Structural Analysis of Muscimol in Chemical Synthesis
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Synthesis Background and Objectives
Muscimol, a potent GABA receptor agonist, has garnered significant attention in the field of chemical synthesis due to its unique structural properties and potential therapeutic applications. The journey of muscimol synthesis began in the 1960s when it was first isolated from the Amanita muscaria mushroom. Since then, researchers have been striving to develop efficient and scalable methods for its production.
The evolution of muscimol synthesis techniques has been driven by the increasing demand for this compound in neuroscience research and drug development. Early synthetic approaches were often complex and low-yielding, limiting the availability of muscimol for extensive studies. However, as analytical tools and synthetic methodologies advanced, chemists have made substantial progress in improving the efficiency and yield of muscimol production.
One of the primary objectives in muscimol synthesis has been to achieve a high degree of stereochemical control. The specific three-dimensional structure of muscimol is crucial for its biological activity, making stereoselectivity a key focus in synthetic strategies. Researchers have explored various approaches, including asymmetric synthesis and the use of chiral auxiliaries, to ensure the production of the correct stereoisomer.
Another important goal in the field has been the development of more environmentally friendly and cost-effective synthesis routes. Traditional methods often involved harsh reagents and multiple steps, leading to significant waste generation and high production costs. Recent efforts have focused on green chemistry principles, aiming to reduce the environmental impact of muscimol synthesis while maintaining or improving yield and purity.
The structural analysis of muscimol in chemical synthesis has played a pivotal role in advancing synthetic methodologies. Techniques such as X-ray crystallography, NMR spectroscopy, and computational modeling have provided detailed insights into the molecule's structure and reactivity. This knowledge has been instrumental in designing more efficient synthetic routes and understanding the structure-activity relationships of muscimol and its analogs.
As research in this area progresses, there is a growing emphasis on developing scalable processes for industrial production of muscimol. This objective is driven by the potential therapeutic applications of muscimol and its derivatives in treating neurological disorders. The challenge lies in translating laboratory-scale synthesis methods to large-scale production while maintaining high purity and stereochemical integrity.
In conclusion, the background and objectives of muscimol synthesis reflect a dynamic and evolving field of research. From its initial discovery to the current state of advanced synthetic methodologies, the journey of muscimol synthesis exemplifies the continuous pursuit of efficiency, selectivity, and scalability in chemical synthesis. As technology and understanding progress, the goals of muscimol synthesis continue to expand, promising new opportunities for both scientific research and potential therapeutic applications.
The evolution of muscimol synthesis techniques has been driven by the increasing demand for this compound in neuroscience research and drug development. Early synthetic approaches were often complex and low-yielding, limiting the availability of muscimol for extensive studies. However, as analytical tools and synthetic methodologies advanced, chemists have made substantial progress in improving the efficiency and yield of muscimol production.
One of the primary objectives in muscimol synthesis has been to achieve a high degree of stereochemical control. The specific three-dimensional structure of muscimol is crucial for its biological activity, making stereoselectivity a key focus in synthetic strategies. Researchers have explored various approaches, including asymmetric synthesis and the use of chiral auxiliaries, to ensure the production of the correct stereoisomer.
Another important goal in the field has been the development of more environmentally friendly and cost-effective synthesis routes. Traditional methods often involved harsh reagents and multiple steps, leading to significant waste generation and high production costs. Recent efforts have focused on green chemistry principles, aiming to reduce the environmental impact of muscimol synthesis while maintaining or improving yield and purity.
The structural analysis of muscimol in chemical synthesis has played a pivotal role in advancing synthetic methodologies. Techniques such as X-ray crystallography, NMR spectroscopy, and computational modeling have provided detailed insights into the molecule's structure and reactivity. This knowledge has been instrumental in designing more efficient synthetic routes and understanding the structure-activity relationships of muscimol and its analogs.
