Expanding Chemical Research Potential with Fluoroantimonic Acid
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Background and Research Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has been a subject of intense scientific interest since its discovery in the 1960s. This compound holds the distinction of being the strongest known superacid, with a Hammett acidity function (H0) estimated to be as low as -31.3. Its exceptional acidity surpasses that of pure sulfuric acid by over a trillion times, making it a unique and powerful tool in chemical research.
The development of fluoroantimonic acid marked a significant milestone in the field of superacid chemistry. Its creation was a result of the continuous pursuit of stronger acids, driven by the need for more effective catalysts and reagents in various chemical processes. The journey towards its discovery involved extensive research into the behavior of strong Lewis acids and their interactions with Brønsted acids.
Throughout the years, fluoroantimonic acid has demonstrated remarkable potential in expanding the boundaries of chemical research. Its extreme acidity allows for the protonation of exceptionally weak bases, enabling reactions that were previously thought impossible. This property has opened up new avenues for synthesis, particularly in the field of organic chemistry, where it has been used to generate highly reactive carbocations and facilitate challenging transformations.
The research objectives surrounding fluoroantimonic acid are multifaceted and ambitious. One primary goal is to fully understand its structure and behavior at the molecular level. Despite its powerful properties, the exact nature of its ionic species in solution and its interaction with various substrates remain subjects of ongoing investigation. Researchers aim to elucidate these mechanisms to optimize its use in chemical processes.
Another critical objective is to explore the full spectrum of its catalytic capabilities. Fluoroantimonic acid has shown promise in various industrial applications, including the isomerization of alkanes, the cracking of hydrocarbons, and the polymerization of olefins. Expanding its use in these areas could lead to more efficient and environmentally friendly chemical processes, potentially revolutionizing sectors of the petrochemical industry.
Safety and handling considerations form a crucial aspect of fluoroantimonic acid research. Due to its extreme reactivity and corrosive nature, developing improved containment and handling protocols is essential. This includes the design of specialized equipment and the formulation of safer, more stable forms of the acid for broader application in both research and industrial settings.
Lastly, there is a growing interest in harnessing the unique properties of fluoroantimonic acid for novel applications beyond traditional chemical synthesis. This includes its potential use in advanced materials science, such as the creation of super-strong materials or the development of new energy storage technologies. The exploration of these frontier applications represents an exciting direction for future research, promising to unlock new possibilities in materials engineering and energy technology.
The development of fluoroantimonic acid marked a significant milestone in the field of superacid chemistry. Its creation was a result of the continuous pursuit of stronger acids, driven by the need for more effective catalysts and reagents in various chemical processes. The journey towards its discovery involved extensive research into the behavior of strong Lewis acids and their interactions with Brønsted acids.
Throughout the years, fluoroantimonic acid has demonstrated remarkable potential in expanding the boundaries of chemical research. Its extreme acidity allows for the protonation of exceptionally weak bases, enabling reactions that were previously thought impossible. This property has opened up new avenues for synthesis, particularly in the field of organic chemistry, where it has been used to generate highly reactive carbocations and facilitate challenging transformations.
The research objectives surrounding fluoroantimonic acid are multifaceted and ambitious. One primary goal is to fully understand its structure and behavior at the molecular level. Despite its powerful properties, the exact nature of its ionic species in solution and its interaction with various substrates remain subjects of ongoing investigation. Researchers aim to elucidate these mechanisms to optimize its use in chemical processes.
Another critical objective is to explore the full spectrum of its catalytic capabilities. Fluoroantimonic acid has shown promise in various industrial applications, including the isomerization of alkanes, the cracking of hydrocarbons, and the polymerization of olefins. Expanding its use in these areas could lead to more efficient and environmentally friendly chemical processes, potentially revolutionizing sectors of the petrochemical industry.
Safety and handling considerations form a crucial aspect of fluoroantimonic acid research. Due to its extreme reactivity and corrosive nature, developing improved containment and handling protocols is essential. This includes the design of specialized equipment and the formulation of safer, more stable forms of the acid for broader application in both research and industrial settings.
Lastly, there is a growing interest in harnessing the unique properties of fluoroantimonic acid for novel applications beyond traditional chemical synthesis. This includes its potential use in advanced materials science, such as the creation of super-strong materials or the development of new energy storage technologies. The exploration of these frontier applications represents an exciting direction for future research, promising to unlock new possibilities in materials engineering and energy technology.
