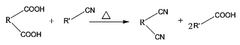How to Achieve Unparalleled Reactions with Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid: Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has garnered significant attention in the field of chemistry due to its exceptional acidity and reactivity. This compound, first synthesized in the 1960s, has been the subject of extensive research and development over the past several decades, driven by its potential applications in various industrial and scientific domains.
The evolution of fluoroantimonic acid technology has been marked by continuous efforts to harness its unique properties for practical use. Initially, the focus was primarily on understanding its fundamental chemical characteristics and behavior. As research progressed, scientists began exploring its potential as a catalyst in organic synthesis, petrochemical processing, and materials science.
The primary objective in the development of fluoroantimonic acid technology has been to achieve unparalleled reaction capabilities. This goal stems from the acid's extraordinary ability to protonate even extremely weak bases, making it a powerful tool for initiating and accelerating chemical reactions that are otherwise difficult or impossible to achieve with conventional acids.
One of the key technological trends in this field has been the optimization of fluoroantimonic acid synthesis and handling techniques. Given its extreme reactivity and corrosive nature, significant advancements have been made in developing specialized containment materials and safety protocols. These improvements have been crucial in enabling broader research and potential industrial applications.
Another important trend has been the exploration of fluoroantimonic acid's role in novel reaction pathways. Researchers have been investigating its use in the activation of typically unreactive compounds, such as alkanes, opening up new possibilities in hydrocarbon processing and fine chemical synthesis.
The technological goals associated with fluoroantimonic acid extend beyond merely achieving powerful reactions. Scientists and engineers are also focused on developing methods to control and fine-tune its reactivity, aiming to create more selective and efficient chemical processes. This includes research into modulating the acid's strength through careful adjustment of the HF:SbF5 ratio and exploring the use of supporting materials or co-catalysts.
Furthermore, there is a growing interest in understanding the fundamental mechanisms of fluoroantimonic acid's extreme acidity at the molecular level. Advanced spectroscopic and computational studies are being employed to elucidate the nature of proton transfer and the behavior of solvated protons in superacidic media. These insights are expected to guide future innovations in superacid chemistry and catalysis.
The evolution of fluoroantimonic acid technology has been marked by continuous efforts to harness its unique properties for practical use. Initially, the focus was primarily on understanding its fundamental chemical characteristics and behavior. As research progressed, scientists began exploring its potential as a catalyst in organic synthesis, petrochemical processing, and materials science.
The primary objective in the development of fluoroantimonic acid technology has been to achieve unparalleled reaction capabilities. This goal stems from the acid's extraordinary ability to protonate even extremely weak bases, making it a powerful tool for initiating and accelerating chemical reactions that are otherwise difficult or impossible to achieve with conventional acids.
One of the key technological trends in this field has been the optimization of fluoroantimonic acid synthesis and handling techniques. Given its extreme reactivity and corrosive nature, significant advancements have been made in developing specialized containment materials and safety protocols. These improvements have been crucial in enabling broader research and potential industrial applications.
Another important trend has been the exploration of fluoroantimonic acid's role in novel reaction pathways. Researchers have been investigating its use in the activation of typically unreactive compounds, such as alkanes, opening up new possibilities in hydrocarbon processing and fine chemical synthesis.
The technological goals associated with fluoroantimonic acid extend beyond merely achieving powerful reactions. Scientists and engineers are also focused on developing methods to control and fine-tune its reactivity, aiming to create more selective and efficient chemical processes. This includes research into modulating the acid's strength through careful adjustment of the HF:SbF5 ratio and exploring the use of supporting materials or co-catalysts.
Furthermore, there is a growing interest in understanding the fundamental mechanisms of fluoroantimonic acid's extreme acidity at the molecular level. Advanced spectroscopic and computational studies are being employed to elucidate the nature of proton transfer and the behavior of solvated protons in superacidic media. These insights are expected to guide future innovations in superacid chemistry and catalysis.
Market Analysis for Superacid Applications
The market for superacid applications, particularly those involving fluoroantimonic acid, has shown significant growth potential in recent years. This powerful superacid, known for its unparalleled reactivity, has found increasing use across various industrial sectors, driving demand and market expansion.
In the petrochemical industry, fluoroantimonic acid has become a crucial catalyst for alkylation processes, enhancing the production of high-octane gasoline components. This application has seen steady growth due to the rising global demand for cleaner-burning, high-performance fuels. The automotive sector's shift towards more efficient engines has further bolstered this trend.