As research in this area progresses, there is a growing emphasis on developing scalable processes for industrial production of muscimol. This objective is driven by the potential therapeutic applications of muscimol and its derivatives in treating neurological disorders. The challenge lies in translating laboratory-scale synthesis methods to large-scale production while maintaining high purity and stereochemical integrity.
In conclusion, the background and objectives of muscimol synthesis reflect a dynamic and evolving field of research. From its initial discovery to the current state of advanced synthetic methodologies, the journey of muscimol synthesis exemplifies the continuous pursuit of efficiency, selectivity, and scalability in chemical synthesis. As technology and understanding progress, the goals of muscimol synthesis continue to expand, promising new opportunities for both scientific research and potential therapeutic applications.
Market Demand for Muscimol and Related Compounds
The market demand for muscimol and related compounds has been steadily growing in recent years, driven by various factors across multiple industries. In the pharmaceutical sector, muscimol's potential as a therapeutic agent for neurological disorders has sparked significant interest. Research into its GABA-mimetic properties has shown promise for treating conditions such as epilepsy, anxiety, and sleep disorders, leading to increased demand from drug development companies and research institutions.
The nutraceutical and functional food industries have also shown a rising interest in muscimol and its derivatives. As consumers become more health-conscious and seek natural alternatives for cognitive enhancement and stress relief, products containing muscimol or its analogs have gained traction. This trend has led to a surge in demand from supplement manufacturers and food companies looking to incorporate these compounds into their product lines.
In the agricultural sector, muscimol's potential as a natural pesticide has attracted attention. With the growing emphasis on sustainable farming practices and the need for alternatives to synthetic pesticides, researchers and agrochemical companies are exploring muscimol-based solutions. This has created a new market segment with promising growth prospects.
The biotechnology industry has also contributed to the increasing demand for muscimol. Advancements in biosynthesis techniques have opened up new possibilities for large-scale production of muscimol and related compounds. This has not only made these substances more accessible but has also stimulated research into novel applications, further driving market growth.
Geographically, North America and Europe currently dominate the market for muscimol and related compounds, primarily due to their advanced pharmaceutical and biotechnology sectors. However, the Asia-Pacific region is emerging as a significant market, with rapid growth in research activities and increasing investments in drug discovery and development.
Despite the growing demand, challenges remain in the muscimol market. Regulatory hurdles, particularly in the pharmaceutical and food industries, can slow down product development and commercialization. Additionally, the complexity of muscimol synthesis and the need for high-purity products present technical challenges that impact supply and pricing.
Looking ahead, the market for muscimol and related compounds is expected to continue its growth trajectory. Factors such as increasing research funding, expanding applications in various industries, and growing consumer awareness of natural health solutions are likely to drive demand. However, the market's evolution will depend on overcoming regulatory and technical challenges, as well as the success of ongoing clinical trials and research initiatives exploring new applications for these fascinating compounds.
The nutraceutical and functional food industries have also shown a rising interest in muscimol and its derivatives. As consumers become more health-conscious and seek natural alternatives for cognitive enhancement and stress relief, products containing muscimol or its analogs have gained traction. This trend has led to a surge in demand from supplement manufacturers and food companies looking to incorporate these compounds into their product lines.
In the agricultural sector, muscimol's potential as a natural pesticide has attracted attention. With the growing emphasis on sustainable farming practices and the need for alternatives to synthetic pesticides, researchers and agrochemical companies are exploring muscimol-based solutions. This has created a new market segment with promising growth prospects.
The biotechnology industry has also contributed to the increasing demand for muscimol. Advancements in biosynthesis techniques have opened up new possibilities for large-scale production of muscimol and related compounds. This has not only made these substances more accessible but has also stimulated research into novel applications, further driving market growth.
Geographically, North America and Europe currently dominate the market for muscimol and related compounds, primarily due to their advanced pharmaceutical and biotechnology sectors. However, the Asia-Pacific region is emerging as a significant market, with rapid growth in research activities and increasing investments in drug discovery and development.
