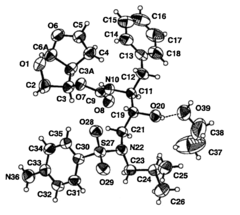
Factors Influencing Muscimol's Bioavailability
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Bioavailability Background and Objectives
Muscimol, a potent GABA receptor agonist, has garnered significant attention in the field of neuropharmacology due to its potential therapeutic applications. The bioavailability of muscimol plays a crucial role in determining its efficacy and safety profile. This technical research report aims to provide a comprehensive overview of the factors influencing muscimol's bioavailability and establish clear objectives for further investigation.
The development of muscimol as a potential therapeutic agent has its roots in the discovery of its natural occurrence in Amanita mushrooms. Over the years, researchers have made substantial progress in understanding the pharmacokinetics and pharmacodynamics of muscimol, leading to increased interest in its potential applications for treating various neurological disorders.
The evolution of muscimol research has been marked by significant milestones, including the elucidation of its molecular structure, the development of synthetic production methods, and the exploration of its interactions with GABA receptors. These advancements have paved the way for more focused studies on optimizing muscimol's bioavailability for therapeutic use.
Current research trends indicate a growing emphasis on enhancing muscimol's bioavailability through various formulation strategies and delivery methods. The primary goal is to overcome the challenges associated with its limited oral bioavailability and poor blood-brain barrier penetration, which have historically hindered its clinical development.
The objectives of this technical research report are multifaceted. Firstly, we aim to provide a comprehensive analysis of the physiological, pharmacological, and formulation factors that influence muscimol's bioavailability. This includes examining aspects such as absorption, distribution, metabolism, and excretion (ADME) properties, as well as exploring the impact of different administration routes.
Secondly, we seek to evaluate the current state of technology and methodologies employed in enhancing muscimol's bioavailability. This involves assessing various drug delivery systems, novel formulation techniques, and potential chemical modifications that could improve its pharmacokinetic profile.
Furthermore, this report aims to identify key challenges and bottlenecks in optimizing muscimol's bioavailability and propose potential solutions or areas for further research. By doing so, we hope to contribute to the development of more effective and efficient muscimol-based therapies.
Lastly, we intend to explore the broader implications of improved muscimol bioavailability on its therapeutic potential. This includes considering how enhanced bioavailability could expand the range of treatable conditions, improve dosing regimens, and potentially reduce side effects associated with higher doses.
The development of muscimol as a potential therapeutic agent has its roots in the discovery of its natural occurrence in Amanita mushrooms. Over the years, researchers have made substantial progress in understanding the pharmacokinetics and pharmacodynamics of muscimol, leading to increased interest in its potential applications for treating various neurological disorders.
The evolution of muscimol research has been marked by significant milestones, including the elucidation of its molecular structure, the development of synthetic production methods, and the exploration of its interactions with GABA receptors. These advancements have paved the way for more focused studies on optimizing muscimol's bioavailability for therapeutic use.
Current research trends indicate a growing emphasis on enhancing muscimol's bioavailability through various formulation strategies and delivery methods. The primary goal is to overcome the challenges associated with its limited oral bioavailability and poor blood-brain barrier penetration, which have historically hindered its clinical development.
The objectives of this technical research report are multifaceted. Firstly, we aim to provide a comprehensive analysis of the physiological, pharmacological, and formulation factors that influence muscimol's bioavailability. This includes examining aspects such as absorption, distribution, metabolism, and excretion (ADME) properties, as well as exploring the impact of different administration routes.
Secondly, we seek to evaluate the current state of technology and methodologies employed in enhancing muscimol's bioavailability. This involves assessing various drug delivery systems, novel formulation techniques, and potential chemical modifications that could improve its pharmacokinetic profile.
Furthermore, this report aims to identify key challenges and bottlenecks in optimizing muscimol's bioavailability and propose potential solutions or areas for further research. By doing so, we hope to contribute to the development of more effective and efficient muscimol-based therapies.
