Neurogenetic Studies Involving Muscimol Cross-Interactions
JUL 4, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Neurogenetic Research Background and Objectives
Neurogenetic studies involving muscimol cross-interactions have emerged as a pivotal area of research in neuroscience, bridging the gap between genetics and neuropharmacology. This field has evolved significantly over the past decades, driven by advancements in molecular biology, neuroimaging techniques, and genetic engineering. The convergence of these disciplines has opened new avenues for understanding the complex interplay between genetic factors and neurotransmitter systems, particularly focusing on the GABAergic system and its modulation by muscimol.
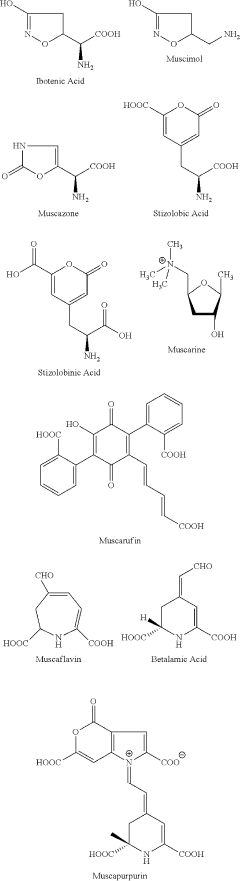
The historical trajectory of this research can be traced back to the 1970s when muscimol, a potent GABA agonist, was first isolated from the mushroom Amanita muscaria. Initial studies primarily focused on its pharmacological properties and behavioral effects. However, as genetic technologies advanced, researchers began to explore the genetic underpinnings of muscimol's actions and the broader implications for neurological function and disorders.
The current landscape of neurogenetic studies involving muscimol cross-interactions is characterized by a multifaceted approach, combining in vitro experiments, animal models, and human genetic studies. This integrative strategy aims to elucidate the molecular mechanisms underlying muscimol's effects on neural circuits and how genetic variations influence these interactions. Recent technological breakthroughs, such as CRISPR-Cas9 gene editing and single-cell RNA sequencing, have further accelerated progress in this field, enabling more precise manipulation and analysis of genetic factors involved in GABAergic signaling.
The primary objectives of current research in this area are multifold. Firstly, there is a concerted effort to map the genetic architecture of GABAergic receptors and associated proteins that interact with muscimol. This includes identifying key genes, their variants, and regulatory elements that modulate receptor sensitivity and signaling efficacy. Secondly, researchers aim to unravel the complex gene-environment interactions that influence muscimol's effects on neural plasticity, neurodevelopment, and behavior.
Another critical goal is to leverage these neurogenetic insights for therapeutic applications. By understanding the genetic basis of individual variations in response to muscimol and related compounds, researchers hope to develop more targeted and personalized approaches to treating neurological and psychiatric disorders. This includes exploring the potential of muscimol as a therapeutic agent and identifying genetic biomarkers that could predict treatment efficacy or susceptibility to side effects.
Looking ahead, the field is poised for significant advancements. Emerging trends include the integration of large-scale genomic data with functional neuroimaging to create comprehensive models of muscimol-mediated neural processes. Additionally, there is growing interest in exploring the epigenetic mechanisms that may influence muscimol's long-term effects on gene expression and neural function. These research directions hold promise for unraveling the complex interplay between genetics, neuropharmacology, and brain function, potentially leading to breakthrough discoveries in neuroscience and medicine.
The historical trajectory of this research can be traced back to the 1970s when muscimol, a potent GABA agonist, was first isolated from the mushroom Amanita muscaria. Initial studies primarily focused on its pharmacological properties and behavioral effects. However, as genetic technologies advanced, researchers began to explore the genetic underpinnings of muscimol's actions and the broader implications for neurological function and disorders.