Despite the growing demand, challenges remain in the muscimol market. Regulatory hurdles, particularly in the pharmaceutical and food industries, can slow down product development and commercialization. Additionally, the complexity of muscimol synthesis and the need for high-purity products present technical challenges that impact supply and pricing.
Looking ahead, the market for muscimol and related compounds is expected to continue its growth trajectory. Factors such as increasing research funding, expanding applications in various industries, and growing consumer awareness of natural health solutions are likely to drive demand. However, the market's evolution will depend on overcoming regulatory and technical challenges, as well as the success of ongoing clinical trials and research initiatives exploring new applications for these fascinating compounds.
Current Challenges in Muscimol Structural Analysis
The structural analysis of muscimol in chemical synthesis faces several significant challenges that hinder its efficient production and characterization. One of the primary obstacles is the molecule's small size and high polarity, which make it difficult to isolate and purify using conventional chromatographic techniques. This challenge is compounded by muscimol's tendency to form hydrogen bonds, leading to potential aggregation and reduced resolution in analytical methods.
Another major hurdle is the molecule's sensitivity to pH changes and oxidative conditions. Muscimol's structure can be easily altered in acidic or basic environments, making it challenging to maintain its integrity throughout the synthesis and analysis processes. This instability necessitates careful control of reaction conditions and storage parameters, which can be both time-consuming and resource-intensive.
The presence of multiple tautomeric forms of muscimol further complicates structural analysis. The interconversion between these forms can occur rapidly, making it difficult to isolate and characterize individual tautomers. This dynamic equilibrium poses challenges in obtaining accurate spectroscopic data and can lead to ambiguities in structural assignments.
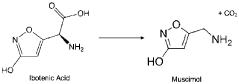
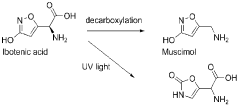
Muscimol's structural similarity to other naturally occurring compounds, such as ibotenic acid, presents another challenge in its analysis. Distinguishing muscimol from these structurally related molecules requires highly selective analytical techniques, which may not always be readily available or cost-effective for routine analysis.
The low natural abundance of muscimol in its biological sources necessitates the development of highly sensitive detection methods. This requirement often pushes the limits of current analytical instrumentation, particularly when working with complex matrices or trace amounts of the compound.
Furthermore, the synthesis of isotopically labeled muscimol for advanced structural studies, such as NMR experiments, presents its own set of challenges. The incorporation of stable isotopes into specific positions of the molecule without altering its overall structure or properties requires sophisticated synthetic strategies and careful optimization.
Lastly, the development of robust and reproducible quantification methods for muscimol remains a significant challenge. The compound's high polarity and potential for non-specific binding to various surfaces can lead to inconsistencies in extraction efficiencies and recovery rates, affecting the accuracy and precision of quantitative analyses.
Another major hurdle is the molecule's sensitivity to pH changes and oxidative conditions. Muscimol's structure can be easily altered in acidic or basic environments, making it challenging to maintain its integrity throughout the synthesis and analysis processes. This instability necessitates careful control of reaction conditions and storage parameters, which can be both time-consuming and resource-intensive.
The presence of multiple tautomeric forms of muscimol further complicates structural analysis. The interconversion between these forms can occur rapidly, making it difficult to isolate and characterize individual tautomers. This dynamic equilibrium poses challenges in obtaining accurate spectroscopic data and can lead to ambiguities in structural assignments.
Muscimol's structural similarity to other naturally occurring compounds, such as ibotenic acid, presents another challenge in its analysis. Distinguishing muscimol from these structurally related molecules requires highly selective analytical techniques, which may not always be readily available or cost-effective for routine analysis.
The low natural abundance of muscimol in its biological sources necessitates the development of highly sensitive detection methods. This requirement often pushes the limits of current analytical instrumentation, particularly when working with complex matrices or trace amounts of the compound.