Lastly, we intend to explore the broader implications of improved muscimol bioavailability on its therapeutic potential. This includes considering how enhanced bioavailability could expand the range of treatable conditions, improve dosing regimens, and potentially reduce side effects associated with higher doses.
Market Analysis for Muscimol-Based Therapeutics
The market for muscimol-based therapeutics is experiencing significant growth potential, driven by increasing research into novel treatments for neurological and psychiatric disorders. Muscimol, a potent GABA-A receptor agonist found naturally in certain mushroom species, has garnered attention for its potential applications in treating conditions such as epilepsy, anxiety, and sleep disorders.
The global neurology drugs market, which encompasses potential muscimol-based therapeutics, was valued at approximately $35 billion in 2020 and is projected to grow at a CAGR of 5.5% through 2027. This growth is fueled by the rising prevalence of neurological disorders, an aging population, and increased healthcare expenditure in both developed and developing countries.
Within this broader market, the segment for GABA receptor modulators, where muscimol-based therapeutics would likely be positioned, is showing particularly strong growth. This is due to the increasing understanding of GABA's role in various neurological and psychiatric conditions, as well as the limitations of current treatment options.
The demand for novel anxiolytics and sleep aids is a key driver for muscimol-based therapeutics. The global anxiety disorder and depression treatment market is expected to reach $19 billion by 2028, with a CAGR of 2.4%. Similarly, the sleep aid market is projected to grow to $101 billion by 2026, with a CAGR of 6.8%.
Factors influencing the market potential of muscimol-based therapeutics include the increasing prevalence of stress-related disorders, the growing awareness of mental health issues, and the need for treatments with fewer side effects compared to traditional benzodiazepines and barbiturates. The COVID-19 pandemic has further accelerated these trends, with a notable increase in anxiety and sleep disorders reported globally.
However, the market for muscimol-based therapeutics faces challenges, including stringent regulatory requirements for novel psychoactive compounds and potential competition from established pharmaceutical products. The success of muscimol-based therapies will largely depend on their ability to demonstrate superior efficacy and safety profiles compared to existing treatments.
Geographically, North America and Europe are expected to be the primary markets for muscimol-based therapeutics due to their advanced healthcare infrastructure and higher healthcare spending. However, emerging markets in Asia-Pacific and Latin America present significant growth opportunities as healthcare access improves and mental health awareness increases.
In conclusion, the market analysis for muscimol-based therapeutics indicates a promising landscape with substantial growth potential. The increasing prevalence of neurological and psychiatric disorders, coupled with the demand for more effective and safer treatment options, creates a favorable environment for the development and commercialization of muscimol-based products.
The global neurology drugs market, which encompasses potential muscimol-based therapeutics, was valued at approximately $35 billion in 2020 and is projected to grow at a CAGR of 5.5% through 2027. This growth is fueled by the rising prevalence of neurological disorders, an aging population, and increased healthcare expenditure in both developed and developing countries.
Within this broader market, the segment for GABA receptor modulators, where muscimol-based therapeutics would likely be positioned, is showing particularly strong growth. This is due to the increasing understanding of GABA's role in various neurological and psychiatric conditions, as well as the limitations of current treatment options.
The demand for novel anxiolytics and sleep aids is a key driver for muscimol-based therapeutics. The global anxiety disorder and depression treatment market is expected to reach $19 billion by 2028, with a CAGR of 2.4%. Similarly, the sleep aid market is projected to grow to $101 billion by 2026, with a CAGR of 6.8%.
Factors influencing the market potential of muscimol-based therapeutics include the increasing prevalence of stress-related disorders, the growing awareness of mental health issues, and the need for treatments with fewer side effects compared to traditional benzodiazepines and barbiturates. The COVID-19 pandemic has further accelerated these trends, with a notable increase in anxiety and sleep disorders reported globally.
However, the market for muscimol-based therapeutics faces challenges, including stringent regulatory requirements for novel psychoactive compounds and potential competition from established pharmaceutical products. The success of muscimol-based therapies will largely depend on their ability to demonstrate superior efficacy and safety profiles compared to existing treatments.