Market Analysis for Superacid Applications
The market for superacid applications, particularly those involving fluoroantimonic acid, has shown significant growth potential in recent years. This powerful superacid, known for its extreme acidity and unique chemical properties, has found increasing use across various industries, driving market expansion and innovation.
In the petrochemical sector, fluoroantimonic acid has become a crucial catalyst in hydrocarbon processing, especially in alkylation reactions for high-octane gasoline production. The growing demand for cleaner, more efficient fuels has led to increased adoption of superacid-based catalytic processes, stimulating market growth in this segment.
The electronics industry has also emerged as a key consumer of fluoroantimonic acid. Its application in the etching of silicon wafers and the production of advanced semiconductors has become more prevalent as the demand for smaller, more powerful electronic devices continues to rise. This trend is expected to contribute significantly to market expansion in the coming years.
In the field of materials science, fluoroantimonic acid has shown promise in the development of novel materials with unique properties. Its ability to facilitate certain polymerization reactions and modify surface characteristics of materials has opened up new avenues for research and development, potentially leading to breakthrough applications in areas such as advanced composites and nanomaterials.
The pharmaceutical industry has also begun exploring the use of fluoroantimonic acid in the synthesis of complex organic compounds. While still in early stages, this application could represent a substantial market opportunity as drug discovery processes become increasingly sophisticated and demand grows for more efficient synthetic routes.
Despite its potential, the market for fluoroantimonic acid faces challenges related to handling and safety concerns. The extreme corrosiveness and reactivity of the acid necessitate specialized equipment and stringent safety protocols, which can limit its widespread adoption. However, ongoing research into safer handling methods and the development of more stable superacid formulations may help mitigate these concerns and further expand market opportunities.
Geographically, North America and Europe currently lead in superacid applications, driven by their well-established chemical and pharmaceutical industries. However, rapid industrialization and increasing research activities in Asia-Pacific countries, particularly China and India, are expected to create new growth opportunities in these regions.
In the petrochemical sector, fluoroantimonic acid has become a crucial catalyst in hydrocarbon processing, especially in alkylation reactions for high-octane gasoline production. The growing demand for cleaner, more efficient fuels has led to increased adoption of superacid-based catalytic processes, stimulating market growth in this segment.
The electronics industry has also emerged as a key consumer of fluoroantimonic acid. Its application in the etching of silicon wafers and the production of advanced semiconductors has become more prevalent as the demand for smaller, more powerful electronic devices continues to rise. This trend is expected to contribute significantly to market expansion in the coming years.
In the field of materials science, fluoroantimonic acid has shown promise in the development of novel materials with unique properties. Its ability to facilitate certain polymerization reactions and modify surface characteristics of materials has opened up new avenues for research and development, potentially leading to breakthrough applications in areas such as advanced composites and nanomaterials.
The pharmaceutical industry has also begun exploring the use of fluoroantimonic acid in the synthesis of complex organic compounds. While still in early stages, this application could represent a substantial market opportunity as drug discovery processes become increasingly sophisticated and demand grows for more efficient synthetic routes.
Despite its potential, the market for fluoroantimonic acid faces challenges related to handling and safety concerns. The extreme corrosiveness and reactivity of the acid necessitate specialized equipment and stringent safety protocols, which can limit its widespread adoption. However, ongoing research into safer handling methods and the development of more stable superacid formulations may help mitigate these concerns and further expand market opportunities.
Geographically, North America and Europe currently lead in superacid applications, driven by their well-established chemical and pharmaceutical industries. However, rapid industrialization and increasing research activities in Asia-Pacific countries, particularly China and India, are expected to create new growth opportunities in these regions.
Current Challenges in Fluoroantimonic Acid Research
Fluoroantimonic acid, known as the world's strongest superacid, presents significant challenges in research and application due to its extreme reactivity and corrosive nature. One of the primary obstacles is the development of suitable containment materials that can withstand its highly acidic properties. Traditional laboratory glassware and most metals are rapidly degraded by fluoroantimonic acid, necessitating the use of specialized materials such as Teflon or certain fluoropolymers.
