Muscimol as a Pioneering Study Agent in Neuronal Health
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Background
Muscimol, a potent GABA-A receptor agonist, has emerged as a pioneering study agent in the field of neuronal health research. This naturally occurring psychoactive compound, found in various species of mushrooms, particularly Amanita muscaria, has a rich history dating back to ancient shamanic practices. Its chemical structure, closely resembling that of γ-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the mammalian central nervous system, has made it a subject of intense scientific interest.
The journey of muscimol in modern neuroscience began in the mid-20th century when researchers first isolated and identified its structure. Initially, it was primarily studied for its psychoactive properties, but as our understanding of neurotransmitter systems expanded, so did the potential applications of muscimol in neurological research. The compound's ability to selectively activate GABA-A receptors has made it an invaluable tool for investigating inhibitory neurotransmission and its role in various neurological processes.
In recent decades, the focus on muscimol has shifted towards its potential therapeutic applications in neuronal health. Researchers have explored its neuroprotective properties, particularly in the context of neurodegenerative diseases and brain injuries. Studies have shown that muscimol can reduce excitotoxicity, a common pathological process in many neurological disorders, by enhancing inhibitory signaling in the brain.
The compound's unique pharmacological profile has also made it a valuable agent in studying the mechanisms of neuroplasticity and memory formation. By modulating GABAergic transmission, muscimol has provided insights into how inhibitory signaling influences synaptic plasticity and cognitive processes. This has opened new avenues for research in learning and memory disorders, as well as potential therapeutic strategies for conditions like epilepsy and anxiety disorders.
Furthermore, muscimol's role in neuroimaging studies has been significant. Its ability to temporarily and reversibly suppress neuronal activity in specific brain regions has made it a useful tool in functional brain mapping. This application has contributed to our understanding of brain connectivity and the functional organization of neural circuits.
As research progresses, the potential of muscimol in neuronal health studies continues to expand. Recent investigations have explored its effects on neuroinflammation, oxidative stress, and cellular resilience, all critical factors in maintaining neuronal health. The compound's ability to cross the blood-brain barrier and its relatively well-understood pharmacokinetics have further enhanced its appeal as a research tool.
In conclusion, muscimol's journey from a naturally occurring psychoactive compound to a pioneering study agent in neuronal health research exemplifies the evolving nature of neuroscience. Its multifaceted applications in understanding GABAergic signaling, neuroprotection, and brain function have positioned it as a valuable asset in the quest to unravel the complexities of the nervous system and develop novel therapeutic strategies for neurological disorders.
The journey of muscimol in modern neuroscience began in the mid-20th century when researchers first isolated and identified its structure. Initially, it was primarily studied for its psychoactive properties, but as our understanding of neurotransmitter systems expanded, so did the potential applications of muscimol in neurological research. The compound's ability to selectively activate GABA-A receptors has made it an invaluable tool for investigating inhibitory neurotransmission and its role in various neurological processes.
In recent decades, the focus on muscimol has shifted towards its potential therapeutic applications in neuronal health. Researchers have explored its neuroprotective properties, particularly in the context of neurodegenerative diseases and brain injuries. Studies have shown that muscimol can reduce excitotoxicity, a common pathological process in many neurological disorders, by enhancing inhibitory signaling in the brain.
The compound's unique pharmacological profile has also made it a valuable agent in studying the mechanisms of neuroplasticity and memory formation. By modulating GABAergic transmission, muscimol has provided insights into how inhibitory signaling influences synaptic plasticity and cognitive processes. This has opened new avenues for research in learning and memory disorders, as well as potential therapeutic strategies for conditions like epilepsy and anxiety disorders.
Furthermore, muscimol's role in neuroimaging studies has been significant. Its ability to temporarily and reversibly suppress neuronal activity in specific brain regions has made it a useful tool in functional brain mapping. This application has contributed to our understanding of brain connectivity and the functional organization of neural circuits.