Geographically, North America and Europe are expected to be the primary markets for muscimol-based therapeutics due to their advanced healthcare infrastructure and higher healthcare spending. However, emerging markets in Asia-Pacific and Latin America present significant growth opportunities as healthcare access improves and mental health awareness increases.
In conclusion, the market analysis for muscimol-based therapeutics indicates a promising landscape with substantial growth potential. The increasing prevalence of neurological and psychiatric disorders, coupled with the demand for more effective and safer treatment options, creates a favorable environment for the development and commercialization of muscimol-based products.
Current Challenges in Muscimol Bioavailability
Despite the promising therapeutic potential of muscimol, its bioavailability presents significant challenges that hinder its widespread clinical application. One of the primary obstacles is muscimol's poor oral bioavailability, which is estimated to be less than 5%. This low bioavailability is primarily attributed to its hydrophilic nature, making it difficult for the compound to cross biological membranes, including the blood-brain barrier (BBB).
The BBB poses a formidable challenge for muscimol delivery to the central nervous system (CNS). As a small, polar molecule, muscimol struggles to penetrate this highly selective barrier, limiting its effectiveness in treating neurological disorders. Researchers have observed that only a small fraction of orally administered muscimol reaches the brain, necessitating higher doses to achieve therapeutic effects, which in turn increases the risk of systemic side effects.
Another significant challenge is muscimol's rapid metabolism and elimination from the body. Studies have shown that muscimol has a relatively short half-life, with peak plasma concentrations occurring within 1-2 hours after oral administration. This rapid clearance further contributes to its low bioavailability and necessitates frequent dosing to maintain therapeutic levels, potentially leading to patient compliance issues.
The stability of muscimol in various physiological conditions also presents challenges. The compound is susceptible to degradation in acidic environments, such as the stomach, which can further reduce its bioavailability when administered orally. This instability necessitates the development of specialized formulations or delivery systems to protect muscimol from degradation and enhance its absorption.
Furthermore, the high water solubility of muscimol presents challenges in developing suitable pharmaceutical formulations. Traditional drug delivery systems often rely on lipophilic properties to enhance absorption and distribution. The hydrophilic nature of muscimol limits its compatibility with many conventional formulation approaches, requiring innovative strategies to improve its bioavailability.
Interindividual variability in muscimol metabolism and response also complicates its clinical use. Factors such as genetic polymorphisms in metabolizing enzymes, age, and concurrent medications can significantly affect muscimol's pharmacokinetics and pharmacodynamics. This variability makes it challenging to establish standardized dosing regimens and predict individual patient responses, necessitating personalized approaches to muscimol therapy.
The BBB poses a formidable challenge for muscimol delivery to the central nervous system (CNS). As a small, polar molecule, muscimol struggles to penetrate this highly selective barrier, limiting its effectiveness in treating neurological disorders. Researchers have observed that only a small fraction of orally administered muscimol reaches the brain, necessitating higher doses to achieve therapeutic effects, which in turn increases the risk of systemic side effects.
Another significant challenge is muscimol's rapid metabolism and elimination from the body. Studies have shown that muscimol has a relatively short half-life, with peak plasma concentrations occurring within 1-2 hours after oral administration. This rapid clearance further contributes to its low bioavailability and necessitates frequent dosing to maintain therapeutic levels, potentially leading to patient compliance issues.
The stability of muscimol in various physiological conditions also presents challenges. The compound is susceptible to degradation in acidic environments, such as the stomach, which can further reduce its bioavailability when administered orally. This instability necessitates the development of specialized formulations or delivery systems to protect muscimol from degradation and enhance its absorption.
Furthermore, the high water solubility of muscimol presents challenges in developing suitable pharmaceutical formulations. Traditional drug delivery systems often rely on lipophilic properties to enhance absorption and distribution. The hydrophilic nature of muscimol limits its compatibility with many conventional formulation approaches, requiring innovative strategies to improve its bioavailability.