Furthermore, the synthesis of isotopically labeled muscimol for advanced structural studies, such as NMR experiments, presents its own set of challenges. The incorporation of stable isotopes into specific positions of the molecule without altering its overall structure or properties requires sophisticated synthetic strategies and careful optimization.
Lastly, the development of robust and reproducible quantification methods for muscimol remains a significant challenge. The compound's high polarity and potential for non-specific binding to various surfaces can lead to inconsistencies in extraction efficiencies and recovery rates, affecting the accuracy and precision of quantitative analyses.
Existing Muscimol Structural Analysis Methods
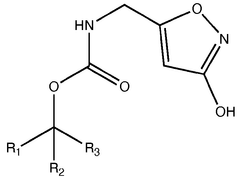
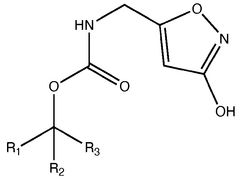
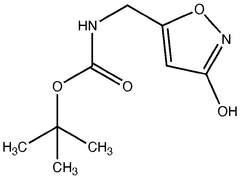
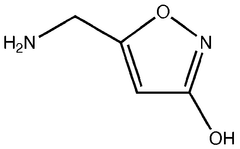
01 Chemical structure of muscimol
Muscimol is a psychoactive compound found in certain mushroom species. Its chemical structure is characterized by a heterocyclic ring system containing nitrogen atoms. The molecule is relatively small and has specific functional groups that contribute to its biological activity.- Chemical structure of muscimol: Muscimol is a psychoactive compound found in certain mushrooms. Its chemical structure is characterized by a heterocyclic ring system containing nitrogen and oxygen atoms. The molecule is relatively small and has a unique arrangement that contributes to its potent effects on the central nervous system.
- Synthesis and derivatives of muscimol: Various methods for synthesizing muscimol and creating structural derivatives have been developed. These approaches aim to modify the core structure to enhance certain properties or reduce side effects. Synthetic routes often involve manipulating precursor molecules to construct the characteristic isoxazole ring of muscimol.
- Pharmacological properties related to structure: The structural features of muscimol are closely linked to its pharmacological effects. Its ability to act as a potent GABA receptor agonist is attributed to specific molecular interactions enabled by its unique structure. Understanding these structure-activity relationships is crucial for developing potential therapeutic applications.
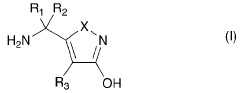
- Structural analogs and their effects: Researchers have explored various structural analogs of muscimol to investigate how modifications to the core structure affect biological activity. These studies have led to the development of compounds with altered potency, selectivity, or pharmacokinetic properties, potentially opening new avenues for drug discovery.
- Analytical methods for structural characterization: Advanced analytical techniques are employed to elucidate and confirm the structure of muscimol and related compounds. These methods include spectroscopic techniques such as NMR, mass spectrometry, and X-ray crystallography, which provide detailed information about the molecular structure and conformation of muscimol.
02 Synthesis and preparation methods
Various methods for synthesizing muscimol have been developed. These include chemical synthesis routes starting from precursor compounds, as well as extraction and purification techniques from natural sources. The synthesis often involves multiple steps and careful control of reaction conditions to achieve high purity.Expand Specific Solutions03 Pharmacological properties and mechanisms of action
Muscimol acts as a potent GABA receptor agonist in the central nervous system. Its structure allows it to bind to specific receptor sites, influencing neurotransmitter activity. The compound's effects on neural signaling contribute to its psychoactive properties and potential therapeutic applications.Expand Specific Solutions04 Structural analogs and derivatives
Researchers have developed various structural analogs and derivatives of muscimol. These compounds maintain key structural features of muscimol but incorporate modifications to enhance specific properties such as potency, selectivity, or pharmacokinetics. Some analogs aim to reduce side effects while retaining therapeutic potential.Expand Specific Solutions05 Analytical methods for structure determination
Advanced analytical techniques are employed to elucidate and confirm the structure of muscimol and related compounds. These methods include spectroscopic techniques such as NMR, mass spectrometry, and X-ray crystallography. Such analyses are crucial for quality control and structure-activity relationship studies.Expand Specific Solutions
Key Players in Muscimol Research and Production
The structural analysis of muscimol in chemical synthesis represents a niche yet evolving field within pharmaceutical research. The market is in its early growth stage, with a relatively small but expanding size due to increasing interest in GABA receptor modulators. Technological maturity varies among key players, with academic institutions like Rutgers University and China Agricultural University leading in fundamental research. Companies such as Takeda Pharmaceutical and Eli Lilly are leveraging their extensive R&D capabilities to advance muscimol-related drug development. Smaller biotechs like Psyched Wellness are exploring novel applications, while established firms like Sorrento Therapeutics are integrating muscimol research into their broader drug discovery platforms.