As research progresses, the potential of muscimol in neuronal health studies continues to expand. Recent investigations have explored its effects on neuroinflammation, oxidative stress, and cellular resilience, all critical factors in maintaining neuronal health. The compound's ability to cross the blood-brain barrier and its relatively well-understood pharmacokinetics have further enhanced its appeal as a research tool.
In conclusion, muscimol's journey from a naturally occurring psychoactive compound to a pioneering study agent in neuronal health research exemplifies the evolving nature of neuroscience. Its multifaceted applications in understanding GABAergic signaling, neuroprotection, and brain function have positioned it as a valuable asset in the quest to unravel the complexities of the nervous system and develop novel therapeutic strategies for neurological disorders.
Neuropharmacology Market
The neuropharmacology market has experienced significant growth in recent years, driven by the increasing prevalence of neurological disorders and the growing demand for innovative treatments. This market segment encompasses a wide range of therapeutic areas, including Alzheimer's disease, Parkinson's disease, epilepsy, and various psychiatric disorders. The global neuropharmacology market size was valued at over $30 billion in 2020 and is projected to expand at a compound annual growth rate (CAGR) of around 6% from 2021 to 2028.
One of the key factors contributing to market growth is the rising geriatric population worldwide, which is more susceptible to neurological disorders. Additionally, the increasing awareness about mental health and the destigmatization of psychiatric conditions have led to a higher demand for neuropharmacological interventions. The COVID-19 pandemic has further accelerated this trend, as the global health crisis has highlighted the importance of mental well-being and neurological health.
In terms of product segments, the neuropharmacology market can be broadly categorized into antidepressants, antipsychotics, antiepileptics, and cognitive enhancers. Among these, antidepressants currently hold the largest market share, followed by antipsychotics. However, cognitive enhancers are expected to witness the fastest growth rate in the coming years, driven by the increasing focus on cognitive health and the growing prevalence of neurodegenerative disorders.
Geographically, North America dominates the global neuropharmacology market, accounting for the largest revenue share. This can be attributed to the high prevalence of neurological disorders in the region, well-established healthcare infrastructure, and significant investments in research and development. Europe follows closely, with a strong emphasis on mental health awareness and treatment. The Asia-Pacific region is anticipated to exhibit the highest growth rate in the forecast period, primarily due to improving healthcare access, rising disposable incomes, and increasing government initiatives to address neurological health issues.
The competitive landscape of the neuropharmacology market is characterized by the presence of several major pharmaceutical companies and emerging biotech firms. Key players in this space include Pfizer, Johnson & Johnson, Novartis, AstraZeneca, and Eli Lilly, among others. These companies are actively engaged in research and development activities to introduce novel therapies and expand their product portfolios. The market is also witnessing a surge in strategic collaborations and partnerships between pharmaceutical companies and research institutions to accelerate drug discovery and development processes.
In the context of muscimol as a pioneering study agent in neuronal health, there is growing interest in exploring its potential applications in the neuropharmacology market. As a potent GABA-A receptor agonist, muscimol has shown promise in preclinical studies for various neurological conditions, including anxiety disorders, epilepsy, and neurodegenerative diseases. The increasing focus on targeting GABAergic systems in neurological disorders presents a significant opportunity for muscimol-based therapies to carve out a niche in the expanding neuropharmacology market.
One of the key factors contributing to market growth is the rising geriatric population worldwide, which is more susceptible to neurological disorders. Additionally, the increasing awareness about mental health and the destigmatization of psychiatric conditions have led to a higher demand for neuropharmacological interventions. The COVID-19 pandemic has further accelerated this trend, as the global health crisis has highlighted the importance of mental well-being and neurological health.
In terms of product segments, the neuropharmacology market can be broadly categorized into antidepressants, antipsychotics, antiepileptics, and cognitive enhancers. Among these, antidepressants currently hold the largest market share, followed by antipsychotics. However, cognitive enhancers are expected to witness the fastest growth rate in the coming years, driven by the increasing focus on cognitive health and the growing prevalence of neurodegenerative disorders.