Interindividual variability in muscimol metabolism and response also complicates its clinical use. Factors such as genetic polymorphisms in metabolizing enzymes, age, and concurrent medications can significantly affect muscimol's pharmacokinetics and pharmacodynamics. This variability makes it challenging to establish standardized dosing regimens and predict individual patient responses, necessitating personalized approaches to muscimol therapy.
Existing Strategies to Enhance Muscimol Bioavailability
01 Formulation strategies for improved muscimol bioavailability
Various formulation strategies are employed to enhance the bioavailability of muscimol. These include the use of nanoparticles, liposomes, and other drug delivery systems to improve absorption and distribution. Additionally, modified release formulations and prodrug approaches are explored to optimize the pharmacokinetic profile of muscimol.- Formulation strategies for improved muscimol bioavailability: Various formulation strategies are employed to enhance the bioavailability of muscimol. These include the use of novel drug delivery systems, such as nanoparticles, liposomes, or cyclodextrins, which can protect the drug from degradation and improve its absorption. Additionally, the development of prodrugs or modified forms of muscimol that are more easily absorbed by the body can significantly increase bioavailability.
- Route of administration optimization for muscimol: The bioavailability of muscimol can be significantly affected by the route of administration. Research focuses on optimizing various routes, including oral, sublingual, transdermal, and intranasal delivery methods. Each route presents unique challenges and opportunities for improving muscimol absorption and bioavailability, with some methods showing promise in bypassing first-pass metabolism.
- Pharmacokinetic studies and bioavailability enhancement: Comprehensive pharmacokinetic studies are conducted to understand the absorption, distribution, metabolism, and excretion of muscimol in the body. These studies help in identifying factors affecting bioavailability and guide the development of strategies to enhance it, such as co-administration with absorption enhancers or metabolic enzyme inhibitors.
- Combination therapies and drug interactions affecting muscimol bioavailability: Research explores the impact of combination therapies on muscimol bioavailability. This includes studying potential drug interactions that may enhance or inhibit muscimol absorption and metabolism. Understanding these interactions is crucial for optimizing treatment regimens and maximizing the therapeutic effects of muscimol.
- Novel muscimol analogs with improved bioavailability: Development of novel muscimol analogs aims to create compounds with improved bioavailability profiles. These analogs are designed to maintain the therapeutic effects of muscimol while overcoming limitations in absorption or metabolism. Structure-activity relationship studies guide the synthesis of these new compounds, potentially leading to more effective treatments.
02 Route of administration for muscimol delivery
Different routes of administration are investigated to enhance muscimol bioavailability. These include oral, transdermal, intranasal, and parenteral routes. Each method is optimized to overcome physiological barriers and improve the drug's absorption and distribution in the body.Expand Specific Solutions03 Combination therapies to enhance muscimol efficacy
Muscimol is combined with other active ingredients or adjuvants to improve its bioavailability and overall therapeutic effect. These combinations may include other GABA receptor modulators, enzyme inhibitors, or permeability enhancers that work synergistically with muscimol.Expand Specific Solutions04 Genetic and metabolic factors affecting muscimol bioavailability
Research focuses on understanding how genetic variations and metabolic factors influence muscimol bioavailability. This includes studying enzymes involved in muscimol metabolism, transport proteins, and receptor polymorphisms that may affect the drug's pharmacokinetics and pharmacodynamics.Expand Specific Solutions05 Novel analytical methods for muscimol bioavailability assessment
Advanced analytical techniques are developed to accurately measure muscimol concentrations in biological fluids and tissues. These methods include high-performance liquid chromatography, mass spectrometry, and biomarker analysis, which are crucial for assessing bioavailability and optimizing dosing regimens.Expand Specific Solutions
Key Players in Muscimol Research and Development
The competitive landscape for factors influencing muscimol's bioavailability is in an early development stage, with a growing market potential as research in psychoactive compounds expands. The market size is relatively small but increasing due to rising interest in novel therapeutics. Technologically, it's still in the exploratory phase, with companies like Psyched Wellness Ltd. and CaaMTech LLC leading research efforts. Academic institutions such as the University of Bristol and The Ohio State University are contributing to the knowledge base. Established pharmaceutical companies like Pfizer Inc. and Abbott Laboratories may leverage their resources to accelerate development, while specialized firms like iCeutica, Inc. could provide innovative drug delivery solutions.