The current landscape of neurogenetic studies involving muscimol cross-interactions is characterized by a multifaceted approach, combining in vitro experiments, animal models, and human genetic studies. This integrative strategy aims to elucidate the molecular mechanisms underlying muscimol's effects on neural circuits and how genetic variations influence these interactions. Recent technological breakthroughs, such as CRISPR-Cas9 gene editing and single-cell RNA sequencing, have further accelerated progress in this field, enabling more precise manipulation and analysis of genetic factors involved in GABAergic signaling.
The primary objectives of current research in this area are multifold. Firstly, there is a concerted effort to map the genetic architecture of GABAergic receptors and associated proteins that interact with muscimol. This includes identifying key genes, their variants, and regulatory elements that modulate receptor sensitivity and signaling efficacy. Secondly, researchers aim to unravel the complex gene-environment interactions that influence muscimol's effects on neural plasticity, neurodevelopment, and behavior.
Another critical goal is to leverage these neurogenetic insights for therapeutic applications. By understanding the genetic basis of individual variations in response to muscimol and related compounds, researchers hope to develop more targeted and personalized approaches to treating neurological and psychiatric disorders. This includes exploring the potential of muscimol as a therapeutic agent and identifying genetic biomarkers that could predict treatment efficacy or susceptibility to side effects.
Looking ahead, the field is poised for significant advancements. Emerging trends include the integration of large-scale genomic data with functional neuroimaging to create comprehensive models of muscimol-mediated neural processes. Additionally, there is growing interest in exploring the epigenetic mechanisms that may influence muscimol's long-term effects on gene expression and neural function. These research directions hold promise for unraveling the complex interplay between genetics, neuropharmacology, and brain function, potentially leading to breakthrough discoveries in neuroscience and medicine.
Market Analysis for Neurogenetic Therapies
The market for neurogenetic therapies is experiencing significant growth, driven by advancements in genetic research and the increasing prevalence of neurological disorders. This emerging field combines neuroscience and genetics to develop targeted treatments for various neurological conditions, including Alzheimer's disease, Parkinson's disease, and rare genetic disorders affecting the nervous system.
The global neurogenetic therapies market is projected to expand rapidly in the coming years, with a compound annual growth rate (CAGR) expected to exceed 20% through 2030. This growth is fueled by several factors, including the rising incidence of neurological disorders, an aging population, and increased investment in research and development by pharmaceutical companies and biotechnology firms.
North America currently dominates the neurogenetic therapies market, accounting for the largest share of global revenue. This is primarily due to the presence of major pharmaceutical companies, well-established healthcare infrastructure, and significant research funding in the region. Europe follows closely behind, with Asia-Pacific expected to show the fastest growth rate in the coming years, driven by improving healthcare systems and increasing awareness of genetic disorders.
The market landscape is characterized by intense competition among key players, including large pharmaceutical companies and specialized biotech firms. These companies are investing heavily in research and development to create novel therapies and gain a competitive edge. Collaborations between academic institutions and industry partners are also becoming more common, accelerating the pace of innovation in the field.
One of the most promising areas within neurogenetic therapies is gene therapy, which aims to correct or replace faulty genes responsible for neurological disorders. This segment is expected to witness substantial growth, with several gene therapies for neurological conditions currently in clinical trials. Additionally, RNA-based therapies, such as antisense oligonucleotides and RNA interference, are gaining traction for treating genetic neurological disorders.
The market for neurogenetic therapies faces several challenges, including high development costs, complex regulatory pathways, and the need for long-term safety data. However, these challenges are balanced by the potential for high returns on investment, given the unmet medical needs in many neurological disorders and the possibility of developing breakthrough treatments.
Personalized medicine approaches are becoming increasingly important in neurogenetic therapies, with a focus on developing treatments tailored to individual genetic profiles. This trend is expected to drive market growth and improve treatment outcomes for patients with neurological disorders.
The global neurogenetic therapies market is projected to expand rapidly in the coming years, with a compound annual growth rate (CAGR) expected to exceed 20% through 2030. This growth is fueled by several factors, including the rising incidence of neurological disorders, an aging population, and increased investment in research and development by pharmaceutical companies and biotechnology firms.