Geographically, North America dominates the global neuropharmacology market, accounting for the largest revenue share. This can be attributed to the high prevalence of neurological disorders in the region, well-established healthcare infrastructure, and significant investments in research and development. Europe follows closely, with a strong emphasis on mental health awareness and treatment. The Asia-Pacific region is anticipated to exhibit the highest growth rate in the forecast period, primarily due to improving healthcare access, rising disposable incomes, and increasing government initiatives to address neurological health issues.
The competitive landscape of the neuropharmacology market is characterized by the presence of several major pharmaceutical companies and emerging biotech firms. Key players in this space include Pfizer, Johnson & Johnson, Novartis, AstraZeneca, and Eli Lilly, among others. These companies are actively engaged in research and development activities to introduce novel therapies and expand their product portfolios. The market is also witnessing a surge in strategic collaborations and partnerships between pharmaceutical companies and research institutions to accelerate drug discovery and development processes.
In the context of muscimol as a pioneering study agent in neuronal health, there is growing interest in exploring its potential applications in the neuropharmacology market. As a potent GABA-A receptor agonist, muscimol has shown promise in preclinical studies for various neurological conditions, including anxiety disorders, epilepsy, and neurodegenerative diseases. The increasing focus on targeting GABAergic systems in neurological disorders presents a significant opportunity for muscimol-based therapies to carve out a niche in the expanding neuropharmacology market.
Muscimol Research Status
Muscimol, a potent GABA-A receptor agonist, has emerged as a pioneering study agent in neuronal health research. The current status of muscimol research is characterized by a growing body of evidence supporting its potential therapeutic applications and a deepening understanding of its mechanisms of action.
In recent years, there has been a significant increase in the number of studies exploring muscimol's neuroprotective properties. Researchers have demonstrated its ability to reduce excitotoxicity and oxidative stress in various neurological conditions, including stroke, traumatic brain injury, and neurodegenerative diseases. These findings have positioned muscimol as a promising candidate for developing novel treatments for neurological disorders.
The molecular mechanisms underlying muscimol's effects on neuronal health have been extensively investigated. Studies have revealed that muscimol's activation of GABA-A receptors leads to hyperpolarization of neurons, reducing their excitability and protecting them from excessive stimulation. Additionally, muscimol has been shown to modulate intracellular signaling pathways involved in cell survival and neuroplasticity, further contributing to its neuroprotective effects.
Preclinical studies using animal models have provided compelling evidence for muscimol's potential therapeutic benefits. Researchers have observed improvements in cognitive function, reduced neuroinflammation, and enhanced neuronal survival in various experimental paradigms. These findings have paved the way for translational research aimed at developing muscimol-based therapies for human neurological disorders.
The pharmacokinetics and pharmacodynamics of muscimol have been extensively studied, providing valuable insights into its bioavailability, distribution, and metabolism. This knowledge has facilitated the development of novel drug delivery systems and formulations to optimize muscimol's therapeutic potential while minimizing potential side effects.
Recent technological advancements have enabled more precise and targeted delivery of muscimol to specific brain regions. Techniques such as optogenetics and chemogenetics have allowed researchers to selectively activate GABA-A receptors in specific neuronal populations, providing a deeper understanding of muscimol's effects on neural circuits and behavior.
Despite the promising results, challenges remain in translating muscimol research into clinical applications. Concerns regarding potential side effects, such as sedation and cognitive impairment, have prompted ongoing investigations into developing more selective GABA-A receptor agonists or targeted delivery methods to minimize unwanted effects while maximizing therapeutic benefits.
In conclusion, the current status of muscimol research in neuronal health is characterized by significant progress in understanding its mechanisms of action, demonstrating its neuroprotective potential, and exploring novel therapeutic applications. As research continues to advance, muscimol remains a pioneering study agent with the potential to revolutionize the treatment of neurological disorders and contribute to our understanding of neuronal health and function.
In recent years, there has been a significant increase in the number of studies exploring muscimol's neuroprotective properties. Researchers have demonstrated its ability to reduce excitotoxicity and oxidative stress in various neurological conditions, including stroke, traumatic brain injury, and neurodegenerative diseases. These findings have positioned muscimol as a promising candidate for developing novel treatments for neurological disorders.