The handling and storage of fluoroantimonic acid pose considerable safety risks, requiring stringent protocols and advanced protective equipment. Researchers must navigate the complexities of working with a substance that reacts violently with water and many organic compounds, limiting the range of potential experiments and applications.
Another significant challenge lies in the precise control and measurement of fluoroantimonic acid's acidity. Its Hammett acidity function exceeds the capabilities of conventional pH measurement techniques, necessitating the development of new methodologies for quantifying its strength and reactivity accurately.
The environmental impact of fluoroantimonic acid research is also a pressing concern. Its production and use generate hazardous waste that requires specialized disposal methods, and any accidental release could have severe consequences for ecosystems and human health. This necessitates the implementation of rigorous safety measures and waste management protocols.
From an analytical perspective, the extreme reactivity of fluoroantimonic acid complicates spectroscopic and structural studies. Many standard analytical techniques are incompatible with its properties, requiring researchers to develop novel approaches for characterizing its behavior and interactions with other substances.
The scalability of fluoroantimonic acid production and application presents another hurdle. While its potential in catalysis and materials processing is significant, transitioning from laboratory-scale experiments to industrial-scale processes involves substantial engineering challenges related to safety, efficiency, and cost-effectiveness.
Lastly, the regulatory landscape surrounding fluoroantimonic acid research is complex and evolving. Researchers must navigate strict guidelines and obtain necessary permits, which can impede the pace of scientific progress and limit collaborative efforts across institutions and international borders.
The handling and storage of fluoroantimonic acid pose considerable safety risks, requiring stringent protocols and advanced protective equipment. Researchers must navigate the complexities of working with a substance that reacts violently with water and many organic compounds, limiting the range of potential experiments and applications.
Another significant challenge lies in the precise control and measurement of fluoroantimonic acid's acidity. Its Hammett acidity function exceeds the capabilities of conventional pH measurement techniques, necessitating the development of new methodologies for quantifying its strength and reactivity accurately.
The environmental impact of fluoroantimonic acid research is also a pressing concern. Its production and use generate hazardous waste that requires specialized disposal methods, and any accidental release could have severe consequences for ecosystems and human health. This necessitates the implementation of rigorous safety measures and waste management protocols.
From an analytical perspective, the extreme reactivity of fluoroantimonic acid complicates spectroscopic and structural studies. Many standard analytical techniques are incompatible with its properties, requiring researchers to develop novel approaches for characterizing its behavior and interactions with other substances.
The scalability of fluoroantimonic acid production and application presents another hurdle. While its potential in catalysis and materials processing is significant, transitioning from laboratory-scale experiments to industrial-scale processes involves substantial engineering challenges related to safety, efficiency, and cost-effectiveness.
Lastly, the regulatory landscape surrounding fluoroantimonic acid research is complex and evolving. Researchers must navigate strict guidelines and obtain necessary permits, which can impede the pace of scientific progress and limit collaborative efforts across institutions and international borders.
Existing Methodologies for Fluoroantimonic Acid Synthesis
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Specialized equipment and safety measures are required due to the extreme acidity and reactivity of the compound.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in catalysis and organic synthesis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acid catalysts, making it valuable in the production of specialty chemicals and advanced materials.
- Use in materials science and surface treatment: Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It is used for etching, cleaning, and activating surfaces of metals, semiconductors, and other materials. The acid's unique properties allow for precise control of surface characteristics, enhancing material performance in specific applications.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, handling fluoroantimonic acid requires stringent safety measures. Specialized equipment, containment systems, and personal protective gear are essential when working with this superacid. Proper storage, transportation, and disposal protocols must be followed to prevent accidents and environmental contamination.