Psyched Wellness Ltd.
Technical Solution: Psyched Wellness Ltd. has developed a proprietary extraction and purification process for muscimol from Amanita muscaria mushrooms. Their approach involves carefully controlled chemical synthesis steps to isolate and purify muscimol while removing other potentially harmful compounds. The company utilizes advanced chromatography and spectroscopy techniques to analyze the structural integrity and purity of the synthesized muscimol[1]. They have also implemented quality control measures to ensure consistent potency and safety of their muscimol-based products. Psyched Wellness is exploring the potential therapeutic applications of muscimol, particularly for sleep, stress, and anxiety disorders[2].
Strengths: Specialized expertise in muscimol extraction and purification; focus on therapeutic applications. Weaknesses: Limited track record compared to larger pharmaceutical companies; regulatory challenges for novel psychoactive compounds.
Takeda Pharmaceutical Co., Ltd.
Technical Solution: Takeda Pharmaceutical has developed advanced analytical techniques for the structural analysis of muscimol and related GABA-ergic compounds. Their approach combines high-resolution mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy, and X-ray crystallography to elucidate the precise molecular structure and conformation of muscimol[3]. Takeda has also explored synthetic routes to produce muscimol analogs with enhanced pharmacological properties. Their research has focused on optimizing the binding affinity and selectivity of muscimol-like compounds to GABA receptors, potentially leading to new therapeutic agents for neurological disorders[4].
Strengths: Extensive R&D capabilities; expertise in neuropharmacology. Weaknesses: Muscimol research may not be a primary focus compared to other drug development programs.
Innovative Approaches in Muscimol Characterization
Pharmaceutical intermediates and methods for preparing the same in the synthesis of muscimol and congeners and derivatives thereof
PatentWO2025128106A1
Innovation
- A novel method for preparing muscimol mono-BOC and muscimol hydrochloride that avoids the use of ion exchange chromatography by modifying the original synthetic route to include a flow reactor for the cyclization step and using BOC anhydride to purify the muscimol, thereby stabilizing the product and improving yields.
Amanita muscaria extracts and compounds and their beneficial and therapeutic use
PatentWO2023015395A1
Innovation
- Development of Amanita muscaria extracts with a muscimol to ibotenic acid weight ratio of at least 1000:1, devoid of stizolobinic acid and heavy metal contaminants, and formulated into dietary supplements, functional foods, and pharmaceutical compositions for various administration methods.
Safety and Handling Protocols for Muscimol
Muscimol, a potent psychoactive compound found in certain mushroom species, requires stringent safety and handling protocols during chemical synthesis and analysis. These protocols are essential to protect researchers, laboratory personnel, and the environment from potential hazards associated with this compound.
Personal protective equipment (PPE) is paramount when working with muscimol. Researchers must wear appropriate gloves, lab coats, and safety goggles at all times. Respiratory protection may be necessary when handling powdered forms of the compound or during processes that may generate aerosols. It is crucial to select gloves made from materials resistant to muscimol and other solvents used in the synthesis process.