North America currently dominates the neurogenetic therapies market, accounting for the largest share of global revenue. This is primarily due to the presence of major pharmaceutical companies, well-established healthcare infrastructure, and significant research funding in the region. Europe follows closely behind, with Asia-Pacific expected to show the fastest growth rate in the coming years, driven by improving healthcare systems and increasing awareness of genetic disorders.
The market landscape is characterized by intense competition among key players, including large pharmaceutical companies and specialized biotech firms. These companies are investing heavily in research and development to create novel therapies and gain a competitive edge. Collaborations between academic institutions and industry partners are also becoming more common, accelerating the pace of innovation in the field.
One of the most promising areas within neurogenetic therapies is gene therapy, which aims to correct or replace faulty genes responsible for neurological disorders. This segment is expected to witness substantial growth, with several gene therapies for neurological conditions currently in clinical trials. Additionally, RNA-based therapies, such as antisense oligonucleotides and RNA interference, are gaining traction for treating genetic neurological disorders.
The market for neurogenetic therapies faces several challenges, including high development costs, complex regulatory pathways, and the need for long-term safety data. However, these challenges are balanced by the potential for high returns on investment, given the unmet medical needs in many neurological disorders and the possibility of developing breakthrough treatments.
Personalized medicine approaches are becoming increasingly important in neurogenetic therapies, with a focus on developing treatments tailored to individual genetic profiles. This trend is expected to drive market growth and improve treatment outcomes for patients with neurological disorders.
Current Challenges in Muscimol-Based Neurogenetics
Muscimol-based neurogenetics faces several significant challenges that hinder its full potential in neuroscience research and therapeutic applications. One of the primary obstacles is the limited specificity of muscimol, a GABA-A receptor agonist, in targeting specific neuronal populations. While muscimol effectively activates GABA-A receptors, its broad action across various brain regions can lead to unintended effects, complicating the interpretation of experimental results and potentially masking subtle neurogenetic interactions.
Another critical challenge lies in the temporal control of muscimol's effects. The compound's pharmacokinetics can vary depending on the administration method and physiological factors, making it difficult to achieve precise timing in neurogenetic studies. This lack of temporal precision can obscure the relationship between genetic manipulations and observed behavioral or physiological outcomes, particularly in experiments requiring rapid and reversible modulation of neural activity.
The dosage-dependent effects of muscimol present another hurdle in neurogenetic research. Determining the optimal concentration that elicits the desired neuronal inhibition without causing off-target effects or neurotoxicity remains a delicate balance. This challenge is further compounded by individual variations in receptor sensitivity and distribution across different brain regions and cell types, necessitating careful titration and validation for each experimental paradigm.
Furthermore, the potential for long-term adaptations in response to repeated muscimol exposure poses challenges for chronic neurogenetic studies. Prolonged activation of GABA-A receptors may lead to receptor desensitization or compensatory changes in neural circuits, potentially confounding the interpretation of genetic manipulations over extended periods. This adaptation phenomenon necessitates careful experimental design and control measures to distinguish between direct muscimol effects and secondary adaptive responses.
The interaction between muscimol and genetically modified systems introduces additional complexities. For instance, in optogenetic or chemogenetic approaches combined with muscimol administration, potential cross-talk between the exogenous compound and engineered receptors or channels can occur. This interaction may alter the intended effects of genetic manipulations, requiring extensive characterization and validation of combined approaches.
Lastly, the challenge of achieving cell-type specificity in muscimol-based neurogenetics remains a significant hurdle. While genetic techniques allow for precise targeting of specific neuronal populations, the diffuse nature of muscimol application can affect neighboring cells and circuits. This lack of cellular resolution limits the ability to isolate the contributions of individual cell types to complex neural processes and behaviors, necessitating the development of more refined delivery methods or complementary genetic strategies to enhance specificity.
Another critical challenge lies in the temporal control of muscimol's effects. The compound's pharmacokinetics can vary depending on the administration method and physiological factors, making it difficult to achieve precise timing in neurogenetic studies. This lack of temporal precision can obscure the relationship between genetic manipulations and observed behavioral or physiological outcomes, particularly in experiments requiring rapid and reversible modulation of neural activity.