The electronics industry represents another key market for fluoroantimonic acid applications. Its use in the etching of silicon wafers and the production of advanced semiconductors has become increasingly important as the demand for smaller, more powerful electronic devices continues to surge. The ongoing development of 5G technology and the Internet of Things (IoT) is expected to further drive this market segment.
In materials science, fluoroantimonic acid's ability to catalyze unique chemical reactions has opened new avenues for the development of advanced materials. This includes the synthesis of novel polymers and the modification of existing materials to enhance their properties. The growing focus on sustainable and high-performance materials in industries such as aerospace, automotive, and consumer goods is likely to fuel demand in this sector.
The pharmaceutical industry has also shown interest in fluoroantimonic acid for its potential in facilitating complex organic syntheses. While its use is currently limited due to handling challenges, ongoing research into safer application methods could unlock significant market potential in drug discovery and development processes.
Despite its promising applications, the market for fluoroantimonic acid faces challenges related to safety concerns and environmental regulations. The extreme corrosiveness and reactivity of the acid necessitate specialized handling and storage facilities, which can be a barrier to widespread adoption. However, innovations in containment technologies and safer handling protocols are gradually addressing these concerns, potentially expanding the market.
The global market for superacids, including fluoroantimonic acid, is geographically diverse, with major demand centers in North America, Europe, and Asia-Pacific. The United States and China, in particular, are key markets due to their large petrochemical and electronics industries. Emerging economies in Southeast Asia and India are also showing increased interest, driven by their growing industrial bases and investments in advanced technologies.
In the petrochemical industry, fluoroantimonic acid has become a crucial catalyst for alkylation processes, enhancing the production of high-octane gasoline components. This application has seen steady growth due to the rising global demand for cleaner-burning, high-performance fuels. The automotive sector's shift towards more efficient engines has further bolstered this trend.
The electronics industry represents another key market for fluoroantimonic acid applications. Its use in the etching of silicon wafers and the production of advanced semiconductors has become increasingly important as the demand for smaller, more powerful electronic devices continues to surge. The ongoing development of 5G technology and the Internet of Things (IoT) is expected to further drive this market segment.
In materials science, fluoroantimonic acid's ability to catalyze unique chemical reactions has opened new avenues for the development of advanced materials. This includes the synthesis of novel polymers and the modification of existing materials to enhance their properties. The growing focus on sustainable and high-performance materials in industries such as aerospace, automotive, and consumer goods is likely to fuel demand in this sector.
The pharmaceutical industry has also shown interest in fluoroantimonic acid for its potential in facilitating complex organic syntheses. While its use is currently limited due to handling challenges, ongoing research into safer application methods could unlock significant market potential in drug discovery and development processes.
Despite its promising applications, the market for fluoroantimonic acid faces challenges related to safety concerns and environmental regulations. The extreme corrosiveness and reactivity of the acid necessitate specialized handling and storage facilities, which can be a barrier to widespread adoption. However, innovations in containment technologies and safer handling protocols are gradually addressing these concerns, potentially expanding the market.
The global market for superacids, including fluoroantimonic acid, is geographically diverse, with major demand centers in North America, Europe, and Asia-Pacific. The United States and China, in particular, are key markets due to their large petrochemical and electronics industries. Emerging economies in Southeast Asia and India are also showing increased interest, driven by their growing industrial bases and investments in advanced technologies.
Current Challenges in Fluoroantimonic Acid Synthesis
Fluoroantimonic acid, known as the world's strongest superacid, presents significant challenges in its synthesis and handling. The primary obstacle lies in its extreme reactivity and corrosiveness, which necessitates specialized equipment and stringent safety protocols. Conventional glassware and most metals are rapidly degraded by this powerful acid, limiting the materials that can be used in its production and containment.
The synthesis process itself is complex and hazardous, involving the combination of hydrogen fluoride and antimony pentafluoride under strictly controlled conditions. Maintaining the precise stoichiometric ratio of these components is crucial for achieving the desired acidity and stability. Any deviation can lead to unpredictable reactions or reduced effectiveness of the final product.
Another major challenge is the extreme moisture sensitivity of fluoroantimonic acid. Even trace amounts of water can cause violent reactions and compromise the acid's strength. This necessitates the use of rigorously anhydrous conditions throughout the synthesis and storage processes, which is technically demanding and resource-intensive.
The volatility of the acid components, particularly hydrogen fluoride, poses significant safety risks and environmental concerns. Stringent containment measures are required to prevent the release of toxic fumes, which can cause severe health hazards and environmental damage. This aspect significantly complicates large-scale production and transportation of fluoroantimonic acid.
