of muscimol, mediated through enhanced GABAergic transmission, may help alleviate symptoms by modulating the brain's fear and stress responses.
Furthermore, muscimol's action on GABA-A receptors has implications for sleep disorders and certain neurodegenerative conditions. By promoting inhibitory neurotransmission, muscimol may facilitate sleep onset and maintenance, addressing insomnia and related sleep disturbances. In neurodegenerative diseases like Parkinson's, where an imbalance between excitatory and inhibitory signaling contributes to motor symptoms, muscimol's GABA-mimetic effects could potentially help restore this balance and alleviate motor deficits.
However, it is crucial to note that while muscimol's mechanism of action offers therapeutic potential, its use also presents challenges. The widespread distribution of GABA-A receptors throughout the CNS means that muscimol's effects are not localized, potentially leading to off-target effects. Additionally, the risk of developing tolerance and dependence with prolonged use necessitates careful consideration in its therapeutic application.
Neurological Disorder Treatment Demand
The demand for effective treatments for neurological disorders has been steadily increasing due to the rising prevalence of these conditions worldwide. Neurological disorders encompass a wide range of conditions affecting the central nervous system (CNS), including Alzheimer's disease, Parkinson's disease, epilepsy, multiple sclerosis, and various forms of anxiety and depression. The global burden of these disorders is substantial, with a significant impact on healthcare systems, economies, and quality of life for millions of individuals.
Market analysis indicates a growing need for innovative therapies that can address the complex nature of CNS disorders. Traditional treatments often fall short in providing comprehensive relief or halting disease progression, creating a substantial unmet medical need. This gap in effective treatments has driven intense research and development efforts in the pharmaceutical and biotechnology sectors, with a particular focus on novel mechanisms of action and targeted therapies.
The potential market for CNS disorder treatments is vast and expanding. Demographic shifts, particularly the aging population in many developed countries, are contributing to an increased incidence of age-related neurological conditions such as Alzheimer's and Parkinson's disease. Additionally, heightened awareness of mental health issues and improved diagnostic capabilities have led to a greater recognition and diagnosis of anxiety disorders and depression, further expanding the potential patient population.
Muscimol, a potent GABA receptor agonist, has emerged as a compound of interest in the treatment of various CNS disorders. Its mechanism of action, which involves enhancing inhibitory neurotransmission in the brain, holds promise for conditions characterized by neuronal hyperexcitability or imbalanced neurotransmitter systems. The exploration of muscimol's therapeutic potential aligns with the broader industry trend towards developing drugs that target specific neural pathways and receptors.
The demand for muscimol-based therapies is driven by several factors, including the need for more effective and better-tolerated treatments for epilepsy, anxiety disorders, and sleep disturbances. Furthermore, the potential neuroprotective effects of muscimol have sparked interest in its application for neurodegenerative diseases. This multifaceted potential underscores the compound's relevance in addressing diverse neurological conditions and meeting the complex needs of patients.
As research into muscimol's mechanism of action in CNS disorders progresses, there is a growing anticipation for novel therapeutic approaches that could offer improved outcomes and quality of life for patients. The development of muscimol-based treatments represents a significant opportunity to address critical gaps in the current neurological disorder treatment landscape, potentially revolutionizing patient care and management strategies in this challenging medical field.
Market analysis indicates a growing need for innovative therapies that can address the complex nature of CNS disorders. Traditional treatments often fall short in providing comprehensive relief or halting disease progression, creating a substantial unmet medical need. This gap in effective treatments has driven intense research and development efforts in the pharmaceutical and biotechnology sectors, with a particular focus on novel mechanisms of action and targeted therapies.
The potential market for CNS disorder treatments is vast and expanding. Demographic shifts, particularly the aging population in many developed countries, are contributing to an increased incidence of age-related neurological conditions such as Alzheimer's and Parkinson's disease. Additionally, heightened awareness of mental health issues and improved diagnostic capabilities have led to a greater recognition and diagnosis of anxiety disorders and depression, further expanding the potential patient population.
Muscimol, a potent GABA receptor agonist, has emerged as a compound of interest in the treatment of various CNS disorders. Its mechanism of action, which involves enhancing inhibitory neurotransmission in the brain, holds promise for conditions characterized by neuronal hyperexcitability or imbalanced neurotransmitter systems. The exploration of muscimol's therapeutic potential aligns with the broader industry trend towards developing drugs that target specific neural pathways and receptors.