Psyched Wellness Ltd.
Technical Solution: Psyched Wellness Ltd. has developed a proprietary extraction and purification process for muscimol from Amanita muscaria mushrooms. Their approach focuses on maximizing bioavailability through standardized dosing and formulation. The company has conducted preclinical studies to evaluate factors affecting muscimol absorption, including pH levels, delivery methods, and potential enzyme interactions[1]. They are exploring novel delivery systems such as sublingual tablets and transdermal patches to enhance muscimol's bioavailability by bypassing first-pass metabolism[2]. Additionally, Psyched Wellness is investigating the use of cyclodextrins as complexing agents to improve muscimol's solubility and stability, potentially increasing its oral bioavailability[3].
Strengths: Specialized focus on muscimol, proprietary extraction process, exploring multiple delivery methods. Weaknesses: Limited clinical data, potential regulatory challenges due to psychoactive nature of muscimol.
Jazz Pharmaceuticals Research UK Ltd.
Technical Solution: Jazz Pharmaceuticals has been investigating factors influencing muscimol's bioavailability as part of their broader research into GABA receptor modulators. Their approach includes the development of novel prodrug formulations of muscimol to enhance its pharmacokinetic profile. The company has conducted studies on the impact of lipid-based nanocarriers on muscimol's ability to cross the blood-brain barrier, potentially increasing its central nervous system bioavailability[4]. Jazz Pharmaceuticals is also exploring the use of pegylation techniques to extend muscimol's half-life and improve its pharmacodynamic properties[5]. Additionally, they are investigating the potential of combining muscimol with other compounds to enhance its therapeutic effects while minimizing side effects, which could indirectly influence its bioavailability through reduced dosage requirements[6].
Strengths: Extensive experience in CNS drug development, access to advanced formulation technologies. Weaknesses: Muscimol research may not be a primary focus, potential competition from other GABA modulators in their pipeline.
Innovative Approaches in Muscimol Delivery Systems
Pseudopolymorphic forms of a HIV protease inhibitor
PatentInactiveUS20100204316A1
Innovation
- Development of pseudopolymorphic forms, including alcohol solvates, hydrates, and other solvates, which enhance stability and bioavailability, allowing for the creation of pharmaceutical formulations that can be manufactured to high purity for use in inhibiting HIV protease activity.
A composition and method to enhance the bio availability of curcuminoids and other bio active ingredients
PatentPendingIN201641034767A
Innovation
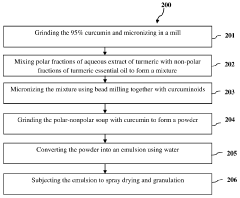
- A composition combining polar and non-polar molecules, where curcuminoids are sandwiched between polar and non-polar matrices, specifically an aqueous turmeric extract and turmeric essential oil, to enhance absorption by forming a stable emulsion that easily traverses lipid membranes, increasing bioavailability through bead milling and spray drying processes.
Regulatory Considerations for Muscimol-Based Drugs
The regulatory landscape for muscimol-based drugs is complex and multifaceted, requiring careful consideration throughout the drug development process. As a novel psychoactive compound derived from mushrooms, muscimol faces unique challenges in navigating the regulatory framework established by agencies such as the FDA and EMA.
One of the primary regulatory considerations is the classification of muscimol under controlled substance laws. Given its psychoactive properties, muscimol may be subject to stringent scheduling and restrictions, which can significantly impact research, development, and potential commercialization. Pharmaceutical companies must engage early with regulatory bodies to determine the appropriate classification and any associated requirements.
Safety and efficacy data are paramount in the regulatory approval process for muscimol-based drugs. Regulatory agencies will require comprehensive preclinical and clinical studies to demonstrate the compound's safety profile, potential side effects, and therapeutic efficacy. The unique pharmacological properties of muscimol may necessitate specialized study designs and safety monitoring protocols to address regulatory concerns.