- Analytical and characterization techniques: Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analysis, and specialized titration procedures. Advanced instrumentation and methodologies are employed to investigate the acid's properties, reaction mechanisms, and interactions with other substances.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid serves as a powerful superacid catalyst in various organic synthesis reactions. It is particularly useful in alkylation, isomerization, and polymerization processes. The extreme acidity of fluoroantimonic acid enables it to catalyze reactions that are difficult or impossible with conventional acids.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science for surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials. The extreme reactivity of the acid allows for unique surface modifications that are not achievable with milder acids.Expand Specific Solutions04 Safety considerations and handling procedures
Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized safety measures and handling procedures. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage, transport, and disposal. Proper training and safety systems are essential for working with this superacid.Expand Specific Solutions05 Analytical and characterization methods
Specialized analytical and characterization methods are employed for fluoroantimonic acid due to its extreme properties. These may include spectroscopic techniques, electrochemical methods, and custom-designed apparatus capable of withstanding the acid's corrosive nature. Such methods are crucial for quality control, purity assessment, and research applications involving fluoroantimonic acid.Expand Specific Solutions
Key Players in Superacid Research and Production
The field of fluoroantimonic acid research is in a nascent stage, with significant potential for growth in chemical and materials science applications. The market size is relatively small but expanding, driven by increasing demand for super-acidic catalysts in industrial processes. Technologically, it's still in the early development phase, with companies like DuPont de Nemours, Inc., 3M Innovative Properties Co., and DAIKIN INDUSTRIES Ltd. leading the way in research and patent filings. Academic institutions such as Liaocheng University and Oxford University Innovation Ltd. are also contributing to the advancement of this technology, indicating a collaborative ecosystem between industry and academia in pushing the boundaries of fluoroantimonic acid applications.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for the safe production and handling of fluoroantimonic acid. Their method involves a controlled reaction between hydrogen fluoride and antimony pentafluoride in specialized corrosion-resistant vessels. The company has also engineered advanced containment systems and safety protocols to manage this superacid's extreme reactivity. DuPont's research focuses on exploring fluoroantimonic acid's potential as a catalyst in various organic synthesis reactions, particularly in the production of high-performance polymers and specialty chemicals.
Strengths: Extensive experience in handling hazardous materials, strong R&D capabilities, and established safety protocols. Weaknesses: High production costs and limited commercial applications due to the acid's extreme reactivity.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN has developed a novel approach to utilizing fluoroantimonic acid in the synthesis of fluoropolymers. Their technique involves using the superacid as a catalyst in controlled polymerization reactions, resulting in fluoropolymers with enhanced properties. DAIKIN's research also extends to the application of fluoroantimonic acid in surface treatment processes, creating highly durable and chemically resistant coatings. The company has invested in specialized equipment and facilities to handle this extremely corrosive substance safely, including advanced containment systems and remote handling technologies.
Strengths: Expertise in fluorine chemistry, innovative applications in polymer science, and advanced handling facilities. Weaknesses: High operational costs and potential environmental concerns associated with fluorine compounds.
Breakthrough Technologies in Superacid Chemistry
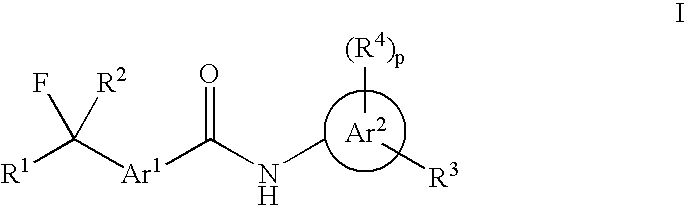
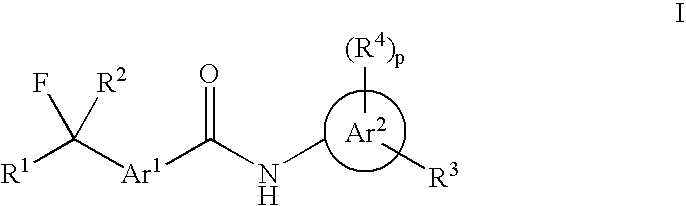
Fluorinated Arylamide Derivatives
PatentInactiveUS20090012075A1
Innovation
- Development of novel fluoroalkylarylamide derivatives that act as potent histone deacetylase inhibitors, capable of inducing terminal differentiation and apoptosis in neoplastic cells, and suitable for treating various diseases including cancer, autoimmune, and neurodegenerative conditions.
Novel Fluorescent Amino Acid Derivative and Production Method Of The Same
PatentInactiveUS20090240026A1
Innovation
- A novel fluorescent amino acid derivative is synthesized using a fluorescent acridone derivative with an electrophilic substitution reaction, incorporating an electron-withdrawing group such as a sulfonyl group, allowing excitation in the blue laser region with improved light stability and simplified production.