The demand for muscimol-based therapies is driven by several factors, including the need for more effective and better-tolerated treatments for epilepsy, anxiety disorders, and sleep disturbances. Furthermore, the potential neuroprotective effects of muscimol have sparked interest in its application for neurodegenerative diseases. This multifaceted potential underscores the compound's relevance in addressing diverse neurological conditions and meeting the complex needs of patients.
As research into muscimol's mechanism of action in CNS disorders progresses, there is a growing anticipation for novel therapeutic approaches that could offer improved outcomes and quality of life for patients. The development of muscimol-based treatments represents a significant opportunity to address critical gaps in the current neurological disorder treatment landscape, potentially revolutionizing patient care and management strategies in this challenging medical field.
Current Muscimol Research Status
Muscimol, a potent GABA-A receptor agonist, has been the subject of extensive research in recent years due to its potential therapeutic applications in various central nervous system (CNS) disorders. Current research on muscimol focuses on elucidating its mechanism of action, optimizing its delivery methods, and exploring its efficacy in treating a wide range of neurological and psychiatric conditions.
One of the primary areas of investigation is the development of novel formulations to enhance muscimol's bioavailability and targeted delivery to the brain. Researchers are exploring nanoparticle-based delivery systems, intranasal administration, and modified molecular structures to improve its pharmacokinetic profile and reduce peripheral side effects. These advancements aim to overcome the limitations associated with muscimol's poor blood-brain barrier penetration and short half-life.
Preclinical studies have demonstrated muscimol's potential in treating epilepsy, anxiety disorders, and neurodegenerative diseases such as Alzheimer's and Parkinson's. The compound's ability to modulate GABAergic neurotransmission has shown promise in reducing seizure activity, alleviating anxiety-like behaviors, and providing neuroprotection in animal models. Ongoing research is focused on translating these findings into clinical applications and determining optimal dosing regimens for different CNS disorders.
Recent investigations have also explored muscimol's role in neuroplasticity and its potential to enhance cognitive function. Studies suggest that low doses of muscimol may facilitate learning and memory processes by modulating synaptic plasticity in specific brain regions. This has led to increased interest in its potential as a cognitive enhancer and its application in treating cognitive deficits associated with various neurological conditions.
The safety profile of muscimol is another area of active research. While the compound has shown a favorable safety profile in preclinical studies, ongoing clinical trials are evaluating its long-term effects and potential interactions with other medications. Researchers are also investigating the risk of tolerance and dependence associated with prolonged muscimol use, particularly in the context of chronic CNS disorders.
Advancements in neuroimaging techniques have enabled researchers to better understand muscimol's effects on brain activity and connectivity. Functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies are providing valuable insights into the compound's impact on neural circuits and its potential to modulate aberrant brain activity in various CNS disorders.
In conclusion, current muscimol research is characterized by a multidisciplinary approach, combining pharmacology, neuroscience, and clinical medicine to unlock its full therapeutic potential. As our understanding of its mechanism of action continues to evolve, muscimol remains a promising candidate for the development of novel treatments for a wide range of CNS disorders.
One of the primary areas of investigation is the development of novel formulations to enhance muscimol's bioavailability and targeted delivery to the brain. Researchers are exploring nanoparticle-based delivery systems, intranasal administration, and modified molecular structures to improve its pharmacokinetic profile and reduce peripheral side effects. These advancements aim to overcome the limitations associated with muscimol's poor blood-brain barrier penetration and short half-life.
Preclinical studies have demonstrated muscimol's potential in treating epilepsy, anxiety disorders, and neurodegenerative diseases such as Alzheimer's and Parkinson's. The compound's ability to modulate GABAergic neurotransmission has shown promise in reducing seizure activity, alleviating anxiety-like behaviors, and providing neuroprotection in animal models. Ongoing research is focused on translating these findings into clinical applications and determining optimal dosing regimens for different CNS disorders.
Recent investigations have also explored muscimol's role in neuroplasticity and its potential to enhance cognitive function. Studies suggest that low doses of muscimol may facilitate learning and memory processes by modulating synaptic plasticity in specific brain regions. This has led to increased interest in its potential as a cognitive enhancer and its application in treating cognitive deficits associated with various neurological conditions.
The safety profile of muscimol is another area of active research. While the compound has shown a favorable safety profile in preclinical studies, ongoing clinical trials are evaluating its long-term effects and potential interactions with other medications. Researchers are also investigating the risk of tolerance and dependence associated with prolonged muscimol use, particularly in the context of chronic CNS disorders.
Advancements in neuroimaging techniques have enabled researchers to better understand muscimol's effects on brain activity and connectivity. Functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies are providing valuable insights into the compound's impact on neural circuits and its potential to modulate aberrant brain activity in various CNS disorders.