Manufacturing and quality control processes for muscimol-based drugs will be subject to rigorous regulatory scrutiny. Good Manufacturing Practice (GMP) guidelines must be strictly adhered to, with particular attention paid to the sourcing of raw materials, extraction methods, and purification processes. Regulatory bodies will likely require detailed documentation of these processes to ensure consistency and safety in drug production.
Given the potential for abuse and misuse of psychoactive compounds, risk evaluation and mitigation strategies (REMS) may be mandated by regulatory agencies for muscimol-based drugs. These strategies could include restricted distribution systems, patient monitoring programs, and healthcare provider education initiatives to ensure safe and appropriate use of the medication.
Labeling and packaging requirements for muscimol-based drugs will need to comply with regulatory guidelines, including clear indications, dosage instructions, and prominent safety warnings. The unique pharmacological profile of muscimol may necessitate specific labeling requirements to address potential drug interactions and contraindications.
International regulatory considerations must also be taken into account for global development and marketing of muscimol-based drugs. Different countries may have varying regulations regarding psychoactive compounds, necessitating tailored regulatory strategies for each target market. Harmonization efforts may be required to navigate these diverse regulatory landscapes effectively.
As research progresses, ongoing pharmacovigilance and post-market surveillance will be critical regulatory requirements for muscimol-based drugs. Regulatory agencies will likely mandate robust systems for monitoring and reporting adverse events, as well as long-term safety studies to assess the drug's impact over extended periods of use.
One of the primary regulatory considerations is the classification of muscimol under controlled substance laws. Given its psychoactive properties, muscimol may be subject to stringent scheduling and restrictions, which can significantly impact research, development, and potential commercialization. Pharmaceutical companies must engage early with regulatory bodies to determine the appropriate classification and any associated requirements.
Safety and efficacy data are paramount in the regulatory approval process for muscimol-based drugs. Regulatory agencies will require comprehensive preclinical and clinical studies to demonstrate the compound's safety profile, potential side effects, and therapeutic efficacy. The unique pharmacological properties of muscimol may necessitate specialized study designs and safety monitoring protocols to address regulatory concerns.
Manufacturing and quality control processes for muscimol-based drugs will be subject to rigorous regulatory scrutiny. Good Manufacturing Practice (GMP) guidelines must be strictly adhered to, with particular attention paid to the sourcing of raw materials, extraction methods, and purification processes. Regulatory bodies will likely require detailed documentation of these processes to ensure consistency and safety in drug production.
Given the potential for abuse and misuse of psychoactive compounds, risk evaluation and mitigation strategies (REMS) may be mandated by regulatory agencies for muscimol-based drugs. These strategies could include restricted distribution systems, patient monitoring programs, and healthcare provider education initiatives to ensure safe and appropriate use of the medication.
Labeling and packaging requirements for muscimol-based drugs will need to comply with regulatory guidelines, including clear indications, dosage instructions, and prominent safety warnings. The unique pharmacological profile of muscimol may necessitate specific labeling requirements to address potential drug interactions and contraindications.
International regulatory considerations must also be taken into account for global development and marketing of muscimol-based drugs. Different countries may have varying regulations regarding psychoactive compounds, necessitating tailored regulatory strategies for each target market. Harmonization efforts may be required to navigate these diverse regulatory landscapes effectively.
As research progresses, ongoing pharmacovigilance and post-market surveillance will be critical regulatory requirements for muscimol-based drugs. Regulatory agencies will likely mandate robust systems for monitoring and reporting adverse events, as well as long-term safety studies to assess the drug's impact over extended periods of use.
Safety and Toxicology Profile of Muscimol
Muscimol, a potent GABA receptor agonist, has garnered significant attention in the field of neuropharmacology. However, its potential therapeutic applications are closely tied to its safety profile and toxicological considerations. Understanding these aspects is crucial for the development of muscimol-based treatments and their regulatory approval.