Safety Protocols for Handling Superacids
Handling superacids, particularly fluoroantimonic acid, requires stringent safety protocols due to their extreme corrosiveness and reactivity. The primary focus of these protocols is to minimize the risk of exposure and ensure proper containment throughout all stages of handling and storage.
Personal protective equipment (PPE) is paramount when working with superacids. This includes chemical-resistant suits, gloves, and boots made from materials such as fluorinated ethylene propylene (FEP) or polytetrafluoroethylene (PTFE). Full-face respirators with appropriate acid gas cartridges or supplied air systems are essential to protect against harmful vapors. Eye protection in the form of chemical splash goggles or a face shield is also crucial.
Workspace design plays a critical role in safety. All handling of superacids must be conducted in a properly functioning fume hood with a face velocity of at least 100 feet per minute. The work area should be equipped with emergency eyewash stations and safety showers within easy reach. Spill containment measures, including acid-resistant trays and absorbent materials, should be readily available.
Storage of superacids requires specialized containers made from materials resistant to their corrosive nature, such as PTFE or FEP. These containers must be kept in a cool, dry, well-ventilated area, away from incompatible substances and sources of heat or ignition. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
Training is a crucial component of safety protocols. All personnel working with superacids must undergo comprehensive training on proper handling techniques, emergency procedures, and the use of safety equipment. This training should be regularly updated and reinforced through practical exercises and simulations.
Emergency response plans must be in place and well-communicated. These plans should outline procedures for dealing with spills, personal exposure, and other potential incidents. Neutralization agents, such as sodium bicarbonate or calcium oxide, should be readily available for spill control, but their use must be carefully considered due to the potential for violent reactions.
Waste disposal of superacids requires specialized procedures. Neutralization should be performed under controlled conditions, followed by proper disposal according to local regulations. This often involves the use of licensed hazardous waste disposal services.
Regular safety audits and risk assessments should be conducted to ensure compliance with protocols and identify areas for improvement. This includes reviewing incident reports, near-misses, and updating procedures based on new research or regulatory requirements.
By implementing and strictly adhering to these comprehensive safety protocols, researchers can minimize the risks associated with handling superacids like fluoroantimonic acid, enabling the expansion of chemical research potential while maintaining a safe working environment.
Personal protective equipment (PPE) is paramount when working with superacids. This includes chemical-resistant suits, gloves, and boots made from materials such as fluorinated ethylene propylene (FEP) or polytetrafluoroethylene (PTFE). Full-face respirators with appropriate acid gas cartridges or supplied air systems are essential to protect against harmful vapors. Eye protection in the form of chemical splash goggles or a face shield is also crucial.
Workspace design plays a critical role in safety. All handling of superacids must be conducted in a properly functioning fume hood with a face velocity of at least 100 feet per minute. The work area should be equipped with emergency eyewash stations and safety showers within easy reach. Spill containment measures, including acid-resistant trays and absorbent materials, should be readily available.
Storage of superacids requires specialized containers made from materials resistant to their corrosive nature, such as PTFE or FEP. These containers must be kept in a cool, dry, well-ventilated area, away from incompatible substances and sources of heat or ignition. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
Training is a crucial component of safety protocols. All personnel working with superacids must undergo comprehensive training on proper handling techniques, emergency procedures, and the use of safety equipment. This training should be regularly updated and reinforced through practical exercises and simulations.
Emergency response plans must be in place and well-communicated. These plans should outline procedures for dealing with spills, personal exposure, and other potential incidents. Neutralization agents, such as sodium bicarbonate or calcium oxide, should be readily available for spill control, but their use must be carefully considered due to the potential for violent reactions.
Waste disposal of superacids requires specialized procedures. Neutralization should be performed under controlled conditions, followed by proper disposal according to local regulations. This often involves the use of licensed hazardous waste disposal services.
Regular safety audits and risk assessments should be conducted to ensure compliance with protocols and identify areas for improvement. This includes reviewing incident reports, near-misses, and updating procedures based on new research or regulatory requirements.
By implementing and strictly adhering to these comprehensive safety protocols, researchers can minimize the risks associated with handling superacids like fluoroantimonic acid, enabling the expansion of chemical research potential while maintaining a safe working environment.