In conclusion, current muscimol research is characterized by a multidisciplinary approach, combining pharmacology, neuroscience, and clinical medicine to unlock its full therapeutic potential. As our understanding of its mechanism of action continues to evolve, muscimol remains a promising candidate for the development of novel treatments for a wide range of CNS disorders.
Muscimol Therapeutic Approaches
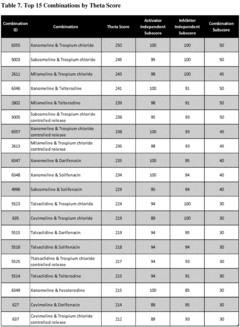
01 Pharmaceutical compositions containing muscimol
Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include different formulations and delivery methods to enhance the efficacy and bioavailability of muscimol. The compositions can be designed for treating neurological disorders, anxiety, or other conditions affected by GABA receptor modulation.- Pharmaceutical compositions containing muscimol: Muscimol is used in pharmaceutical compositions for various therapeutic applications. These formulations may include specific dosage forms, delivery methods, or combinations with other active ingredients to enhance efficacy or reduce side effects.
- Muscimol for neurological disorders: Muscimol is investigated for its potential in treating neurological disorders. Research focuses on its GABA-mimetic properties and ability to modulate neural activity, which may be beneficial in conditions such as epilepsy, anxiety, or sleep disorders.
- Synthesis and production methods for muscimol: Various methods for synthesizing and producing muscimol are developed to improve yield, purity, or cost-effectiveness. These may include novel chemical pathways, biotechnological approaches, or extraction techniques from natural sources.
- Muscimol analogs and derivatives: Research into muscimol analogs and derivatives aims to create compounds with improved pharmacological profiles. These modified versions may offer enhanced selectivity, bioavailability, or reduced side effects compared to the parent compound.
- Drug delivery systems for muscimol: Innovative drug delivery systems are developed to optimize the administration of muscimol. These may include controlled release formulations, targeted delivery mechanisms, or novel routes of administration to enhance therapeutic efficacy and patient compliance.
02 Muscimol analogs and derivatives
Research focuses on developing and synthesizing muscimol analogs and derivatives. These modified compounds aim to improve upon the properties of muscimol, such as enhanced potency, selectivity, or reduced side effects. The analogs may be designed to target specific GABA receptor subtypes or to have improved pharmacokinetic profiles.Expand Specific Solutions03 Use of muscimol in neurostimulation therapies
Muscimol is explored in combination with neurostimulation techniques for treating neurological and psychiatric disorders. This approach may involve the local delivery of muscimol to specific brain regions in conjunction with electrical or magnetic stimulation, potentially enhancing the therapeutic effects of both interventions.Expand Specific Solutions04 Muscimol in combination therapies
Muscimol is investigated as part of combination therapies with other active compounds. These combinations may target multiple pathways or receptors simultaneously, potentially leading to synergistic effects in treating various disorders. The combinations could include other GABA modulators, antidepressants, or anxiolytics.Expand Specific Solutions05 Novel delivery systems for muscimol
Innovative delivery systems are being developed to improve the administration and efficacy of muscimol. These may include nanoparticle formulations, transdermal patches, or controlled-release mechanisms. The goal is to enhance the bioavailability of muscimol, reduce dosing frequency, or target specific tissues or organs more effectively.Expand Specific Solutions
Key Neuropharmacology Players
The research into the mechanism of action of muscimol in CNS disorders is in a relatively early stage of development, with the market showing significant growth potential. The global CNS therapeutics market is expanding, driven by increasing prevalence of neurological disorders and growing demand for innovative treatments. Companies like ACADIA Pharmaceuticals, Allergan, and Supernus Pharmaceuticals are at the forefront of this research, leveraging their expertise in CNS drug development. The technology is still evolving, with varying degrees of maturity across different applications. Larger pharmaceutical companies such as Merck & Co. and Eli Lilly & Co. are also investing in this area, indicating the potential for future breakthroughs and market expansion.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals has developed a novel approach to targeting GABAergic signaling in CNS disorders using muscimol-inspired compounds. Their lead candidate, ACP-101, is a selective GABAA receptor positive allosteric modulator designed to enhance inhibitory neurotransmission[1]. Preclinical studies have shown that ACP-101 exhibits anxiolytic and anticonvulsant effects similar to muscimol, but with an improved safety profile due to its selective targeting of specific GABAA receptor subtypes[2]. The company is currently conducting Phase 2 clinical trials to evaluate ACP-101's efficacy in treating anxiety disorders and epilepsy[3].