The acute toxicity of muscimol has been well-documented in animal studies. The LD50 in mice ranges from 2.5 to 4.5 mg/kg when administered intraperitoneally, indicating a relatively narrow therapeutic window. Oral administration typically results in lower toxicity due to reduced bioavailability, but precise human toxicity data are limited due to ethical constraints on clinical trials.
Muscimol's primary mechanism of action involves potentiation of GABA-mediated inhibitory neurotransmission. While this can lead to desired therapeutic effects, it also contributes to its toxicological profile. Overdose symptoms may include sedation, confusion, ataxia, and in severe cases, respiratory depression. The risk of these adverse effects necessitates careful dosing and monitoring in any potential clinical applications.
Chronic toxicity studies in animals have revealed potential concerns regarding long-term muscimol use. Prolonged exposure has been associated with changes in GABA receptor expression and function, which could lead to tolerance or altered neuronal excitability. However, the translational relevance of these findings to human subjects remains uncertain and requires further investigation.
Muscimol's psychoactive properties, including its ability to induce hallucinations and alter perception, present additional safety considerations. These effects, while potentially therapeutic in certain contexts, also raise concerns about abuse potential and cognitive impairment. Regulatory bodies will likely require robust safeguards and risk management strategies for any muscimol-based therapeutics.
Interactions with other medications, particularly those affecting GABAergic neurotransmission, represent another critical aspect of muscimol's safety profile. Concomitant use with benzodiazepines, barbiturates, or alcohol could potentiate CNS depression, necessitating careful consideration in polypharmacy scenarios.
Reproductive and developmental toxicity studies have yielded mixed results, with some animal models suggesting potential risks during pregnancy. These findings underscore the need for comprehensive preclinical and clinical investigations before considering muscimol use in vulnerable populations.
In conclusion, while muscimol shows promise in various therapeutic areas, its safety and toxicology profile presents significant challenges for drug development. Addressing these concerns through rigorous research and innovative formulation strategies will be crucial for realizing the potential of muscimol-based therapies while ensuring patient safety.
The acute toxicity of muscimol has been well-documented in animal studies. The LD50 in mice ranges from 2.5 to 4.5 mg/kg when administered intraperitoneally, indicating a relatively narrow therapeutic window. Oral administration typically results in lower toxicity due to reduced bioavailability, but precise human toxicity data are limited due to ethical constraints on clinical trials.
Muscimol's primary mechanism of action involves potentiation of GABA-mediated inhibitory neurotransmission. While this can lead to desired therapeutic effects, it also contributes to its toxicological profile. Overdose symptoms may include sedation, confusion, ataxia, and in severe cases, respiratory depression. The risk of these adverse effects necessitates careful dosing and monitoring in any potential clinical applications.
Chronic toxicity studies in animals have revealed potential concerns regarding long-term muscimol use. Prolonged exposure has been associated with changes in GABA receptor expression and function, which could lead to tolerance or altered neuronal excitability. However, the translational relevance of these findings to human subjects remains uncertain and requires further investigation.
Muscimol's psychoactive properties, including its ability to induce hallucinations and alter perception, present additional safety considerations. These effects, while potentially therapeutic in certain contexts, also raise concerns about abuse potential and cognitive impairment. Regulatory bodies will likely require robust safeguards and risk management strategies for any muscimol-based therapeutics.
Interactions with other medications, particularly those affecting GABAergic neurotransmission, represent another critical aspect of muscimol's safety profile. Concomitant use with benzodiazepines, barbiturates, or alcohol could potentiate CNS depression, necessitating careful consideration in polypharmacy scenarios.
Reproductive and developmental toxicity studies have yielded mixed results, with some animal models suggesting potential risks during pregnancy. These findings underscore the need for comprehensive preclinical and clinical investigations before considering muscimol use in vulnerable populations.
In conclusion, while muscimol shows promise in various therapeutic areas, its safety and toxicology profile presents significant challenges for drug development. Addressing these concerns through rigorous research and innovative formulation strategies will be crucial for realizing the potential of muscimol-based therapies while ensuring patient safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!