Proper ventilation is critical in laboratories where muscimol is synthesized or analyzed. Fume hoods should be used for all procedures involving the compound, ensuring that any vapors or particulates are safely contained and exhausted. Regular maintenance and testing of ventilation systems are necessary to guarantee their effectiveness.
Storage of muscimol and its precursors requires careful consideration. The compound should be kept in tightly sealed containers, protected from light, and stored in a cool, dry place. Access to storage areas must be restricted to authorized personnel only. Proper labeling of all containers is essential, including hazard warnings and safety data sheet references.
Waste management protocols for muscimol-related materials are crucial. All waste, including contaminated PPE and disposable laboratory equipment, must be collected in designated containers and disposed of according to local regulations for hazardous chemical waste. Decontamination procedures for work surfaces and equipment should be established and strictly followed.
Emergency response plans specific to muscimol incidents should be developed and regularly reviewed. This includes procedures for spill containment and cleanup, as well as first aid measures in case of accidental exposure. All laboratory personnel must be trained in these procedures and have easy access to safety equipment such as eyewash stations and emergency showers.
Documentation and record-keeping are vital components of safety protocols. Detailed logs of muscimol usage, synthesis procedures, and disposal should be maintained. Regular safety audits and inspections should be conducted to ensure compliance with established protocols and to identify areas for improvement in safety practices.
Training programs for all personnel involved in muscimol-related work are essential. These should cover the specific hazards of muscimol, proper handling techniques, emergency procedures, and the importance of adhering to safety protocols. Refresher training should be provided periodically to reinforce safe practices and introduce any updates to procedures.
Personal protective equipment (PPE) is paramount when working with muscimol. Researchers must wear appropriate gloves, lab coats, and safety goggles at all times. Respiratory protection may be necessary when handling powdered forms of the compound or during processes that may generate aerosols. It is crucial to select gloves made from materials resistant to muscimol and other solvents used in the synthesis process.
Proper ventilation is critical in laboratories where muscimol is synthesized or analyzed. Fume hoods should be used for all procedures involving the compound, ensuring that any vapors or particulates are safely contained and exhausted. Regular maintenance and testing of ventilation systems are necessary to guarantee their effectiveness.
Storage of muscimol and its precursors requires careful consideration. The compound should be kept in tightly sealed containers, protected from light, and stored in a cool, dry place. Access to storage areas must be restricted to authorized personnel only. Proper labeling of all containers is essential, including hazard warnings and safety data sheet references.
Waste management protocols for muscimol-related materials are crucial. All waste, including contaminated PPE and disposable laboratory equipment, must be collected in designated containers and disposed of according to local regulations for hazardous chemical waste. Decontamination procedures for work surfaces and equipment should be established and strictly followed.
Emergency response plans specific to muscimol incidents should be developed and regularly reviewed. This includes procedures for spill containment and cleanup, as well as first aid measures in case of accidental exposure. All laboratory personnel must be trained in these procedures and have easy access to safety equipment such as eyewash stations and emergency showers.
Documentation and record-keeping are vital components of safety protocols. Detailed logs of muscimol usage, synthesis procedures, and disposal should be maintained. Regular safety audits and inspections should be conducted to ensure compliance with established protocols and to identify areas for improvement in safety practices.
Training programs for all personnel involved in muscimol-related work are essential. These should cover the specific hazards of muscimol, proper handling techniques, emergency procedures, and the importance of adhering to safety protocols. Refresher training should be provided periodically to reinforce safe practices and introduce any updates to procedures.
Regulatory Framework for Muscimol Research
The regulatory framework for muscimol research is complex and multifaceted, reflecting the compound's unique pharmacological properties and potential for both therapeutic use and misuse. At the federal level in the United States, muscimol is not specifically scheduled under the Controlled Substances Act. However, its presence in certain mushroom species, particularly Amanita muscaria, places it in a regulatory gray area.