Strengths: Selective targeting of specific GABAA receptor subtypes may provide better efficacy and reduced side effects compared to non-selective GABAergic drugs. Weaknesses: Still in clinical development stages, efficacy and safety in humans yet to be fully established.
Allergan, Inc.
Technical Solution: Allergan has developed a proprietary muscimol prodrug technology platform called GABA-A Receptor Subtype-Selective (GARSS) compounds. Their lead candidate, AGN-241751, is designed to selectively activate α2/α3 subunit-containing GABAA receptors, which are implicated in anxiety and pain processing[1]. This approach aims to harness the therapeutic potential of muscimol while minimizing side effects associated with non-selective GABA activation. Preclinical studies have demonstrated AGN-241751's ability to reduce anxiety-like behaviors and alleviate neuropathic pain in animal models[2]. The compound has shown improved bioavailability and brain penetration compared to muscimol, potentially allowing for lower doses and reduced systemic side effects[3].
Strengths: Subtype-selective approach may offer improved therapeutic index. Enhanced bioavailability and brain penetration could lead to more effective treatment. Weaknesses: Clinical efficacy and long-term safety in humans still need to be established.
GABA Receptor Modulation Insights
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
Compositions for treatment of disorders ameliorated by muscarinic receptor activation
PatentActiveHK1228453A
Innovation
- Combination therapy using muscarinic activators and inhibitors for treating CNS disorders.
- Utilizing the muscarinic system as a therapeutic target for schizophrenia and other mental illnesses.
- Addressing multiple symptom categories of schizophrenia (positive, negative, and cognitive) through muscarinic receptor modulation.
Muscimol Safety Profile
Muscimol, a potent GABA-A receptor agonist, has demonstrated a generally favorable safety profile in preclinical and clinical studies. However, as with any pharmacological agent, its use is associated with certain risks and potential adverse effects that require careful consideration.
In animal studies, muscimol has shown a wide margin of safety, with lethal doses significantly higher than those required for therapeutic effects. Acute toxicity studies in rodents have reported LD50 values ranging from 3.8 to 4.5 mg/kg when administered intraperitoneally, indicating a relatively low risk of acute toxicity at therapeutic doses.
The most common side effects observed in clinical trials involving muscimol are related to its GABAergic activity. These include sedation, dizziness, and cognitive impairment, which are typically dose-dependent and reversible upon discontinuation of the drug. In some cases, muscimol administration has been associated with mild to moderate ataxia and motor incoordination, particularly at higher doses.
Respiratory depression, a concern with many CNS depressants, appears to be minimal with muscimol at therapeutic doses. However, caution is warranted in patients with compromised respiratory function or when used in combination with other CNS depressants.
Long-term safety data on muscimol is limited, as most studies have focused on acute or short-term administration. Chronic toxicity studies in animals have not revealed significant organ toxicity or carcinogenic potential, but further research is needed to fully elucidate the long-term safety profile in humans.
Muscimol does not appear to have significant abuse potential, unlike some other GABAergic drugs such as benzodiazepines. This is likely due to its rapid metabolism and lack of reinforcing effects. However, abrupt discontinuation after prolonged use may lead to withdrawal symptoms, suggesting the need for gradual dose tapering.
Pharmacokinetic studies have shown that muscimol is rapidly absorbed and distributed throughout the body, with a relatively short half-life of 1-2 hours. It is primarily eliminated through renal excretion, with minimal hepatic metabolism. This pharmacokinetic profile contributes to its favorable safety profile by reducing the risk of drug accumulation and prolonged side effects.
In conclusion, while muscimol demonstrates a promising safety profile, its use in CNS disorders requires careful monitoring and individualized dosing to minimize potential adverse effects. Further research, particularly long-term safety studies in diverse patient populations, will be crucial in fully establishing its safety profile for therapeutic use.
In animal studies, muscimol has shown a wide margin of safety, with lethal doses significantly higher than those required for therapeutic effects. Acute toxicity studies in rodents have reported LD50 values ranging from 3.8 to 4.5 mg/kg when administered intraperitoneally, indicating a relatively low risk of acute toxicity at therapeutic doses.
The most common side effects observed in clinical trials involving muscimol are related to its GABAergic activity. These include sedation, dizziness, and cognitive impairment, which are typically dose-dependent and reversible upon discontinuation of the drug. In some cases, muscimol administration has been associated with mild to moderate ataxia and motor incoordination, particularly at higher doses.
Respiratory depression, a concern with many CNS depressants, appears to be minimal with muscimol at therapeutic doses. However, caution is warranted in patients with compromised respiratory function or when used in combination with other CNS depressants.