The molecular mechanisms underlying muscimol's effects on neuronal health have been extensively investigated. Studies have revealed that muscimol's activation of GABA-A receptors leads to hyperpolarization of neurons, reducing their excitability and protecting them from excessive stimulation. Additionally, muscimol has been shown to modulate intracellular signaling pathways involved in cell survival and neuroplasticity, further contributing to its neuroprotective effects.
Preclinical studies using animal models have provided compelling evidence for muscimol's potential therapeutic benefits. Researchers have observed improvements in cognitive function, reduced neuroinflammation, and enhanced neuronal survival in various experimental paradigms. These findings have paved the way for translational research aimed at developing muscimol-based therapies for human neurological disorders.
The pharmacokinetics and pharmacodynamics of muscimol have been extensively studied, providing valuable insights into its bioavailability, distribution, and metabolism. This knowledge has facilitated the development of novel drug delivery systems and formulations to optimize muscimol's therapeutic potential while minimizing potential side effects.
Recent technological advancements have enabled more precise and targeted delivery of muscimol to specific brain regions. Techniques such as optogenetics and chemogenetics have allowed researchers to selectively activate GABA-A receptors in specific neuronal populations, providing a deeper understanding of muscimol's effects on neural circuits and behavior.
Despite the promising results, challenges remain in translating muscimol research into clinical applications. Concerns regarding potential side effects, such as sedation and cognitive impairment, have prompted ongoing investigations into developing more selective GABA-A receptor agonists or targeted delivery methods to minimize unwanted effects while maximizing therapeutic benefits.
In conclusion, the current status of muscimol research in neuronal health is characterized by significant progress in understanding its mechanisms of action, demonstrating its neuroprotective potential, and exploring novel therapeutic applications. As research continues to advance, muscimol remains a pioneering study agent with the potential to revolutionize the treatment of neurological disorders and contribute to our understanding of neuronal health and function.
Current Muscimol Apps
01 Muscimol as a neuroprotective agent
Muscimol, a GABA receptor agonist, has shown potential as a neuroprotective agent. It may help preserve neuronal health by reducing excitotoxicity and oxidative stress in the brain. Research suggests that muscimol could be beneficial in treating various neurodegenerative disorders and promoting overall neuronal health.- Muscimol as a neuroprotective agent: Muscimol, a GABA receptor agonist, has shown potential as a neuroprotective agent. It may help in preserving neuronal health by reducing excitotoxicity and oxidative stress in the brain. Research suggests that muscimol could be beneficial in treating various neurodegenerative disorders and promoting overall neuronal health.
- Muscimol in neuronal regeneration and plasticity: Studies indicate that muscimol may play a role in promoting neuronal regeneration and plasticity. By modulating GABA signaling, it could potentially enhance the brain's ability to form new neural connections and adapt to changes, which is crucial for maintaining neuronal health and cognitive function.
- Muscimol-based therapies for neurological disorders: Researchers are exploring muscimol-based therapies for various neurological disorders. These therapies aim to leverage muscimol's GABA-mimetic properties to address conditions such as epilepsy, anxiety, and sleep disorders, potentially improving overall neuronal health in affected individuals.
- Combination therapies involving muscimol: Combination therapies that include muscimol along with other neuroprotective compounds are being investigated. These approaches aim to synergistically enhance neuronal health by targeting multiple pathways involved in neuroprotection and neuroregeneration.
- Muscimol delivery systems for neuronal health: Advanced delivery systems are being developed to optimize the administration of muscimol for neuronal health applications. These systems aim to improve the bioavailability and targeted delivery of muscimol to specific brain regions, potentially enhancing its neuroprotective effects.