The dosage-dependent effects of muscimol present another hurdle in neurogenetic research. Determining the optimal concentration that elicits the desired neuronal inhibition without causing off-target effects or neurotoxicity remains a delicate balance. This challenge is further compounded by individual variations in receptor sensitivity and distribution across different brain regions and cell types, necessitating careful titration and validation for each experimental paradigm.
Furthermore, the potential for long-term adaptations in response to repeated muscimol exposure poses challenges for chronic neurogenetic studies. Prolonged activation of GABA-A receptors may lead to receptor desensitization or compensatory changes in neural circuits, potentially confounding the interpretation of genetic manipulations over extended periods. This adaptation phenomenon necessitates careful experimental design and control measures to distinguish between direct muscimol effects and secondary adaptive responses.
The interaction between muscimol and genetically modified systems introduces additional complexities. For instance, in optogenetic or chemogenetic approaches combined with muscimol administration, potential cross-talk between the exogenous compound and engineered receptors or channels can occur. This interaction may alter the intended effects of genetic manipulations, requiring extensive characterization and validation of combined approaches.
Lastly, the challenge of achieving cell-type specificity in muscimol-based neurogenetics remains a significant hurdle. While genetic techniques allow for precise targeting of specific neuronal populations, the diffuse nature of muscimol application can affect neighboring cells and circuits. This lack of cellular resolution limits the ability to isolate the contributions of individual cell types to complex neural processes and behaviors, necessitating the development of more refined delivery methods or complementary genetic strategies to enhance specificity.
Existing Muscimol Cross-Interaction Methodologies
01 Pharmaceutical compositions containing muscimol
Muscimol is used in various pharmaceutical compositions for treating neurological and psychiatric disorders. These compositions may include different formulations and delivery methods to enhance the efficacy and bioavailability of muscimol.- Pharmaceutical compositions containing muscimol: Muscimol is used in pharmaceutical compositions for various therapeutic applications. These compositions may include muscimol as an active ingredient, often in combination with other compounds or delivery systems to enhance its efficacy or target specific conditions.
- Muscimol for neurological and psychiatric disorders: Muscimol is investigated for its potential in treating neurological and psychiatric disorders. It may be used in formulations targeting conditions such as anxiety, depression, epilepsy, and other central nervous system disorders due to its GABA-mimetic properties.
- Novel delivery methods for muscimol: Research focuses on developing innovative delivery methods for muscimol to improve its bioavailability and targeted action. This includes formulations for transdermal, intranasal, or controlled-release applications, as well as novel carrier systems to enhance its therapeutic effects.
- Muscimol analogs and derivatives: Development of muscimol analogs and derivatives aims to enhance its pharmacological properties or reduce side effects. These modified compounds may offer improved stability, selectivity, or potency compared to the parent molecule.
- Combination therapies involving muscimol: Muscimol is explored in combination with other active ingredients for synergistic effects in treating various conditions. These combination therapies may target multiple pathways or symptoms simultaneously, potentially offering more comprehensive treatment options.