Environmental Impact of Fluoroantimonic Acid Production
The production of fluoroantimonic acid, one of the strongest known superacids, poses significant environmental challenges that require careful consideration and management. The primary environmental concerns stem from the raw materials used in its production, the manufacturing process itself, and the potential for accidental release.
Fluoroantimonic acid is typically synthesized by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5). Both of these precursors are highly toxic and corrosive substances. The production and handling of HF, in particular, can lead to air and water pollution if not properly contained. HF emissions can contribute to acid rain and have detrimental effects on vegetation and aquatic ecosystems.
The manufacturing process of fluoroantimonic acid requires specialized equipment and stringent safety measures due to its extreme reactivity. Any leaks or spills during production can result in severe environmental contamination. The acid's ability to react violently with water and many other substances means that even small releases can have far-reaching consequences on soil and water quality.
Waste management is another critical environmental concern. The byproducts and residues from fluoroantimonic acid production must be carefully treated and disposed of to prevent environmental contamination. Improper disposal can lead to long-term soil and groundwater pollution, potentially affecting ecosystems and human health.
The transportation of raw materials and the finished product also presents environmental risks. Accidents during transport could result in widespread environmental damage, particularly if the acid comes into contact with water bodies or sensitive ecosystems. This necessitates robust containment systems and emergency response protocols.
Long-term environmental impacts of fluoroantimonic acid production include the potential accumulation of fluoride and antimony compounds in the environment. These elements can persist in soil and water, potentially entering the food chain and affecting wildlife and human health over time. Bioaccumulation of these substances in aquatic organisms is a particular concern, as it can lead to ecosystem disruption and biodiversity loss.
To mitigate these environmental risks, stringent regulations and best practices must be implemented throughout the production lifecycle. This includes using closed-loop systems to minimize emissions, implementing advanced waste treatment technologies, and developing safer handling and transportation methods. Continuous environmental monitoring around production facilities is essential to detect and address any potential contamination quickly.
Research into greener production methods and alternative superacids with lower environmental impact is ongoing. These efforts aim to reduce the reliance on fluoroantimonic acid or develop more environmentally friendly synthesis routes. However, given the unique properties of fluoroantimonic acid, finding suitable alternatives that match its performance remains a significant challenge for the chemical industry.
Fluoroantimonic acid is typically synthesized by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5). Both of these precursors are highly toxic and corrosive substances. The production and handling of HF, in particular, can lead to air and water pollution if not properly contained. HF emissions can contribute to acid rain and have detrimental effects on vegetation and aquatic ecosystems.
The manufacturing process of fluoroantimonic acid requires specialized equipment and stringent safety measures due to its extreme reactivity. Any leaks or spills during production can result in severe environmental contamination. The acid's ability to react violently with water and many other substances means that even small releases can have far-reaching consequences on soil and water quality.
Waste management is another critical environmental concern. The byproducts and residues from fluoroantimonic acid production must be carefully treated and disposed of to prevent environmental contamination. Improper disposal can lead to long-term soil and groundwater pollution, potentially affecting ecosystems and human health.
The transportation of raw materials and the finished product also presents environmental risks. Accidents during transport could result in widespread environmental damage, particularly if the acid comes into contact with water bodies or sensitive ecosystems. This necessitates robust containment systems and emergency response protocols.
Long-term environmental impacts of fluoroantimonic acid production include the potential accumulation of fluoride and antimony compounds in the environment. These elements can persist in soil and water, potentially entering the food chain and affecting wildlife and human health over time. Bioaccumulation of these substances in aquatic organisms is a particular concern, as it can lead to ecosystem disruption and biodiversity loss.
To mitigate these environmental risks, stringent regulations and best practices must be implemented throughout the production lifecycle. This includes using closed-loop systems to minimize emissions, implementing advanced waste treatment technologies, and developing safer handling and transportation methods. Continuous environmental monitoring around production facilities is essential to detect and address any potential contamination quickly.
Research into greener production methods and alternative superacids with lower environmental impact is ongoing. These efforts aim to reduce the reliance on fluoroantimonic acid or develop more environmentally friendly synthesis routes. However, given the unique properties of fluoroantimonic acid, finding suitable alternatives that match its performance remains a significant challenge for the chemical industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!