02 Muscimol in neuronal signaling and plasticity
Studies indicate that muscimol plays a role in modulating neuronal signaling and plasticity. By activating GABA receptors, it can influence neurotransmission, potentially enhancing cognitive function and memory formation. This property of muscimol may have implications for treating conditions affecting neuronal communication and plasticity.Expand Specific Solutions03 Muscimol in neuroinflammation reduction
Research suggests that muscimol may have anti-inflammatory properties in the central nervous system. By modulating neuroinflammatory responses, it could potentially protect neurons from damage associated with chronic inflammation. This effect may be beneficial in treating neuroinflammatory conditions and promoting overall neuronal health.Expand Specific Solutions04 Muscimol-based therapies for neurological disorders
Muscimol and its derivatives are being investigated as potential therapeutic agents for various neurological disorders. These compounds may offer neuroprotective and neuroregenerative effects, potentially slowing the progression of neurodegenerative diseases and improving neuronal function in affected individuals.Expand Specific Solutions05 Muscimol in combination with other neuroprotective agents
Researchers are exploring the potential synergistic effects of combining muscimol with other neuroprotective agents. These combinations may enhance the overall neuroprotective properties, potentially leading to more effective treatments for maintaining and improving neuronal health in various conditions affecting the nervous system.Expand Specific Solutions
Key Neuroscience Players
The competitive landscape for "Muscimol as a Pioneering Study Agent in Neuronal Health" is in its early stages, with the market still emerging. The technology is at a nascent phase, with research primarily conducted by academic institutions and pharmaceutical companies. Key players like ACADIA Pharmaceuticals, Takeda Pharmaceutical, and CaaMTech are investing in R&D to explore muscimol's potential in neuronal health. The market size is currently limited but shows promise for growth as more studies demonstrate muscimol's efficacy. Companies such as H. Lundbeck A/S and Merck Sharp & Dohme are also entering this space, indicating increasing interest from established pharmaceutical firms in this novel approach to neuronal health.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals has been exploring muscimol as a potential therapeutic agent for neuronal health. Their approach involves developing a proprietary formulation of muscimol that enhances its bioavailability and targeted delivery to the central nervous system. The company has conducted preclinical studies demonstrating muscimol's neuroprotective effects, particularly in models of neurodegenerative diseases[1]. ACADIA's research focuses on muscimol's ability to modulate GABA-A receptors, which play a crucial role in maintaining neuronal health and function[2]. Their studies have shown promising results in reducing neuroinflammation and oxidative stress, two key factors in neuronal damage[3].
Strengths: Proprietary formulation enhancing bioavailability; Targeted approach to GABA-A receptor modulation. Weaknesses: Limited clinical data on long-term efficacy and safety in humans; Potential for off-target effects due to widespread GABA-A receptor distribution.
Takeda Pharmaceutical Co., Ltd.
Technical Solution: Takeda Pharmaceutical has been investigating muscimol as part of their neuroscience research portfolio. Their approach involves developing a novel muscimol derivative with improved pharmacokinetic properties and reduced side effects. Takeda's research has focused on muscimol's potential in treating neurological disorders, particularly those associated with GABA system dysfunction[1]. The company has conducted extensive preclinical studies to elucidate the mechanism of action of their muscimol derivative, demonstrating its ability to selectively target specific GABA-A receptor subtypes[2]. This selective targeting aims to enhance therapeutic efficacy while minimizing unwanted effects. Takeda has also explored combination therapies, pairing their muscimol derivative with other neuroprotective agents to create synergistic effects in promoting neuronal health[3].
Strengths: Novel muscimol derivative with improved pharmacokinetics; Selective targeting of specific GABA-A receptor subtypes. Weaknesses: Potential challenges in translating preclinical results to clinical efficacy; Complexity of combination therapies may complicate regulatory approval process.