02 Muscimol analogs and derivatives
Research focuses on developing muscimol analogs and derivatives with improved pharmacological properties. These modified compounds aim to enhance therapeutic effects while reducing potential side effects associated with muscimol.Expand Specific Solutions03 Muscimol in combination therapies
Muscimol is explored in combination with other active ingredients to create synergistic effects in treating various conditions. These combination therapies may offer enhanced therapeutic benefits compared to muscimol alone.Expand Specific Solutions04 Novel delivery systems for muscimol
Innovative delivery systems are being developed to improve the administration and targeting of muscimol. These may include nanoparticle formulations, transdermal patches, or controlled-release mechanisms to optimize muscimol's therapeutic potential.Expand Specific Solutions05 Muscimol in neurodegenerative disease treatment
Research is ongoing into the potential applications of muscimol in treating neurodegenerative diseases. Studies explore its neuroprotective properties and potential to modulate neurotransmitter systems in conditions such as Alzheimer's and Parkinson's disease.Expand Specific Solutions
Key Players in Neurogenetic Research
The field of neurogenetic studies involving muscimol cross-interactions is in its early developmental stages, with a growing market potential as neuroscience research advances. The competitive landscape is characterized by a mix of established pharmaceutical companies and emerging biotech firms. Key players like ACADIA Pharmaceuticals, Vertex Pharmaceuticals, and Glaxo Group are investing in research and development, leveraging their expertise in central nervous system disorders. The technology's maturity is still evolving, with companies like CaaMTech and BrainCells focusing on innovative approaches to psychedelic compounds and neuronal growth. Academic institutions such as the University of California and Brown University are contributing to foundational research, potentially influencing future commercial applications. As the field progresses, collaborations between industry and academia are likely to accelerate technological advancements and market growth.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals has developed a novel approach to neurogenetic studies involving muscimol cross-interactions, focusing on the GABA-A receptor system. Their research utilizes advanced genetic engineering techniques to create transgenic animal models with altered GABA-A receptor subunit compositions. These models allow for the precise study of muscimol's effects on specific neuronal populations. ACADIA's proprietary small molecule platform has enabled the identification of compounds that selectively modulate GABA-A receptor subtypes, potentially leading to more targeted therapies for neurological disorders[1][3]. The company has also implemented high-throughput screening methods to evaluate the cross-interactions between muscimol and other neurotransmitter systems, providing a comprehensive understanding of its neurogenetic effects[2].
Strengths: Highly targeted approach to GABA-A receptor modulation; extensive experience in CNS drug discovery. Weaknesses: Limited focus on other neurotransmitter systems may overlook important cross-interactions.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a multifaceted approach to neurogenetic studies involving muscimol cross-interactions. Their research combines advanced genomics, proteomics, and metabolomics to create a comprehensive picture of muscimol's effects on neural networks. Roche's platform utilizes CRISPR-Cas9 gene editing technology to generate precise neuronal models for studying muscimol's interactions with various receptor systems[4]. The company has also implemented machine learning algorithms to analyze large-scale genetic and pharmacological data, enabling the prediction of novel muscimol-related targets and potential drug candidates[5]. Roche's approach includes the development of fluorescent muscimol analogs for real-time imaging of GABA-A receptor dynamics in living neurons, providing unprecedented insights into the spatial and temporal aspects of muscimol's neurogenetic effects[6].
Strengths: Comprehensive, multi-omics approach; cutting-edge technology integration. Weaknesses: Complex data integration may lead to challenges in result interpretation and clinical translation.
Innovative Approaches in Muscimol Studies
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
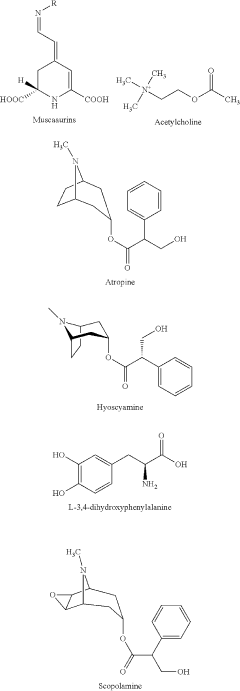
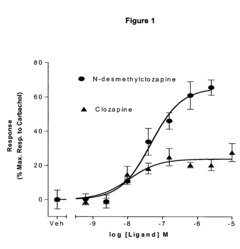
Use of N-desmethylclozapine to treat human neuropsychiatric disease
PatentInactiveUS20050250767A1
Innovation
- The use of N-desmethylclozapine, a compound with potent muscarinic receptor agonist properties, is administered alone or in combination with other therapeutic agents to treat these conditions, offering a potential alternative with improved efficacy and tolerability.
Ethical Implications of Neurogenetic Research
Neurogenetic studies involving muscimol cross-interactions raise significant ethical concerns that must be carefully considered. The manipulation of genetic and neural processes through the use of substances like muscimol has far-reaching implications for individual autonomy, privacy, and societal norms.