The Food and Drug Administration (FDA) plays a crucial role in overseeing muscimol research, especially in the context of potential pharmaceutical applications. Any clinical trials involving muscimol or its derivatives must adhere to strict FDA guidelines, including obtaining Investigational New Drug (IND) approval before human studies can commence. The FDA's regulatory pathway for novel psychoactive compounds like muscimol typically involves extensive preclinical studies to establish safety profiles and potential therapeutic benefits.
The Drug Enforcement Administration (DEA) also maintains interest in muscimol due to its psychoactive properties. While not explicitly controlled, researchers working with muscimol may need to register with the DEA and comply with reporting requirements, particularly if the compound is synthesized or extracted in significant quantities.
At the state level, regulations surrounding muscimol can vary significantly. Some states have enacted laws specifically addressing Amanita muscaria and its constituents, while others rely on broader statutes governing hallucinogenic substances. This patchwork of state regulations creates challenges for researchers operating across multiple jurisdictions.
Internationally, the regulatory landscape for muscimol research is equally diverse. The United Nations Convention on Psychotropic Substances does not explicitly list muscimol, but individual countries may impose their own restrictions. In the European Union, the European Medicines Agency (EMA) provides guidance for research involving novel psychoactive substances, which would encompass muscimol studies aimed at therapeutic applications.
Research institutions and pharmaceutical companies engaged in muscimol research must navigate these complex regulatory frameworks carefully. Compliance often requires robust internal protocols, meticulous record-keeping, and ongoing communication with regulatory bodies. As interest in the therapeutic potential of psychoactive compounds grows, there is an increasing push for regulatory clarity and harmonization to facilitate responsible research while maintaining necessary safeguards.
The evolving nature of drug policy and increasing scientific interest in compounds like muscimol may lead to regulatory changes in the coming years. Researchers and institutions involved in muscimol studies should stay abreast of these developments and be prepared to adapt their practices to ensure ongoing compliance with evolving regulatory requirements.
The Food and Drug Administration (FDA) plays a crucial role in overseeing muscimol research, especially in the context of potential pharmaceutical applications. Any clinical trials involving muscimol or its derivatives must adhere to strict FDA guidelines, including obtaining Investigational New Drug (IND) approval before human studies can commence. The FDA's regulatory pathway for novel psychoactive compounds like muscimol typically involves extensive preclinical studies to establish safety profiles and potential therapeutic benefits.
The Drug Enforcement Administration (DEA) also maintains interest in muscimol due to its psychoactive properties. While not explicitly controlled, researchers working with muscimol may need to register with the DEA and comply with reporting requirements, particularly if the compound is synthesized or extracted in significant quantities.
At the state level, regulations surrounding muscimol can vary significantly. Some states have enacted laws specifically addressing Amanita muscaria and its constituents, while others rely on broader statutes governing hallucinogenic substances. This patchwork of state regulations creates challenges for researchers operating across multiple jurisdictions.
Internationally, the regulatory landscape for muscimol research is equally diverse. The United Nations Convention on Psychotropic Substances does not explicitly list muscimol, but individual countries may impose their own restrictions. In the European Union, the European Medicines Agency (EMA) provides guidance for research involving novel psychoactive substances, which would encompass muscimol studies aimed at therapeutic applications.
Research institutions and pharmaceutical companies engaged in muscimol research must navigate these complex regulatory frameworks carefully. Compliance often requires robust internal protocols, meticulous record-keeping, and ongoing communication with regulatory bodies. As interest in the therapeutic potential of psychoactive compounds grows, there is an increasing push for regulatory clarity and harmonization to facilitate responsible research while maintaining necessary safeguards.
The evolving nature of drug policy and increasing scientific interest in compounds like muscimol may lead to regulatory changes in the coming years. Researchers and institutions involved in muscimol studies should stay abreast of these developments and be prepared to adapt their practices to ensure ongoing compliance with evolving regulatory requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!