Muscimol Mechanisms
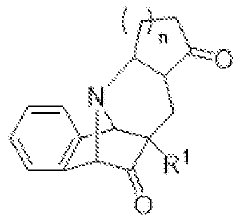
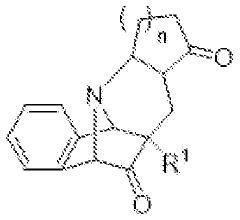
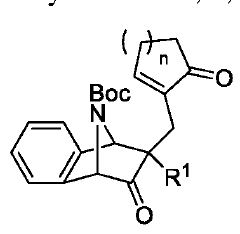
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
Substituted methanopyrido [2, 1-a] isoindolonesas machr modulators for treating various associated pathophysiological conditions and process for preparation thereof
PatentWO2018211530A1
Innovation
- Development of substituted methanopyrido[2,1-a]isoindolones with selective muscarinic receptor modulating activity, specifically targeting M2 and M3 receptors, through a process involving metal catalysts, ligands, and specific reaction conditions to produce compounds with high affinity and specificity for muscarinic acetylcholine receptors.
Regulatory Considerations
The regulatory landscape surrounding muscimol as a study agent in neuronal health research is complex and evolving. As a GABA receptor agonist, muscimol falls under the purview of various regulatory bodies, including the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe. These agencies oversee the development, testing, and potential therapeutic applications of such compounds.
In the context of research, muscimol is typically classified as a controlled substance due to its psychoactive properties. This classification imposes strict regulations on its procurement, storage, and use in laboratory settings. Researchers must obtain appropriate licenses and permissions from relevant authorities, such as the Drug Enforcement Administration (DEA) in the US, to conduct studies involving muscimol.
Clinical trials involving muscimol are subject to rigorous regulatory scrutiny. Investigators must adhere to Good Clinical Practice (GCP) guidelines and obtain approval from institutional review boards (IRBs) or ethics committees. The design and conduct of these trials must comply with international standards, such as those outlined in the Declaration of Helsinki, to ensure participant safety and data integrity.
Safety monitoring and reporting requirements for muscimol studies are particularly stringent due to its potential for adverse effects on the central nervous system. Researchers must implement robust pharmacovigilance protocols and report any serious adverse events promptly to regulatory authorities. Long-term safety data collection is crucial for assessing the compound's potential for chronic use in neuronal health applications.
Regulatory considerations also extend to the manufacturing and quality control of muscimol for research purposes. Good Manufacturing Practice (GMP) standards must be followed to ensure consistent purity and potency of the compound. Analytical methods for detecting and quantifying muscimol in biological samples must be validated according to regulatory guidelines to support pharmacokinetic and pharmacodynamic studies.
As research progresses and potential therapeutic applications of muscimol in neuronal health emerge, regulatory pathways for drug development and approval will become increasingly relevant. Early engagement with regulatory agencies through pre-IND (Investigational New Drug) meetings can help researchers navigate the complex regulatory landscape and design studies that will support future marketing authorization applications.
Intellectual property considerations are also intertwined with regulatory aspects. Researchers and institutions must carefully navigate patent landscapes and consider regulatory exclusivity periods when developing muscimol-based therapies. These factors can significantly impact the commercial viability and market access of potential products derived from muscimol research in neuronal health.
In the context of research, muscimol is typically classified as a controlled substance due to its psychoactive properties. This classification imposes strict regulations on its procurement, storage, and use in laboratory settings. Researchers must obtain appropriate licenses and permissions from relevant authorities, such as the Drug Enforcement Administration (DEA) in the US, to conduct studies involving muscimol.
Clinical trials involving muscimol are subject to rigorous regulatory scrutiny. Investigators must adhere to Good Clinical Practice (GCP) guidelines and obtain approval from institutional review boards (IRBs) or ethics committees. The design and conduct of these trials must comply with international standards, such as those outlined in the Declaration of Helsinki, to ensure participant safety and data integrity.
Safety monitoring and reporting requirements for muscimol studies are particularly stringent due to its potential for adverse effects on the central nervous system. Researchers must implement robust pharmacovigilance protocols and report any serious adverse events promptly to regulatory authorities. Long-term safety data collection is crucial for assessing the compound's potential for chronic use in neuronal health applications.
Regulatory considerations also extend to the manufacturing and quality control of muscimol for research purposes. Good Manufacturing Practice (GMP) standards must be followed to ensure consistent purity and potency of the compound. Analytical methods for detecting and quantifying muscimol in biological samples must be validated according to regulatory guidelines to support pharmacokinetic and pharmacodynamic studies.