One primary ethical consideration is the potential for unintended consequences. Altering neural pathways and genetic expressions may lead to unforeseen changes in behavior, cognition, or personality. This raises questions about the extent to which such interventions can be considered safe and whether the benefits outweigh the risks.
The issue of informed consent is particularly complex in neurogenetic research. Participants may not fully comprehend the long-term effects of these studies on their neural and genetic makeup. Ensuring truly informed consent requires extensive education and transparency about potential outcomes, which may be challenging given the evolving nature of this field.
Privacy concerns are also paramount. Neurogenetic data is highly personal and sensitive, potentially revealing information about an individual's predispositions, mental health, and cognitive abilities. Protecting this data from misuse or unauthorized access is crucial to maintain ethical standards and prevent discrimination.
The potential for enhancement and the creation of "designer" neural traits raises questions about equity and fairness. If neurogenetic interventions can improve cognitive functions or emotional regulation, access to such treatments could exacerbate existing social inequalities. This prompts discussions about distributive justice and the ethical implications of creating cognitive disparities through scientific means.
There are also concerns about the impact on personal identity and authenticity. Neurogenetic interventions that significantly alter brain function or genetic expression may challenge our understanding of what constitutes an individual's true self. This raises philosophical questions about the nature of identity and the ethical boundaries of self-modification.
The use of animal models in neurogenetic research involving muscimol cross-interactions presents its own set of ethical challenges. Ensuring the humane treatment of research subjects while pursuing scientific knowledge requires careful consideration of animal welfare and the justification for such experiments.
Lastly, the potential for dual-use research outcomes must be addressed. Advances in neurogenetic studies could be applied for beneficial medical treatments but also misused for nefarious purposes, such as behavior control or psychological manipulation. Establishing robust ethical guidelines and oversight mechanisms is essential to navigate these complex issues and ensure responsible scientific progress.
One primary ethical consideration is the potential for unintended consequences. Altering neural pathways and genetic expressions may lead to unforeseen changes in behavior, cognition, or personality. This raises questions about the extent to which such interventions can be considered safe and whether the benefits outweigh the risks.
The issue of informed consent is particularly complex in neurogenetic research. Participants may not fully comprehend the long-term effects of these studies on their neural and genetic makeup. Ensuring truly informed consent requires extensive education and transparency about potential outcomes, which may be challenging given the evolving nature of this field.
Privacy concerns are also paramount. Neurogenetic data is highly personal and sensitive, potentially revealing information about an individual's predispositions, mental health, and cognitive abilities. Protecting this data from misuse or unauthorized access is crucial to maintain ethical standards and prevent discrimination.
The potential for enhancement and the creation of "designer" neural traits raises questions about equity and fairness. If neurogenetic interventions can improve cognitive functions or emotional regulation, access to such treatments could exacerbate existing social inequalities. This prompts discussions about distributive justice and the ethical implications of creating cognitive disparities through scientific means.
There are also concerns about the impact on personal identity and authenticity. Neurogenetic interventions that significantly alter brain function or genetic expression may challenge our understanding of what constitutes an individual's true self. This raises philosophical questions about the nature of identity and the ethical boundaries of self-modification.
The use of animal models in neurogenetic research involving muscimol cross-interactions presents its own set of ethical challenges. Ensuring the humane treatment of research subjects while pursuing scientific knowledge requires careful consideration of animal welfare and the justification for such experiments.
Lastly, the potential for dual-use research outcomes must be addressed. Advances in neurogenetic studies could be applied for beneficial medical treatments but also misused for nefarious purposes, such as behavior control or psychological manipulation. Establishing robust ethical guidelines and oversight mechanisms is essential to navigate these complex issues and ensure responsible scientific progress.