Long-term safety data on muscimol is limited, as most studies have focused on acute or short-term administration. Chronic toxicity studies in animals have not revealed significant organ toxicity or carcinogenic potential, but further research is needed to fully elucidate the long-term safety profile in humans.
Muscimol does not appear to have significant abuse potential, unlike some other GABAergic drugs such as benzodiazepines. This is likely due to its rapid metabolism and lack of reinforcing effects. However, abrupt discontinuation after prolonged use may lead to withdrawal symptoms, suggesting the need for gradual dose tapering.
Pharmacokinetic studies have shown that muscimol is rapidly absorbed and distributed throughout the body, with a relatively short half-life of 1-2 hours. It is primarily eliminated through renal excretion, with minimal hepatic metabolism. This pharmacokinetic profile contributes to its favorable safety profile by reducing the risk of drug accumulation and prolonged side effects.
In conclusion, while muscimol demonstrates a promising safety profile, its use in CNS disorders requires careful monitoring and individualized dosing to minimize potential adverse effects. Further research, particularly long-term safety studies in diverse patient populations, will be crucial in fully establishing its safety profile for therapeutic use.
Regulatory Pathway for Muscimol
The regulatory pathway for muscimol, a potent GABA-A receptor agonist, involves a complex process of drug development and approval. This pathway is crucial for ensuring the safety and efficacy of muscimol-based treatments for CNS disorders. The first step in this process is preclinical research, which includes in vitro and animal studies to establish the pharmacological profile and potential therapeutic effects of muscimol.
Following successful preclinical studies, the next phase involves submitting an Investigational New Drug (IND) application to the relevant regulatory authority, such as the FDA in the United States or the EMA in Europe. This application must include comprehensive data on the drug's chemistry, manufacturing, and controls (CMC), as well as results from preclinical studies and proposed clinical trial protocols.
Once the IND is approved, clinical trials can commence. These trials typically progress through three phases, each assessing different aspects of the drug's safety and efficacy. Phase I trials focus on safety and dosage in healthy volunteers, while Phase II trials evaluate efficacy and side effects in a small group of patients with the target CNS disorder. Phase III trials involve larger patient populations and longer treatment durations to confirm efficacy and monitor adverse reactions.
Throughout the clinical trial process, regular communication with regulatory authorities is essential. This includes submitting periodic safety reports and updates on trial progress. If the clinical trials demonstrate favorable results, the next step is to prepare and submit a New Drug Application (NDA) or Marketing Authorization Application (MAA), depending on the regulatory jurisdiction.
The regulatory review process for the NDA/MAA is rigorous and typically takes 6-12 months. During this time, regulatory authorities may request additional data or clarifications. If approved, the drug can be marketed, but post-marketing surveillance (Phase IV) is required to monitor long-term safety and efficacy in real-world settings.
It's important to note that the regulatory pathway for muscimol may be influenced by its classification as a controlled substance in many jurisdictions due to its psychoactive properties. This classification can add additional layers of regulatory scrutiny and requirements throughout the development and approval process.
Following successful preclinical studies, the next phase involves submitting an Investigational New Drug (IND) application to the relevant regulatory authority, such as the FDA in the United States or the EMA in Europe. This application must include comprehensive data on the drug's chemistry, manufacturing, and controls (CMC), as well as results from preclinical studies and proposed clinical trial protocols.
Once the IND is approved, clinical trials can commence. These trials typically progress through three phases, each assessing different aspects of the drug's safety and efficacy. Phase I trials focus on safety and dosage in healthy volunteers, while Phase II trials evaluate efficacy and side effects in a small group of patients with the target CNS disorder. Phase III trials involve larger patient populations and longer treatment durations to confirm efficacy and monitor adverse reactions.
Throughout the clinical trial process, regular communication with regulatory authorities is essential. This includes submitting periodic safety reports and updates on trial progress. If the clinical trials demonstrate favorable results, the next step is to prepare and submit a New Drug Application (NDA) or Marketing Authorization Application (MAA), depending on the regulatory jurisdiction.
The regulatory review process for the NDA/MAA is rigorous and typically takes 6-12 months. During this time, regulatory authorities may request additional data or clarifications. If approved, the drug can be marketed, but post-marketing surveillance (Phase IV) is required to monitor long-term safety and efficacy in real-world settings.
It's important to note that the regulatory pathway for muscimol may be influenced by its classification as a controlled substance in many jurisdictions due to its psychoactive properties. This classification can add additional layers of regulatory scrutiny and requirements throughout the development and approval process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!