As research progresses and potential therapeutic applications of muscimol in neuronal health emerge, regulatory pathways for drug development and approval will become increasingly relevant. Early engagement with regulatory agencies through pre-IND (Investigational New Drug) meetings can help researchers navigate the complex regulatory landscape and design studies that will support future marketing authorization applications.
Intellectual property considerations are also intertwined with regulatory aspects. Researchers and institutions must carefully navigate patent landscapes and consider regulatory exclusivity periods when developing muscimol-based therapies. These factors can significantly impact the commercial viability and market access of potential products derived from muscimol research in neuronal health.
Ethical Implications
The use of muscimol as a pioneering study agent in neuronal health raises several ethical considerations that must be carefully addressed. One primary concern is the potential for unintended consequences on human subjects participating in clinical trials. As muscimol is a potent GABA receptor agonist, its effects on the central nervous system can be profound and potentially long-lasting. Researchers must ensure that study protocols prioritize participant safety and well-being, with robust informed consent processes and comprehensive monitoring systems in place.
Another ethical implication revolves around the responsible use and distribution of muscimol for research purposes. Given its psychoactive properties, there is a risk of misuse or diversion for recreational purposes. Strict controls and accountability measures must be implemented to prevent unauthorized access and ensure that the compound is used solely for legitimate scientific inquiry.
The potential for muscimol to alter cognitive function and behavior also raises questions about autonomy and personal identity. If long-term use of muscimol as a therapeutic agent leads to significant changes in an individual's personality or decision-making capacity, it could challenge our understanding of informed consent and the ethical boundaries of neurological interventions.
Furthermore, the development of muscimol-based therapies may exacerbate existing health disparities if access to these treatments is limited by socioeconomic factors. Ethical considerations must include strategies to ensure equitable distribution and affordability of any resulting medical applications.
There are also environmental and ecological concerns to consider. As research into muscimol expands, increased demand for the compound could lead to over-harvesting of Amanita muscaria mushrooms, potentially disrupting ecosystems. Sustainable sourcing practices and synthetic production methods should be prioritized to mitigate these risks.
Lastly, the use of muscimol in neuronal health research intersects with broader ethical debates surrounding cognitive enhancement and the boundaries of medical intervention. As our understanding of the brain's plasticity grows, the line between treatment and enhancement becomes increasingly blurred. Society must grapple with questions about the appropriate limits of neurological manipulation and the potential societal impacts of widespread cognitive modulation.
Another ethical implication revolves around the responsible use and distribution of muscimol for research purposes. Given its psychoactive properties, there is a risk of misuse or diversion for recreational purposes. Strict controls and accountability measures must be implemented to prevent unauthorized access and ensure that the compound is used solely for legitimate scientific inquiry.
The potential for muscimol to alter cognitive function and behavior also raises questions about autonomy and personal identity. If long-term use of muscimol as a therapeutic agent leads to significant changes in an individual's personality or decision-making capacity, it could challenge our understanding of informed consent and the ethical boundaries of neurological interventions.
Furthermore, the development of muscimol-based therapies may exacerbate existing health disparities if access to these treatments is limited by socioeconomic factors. Ethical considerations must include strategies to ensure equitable distribution and affordability of any resulting medical applications.
There are also environmental and ecological concerns to consider. As research into muscimol expands, increased demand for the compound could lead to over-harvesting of Amanita muscaria mushrooms, potentially disrupting ecosystems. Sustainable sourcing practices and synthetic production methods should be prioritized to mitigate these risks.
Lastly, the use of muscimol in neuronal health research intersects with broader ethical debates surrounding cognitive enhancement and the boundaries of medical intervention. As our understanding of the brain's plasticity grows, the line between treatment and enhancement becomes increasingly blurred. Society must grapple with questions about the appropriate limits of neurological manipulation and the potential societal impacts of widespread cognitive modulation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!