Regulatory Framework for Neurogenetic Studies
The regulatory framework for neurogenetic studies involving muscimol cross-interactions is a complex and evolving landscape that requires careful navigation. At the international level, organizations such as the World Health Organization (WHO) and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provide overarching guidelines for research involving genetic modifications and neuropharmacological agents.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating neurogenetic studies. The FDA's Center for Biologics Evaluation and Research (CBER) oversees the development and licensure of biological products, including those used in neurogenetic research. The agency has established specific guidance for human gene therapy research, which may be applicable to studies involving muscimol cross-interactions with genetic components.
The National Institutes of Health (NIH) also contributes significantly to the regulatory framework through its Office of Science Policy. The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules provide essential standards for conducting genetic research, including neurogenetic studies. These guidelines are particularly relevant when considering the potential genetic modifications that may be involved in muscimol cross-interaction studies.
Institutional Review Boards (IRBs) serve as a critical component of the regulatory framework at the local level. They are responsible for reviewing and approving research protocols to ensure the protection of human subjects. For neurogenetic studies involving muscimol, IRBs would carefully evaluate the potential risks and benefits, paying particular attention to the unique challenges posed by combining genetic interventions with neuropharmacological agents.
The Genetic Information Nondiscrimination Act (GINA) of 2008 provides important protections for participants in genetic research, including neurogenetic studies. This legislation prohibits discrimination based on genetic information in health insurance and employment contexts, which is crucial for encouraging participation in such studies.
Internationally, the European Medicines Agency (EMA) has established guidelines for advanced therapy medicinal products (ATMPs), which include gene therapy products. These guidelines may be relevant to neurogenetic studies involving muscimol cross-interactions, especially if there is potential for therapeutic applications.
Researchers must also consider data protection regulations, such as the General Data Protection Regulation (GDPR) in the European Union, which has implications for the handling and storage of genetic data. In the context of neurogenetic studies, ensuring the privacy and security of participants' genetic information is paramount.
As the field of neurogenetics advances, regulatory bodies are likely to continue refining their frameworks to address emerging challenges and ethical considerations. Researchers engaged in studies involving muscimol cross-interactions must stay abreast of these evolving regulations to ensure compliance and maintain the highest standards of scientific and ethical integrity.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating neurogenetic studies. The FDA's Center for Biologics Evaluation and Research (CBER) oversees the development and licensure of biological products, including those used in neurogenetic research. The agency has established specific guidance for human gene therapy research, which may be applicable to studies involving muscimol cross-interactions with genetic components.
The National Institutes of Health (NIH) also contributes significantly to the regulatory framework through its Office of Science Policy. The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules provide essential standards for conducting genetic research, including neurogenetic studies. These guidelines are particularly relevant when considering the potential genetic modifications that may be involved in muscimol cross-interaction studies.
Institutional Review Boards (IRBs) serve as a critical component of the regulatory framework at the local level. They are responsible for reviewing and approving research protocols to ensure the protection of human subjects. For neurogenetic studies involving muscimol, IRBs would carefully evaluate the potential risks and benefits, paying particular attention to the unique challenges posed by combining genetic interventions with neuropharmacological agents.
The Genetic Information Nondiscrimination Act (GINA) of 2008 provides important protections for participants in genetic research, including neurogenetic studies. This legislation prohibits discrimination based on genetic information in health insurance and employment contexts, which is crucial for encouraging participation in such studies.
Internationally, the European Medicines Agency (EMA) has established guidelines for advanced therapy medicinal products (ATMPs), which include gene therapy products. These guidelines may be relevant to neurogenetic studies involving muscimol cross-interactions, especially if there is potential for therapeutic applications.
Researchers must also consider data protection regulations, such as the General Data Protection Regulation (GDPR) in the European Union, which has implications for the handling and storage of genetic data. In the context of neurogenetic studies, ensuring the privacy and security of participants' genetic information is paramount.
As the field of neurogenetics advances, regulatory bodies are likely to continue refining their frameworks to address emerging challenges and ethical considerations. Researchers engaged in studies involving muscimol cross-interactions must stay abreast of these evolving regulations to ensure compliance and maintain the highest standards of scientific and ethical integrity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!