Furthermore, the characterization and analysis of fluoroantimonic acid present unique challenges due to its extreme reactivity. Traditional analytical techniques often fail or provide unreliable results when applied to this superacid. Developing accurate and safe methods for assessing the purity and strength of fluoroantimonic acid remains an ongoing challenge in the field.
The disposal of fluoroantimonic acid and its byproducts is another critical issue. The acid's extreme corrosiveness and environmental impact necessitate specialized disposal procedures, which are often costly and complex. Developing more efficient and environmentally friendly methods for neutralizing and disposing of this superacid is an area of active research.
Lastly, the scaling up of fluoroantimonic acid production from laboratory to industrial levels presents significant engineering challenges. Designing reactors and processing equipment that can withstand the acid's corrosive nature while maintaining safety and efficiency is a complex task that requires innovative materials science and engineering solutions.
The synthesis process itself is complex and hazardous, involving the combination of hydrogen fluoride and antimony pentafluoride under strictly controlled conditions. Maintaining the precise stoichiometric ratio of these components is crucial for achieving the desired acidity and stability. Any deviation can lead to unpredictable reactions or reduced effectiveness of the final product.
Another major challenge is the extreme moisture sensitivity of fluoroantimonic acid. Even trace amounts of water can cause violent reactions and compromise the acid's strength. This necessitates the use of rigorously anhydrous conditions throughout the synthesis and storage processes, which is technically demanding and resource-intensive.
The volatility of the acid components, particularly hydrogen fluoride, poses significant safety risks and environmental concerns. Stringent containment measures are required to prevent the release of toxic fumes, which can cause severe health hazards and environmental damage. This aspect significantly complicates large-scale production and transportation of fluoroantimonic acid.
Furthermore, the characterization and analysis of fluoroantimonic acid present unique challenges due to its extreme reactivity. Traditional analytical techniques often fail or provide unreliable results when applied to this superacid. Developing accurate and safe methods for assessing the purity and strength of fluoroantimonic acid remains an ongoing challenge in the field.
The disposal of fluoroantimonic acid and its byproducts is another critical issue. The acid's extreme corrosiveness and environmental impact necessitate specialized disposal procedures, which are often costly and complex. Developing more efficient and environmentally friendly methods for neutralizing and disposing of this superacid is an area of active research.
Lastly, the scaling up of fluoroantimonic acid production from laboratory to industrial levels presents significant engineering challenges. Designing reactors and processing equipment that can withstand the acid's corrosive nature while maintaining safety and efficiency is a complex task that requires innovative materials science and engineering solutions.
Existing Methodologies for Fluoroantimonic Acid Reactions
01 Fluoroantimonic acid as a superacid catalyst
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. Its extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids. This includes isomerization, alkylation, and polymerization reactions, particularly in the petrochemical industry.- Fluoroantimonic acid as a superacid catalyst: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. Its extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids. This includes isomerization, alkylation, and polymerization reactions, particularly in the petrochemical industry.
- Analytical applications of fluoroantimonic acid: Fluoroantimonic acid finds use in analytical chemistry for sample preparation and analysis. It can be employed in the digestion of complex matrices, extraction of certain analytes, or as a component in specialized analytical techniques. Its strong acidic properties make it valuable for breaking down resistant materials for analysis.
- Fluoroantimonic acid in materials processing: The extreme reactivity of fluoroantimonic acid is harnessed in materials processing applications. It can be used for etching or surface modification of certain materials, particularly in the semiconductor industry. The acid's ability to react with a wide range of substances makes it useful in specialized material treatments.
- Safety and handling considerations: Due to its extremely corrosive and reactive nature, special safety measures and handling procedures are required when working with fluoroantimonic acid. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage, transport, and disposal to prevent accidents and environmental contamination.
- Synthesis and characterization of fluoroantimonic acid: Methods for synthesizing fluoroantimonic acid and characterizing its properties are important areas of research. This includes developing new preparation techniques, studying its molecular structure and behavior, and investigating its interactions with various substances. Understanding these aspects is crucial for expanding its applications and improving safety in its use.
02 Analytical applications of fluoroantimonic acid
Fluoroantimonic acid finds use in analytical chemistry for sample preparation and analysis. It can be employed in the digestion of complex matrices, extraction of certain compounds, and as a reagent in specialized analytical techniques. Its strong protonating ability makes it valuable for analyzing resistant materials.Expand Specific Solutions03 Synthesis and handling of fluoroantimonic acid
The synthesis and handling of fluoroantimonic acid require specialized equipment and techniques due to its extreme reactivity and corrosiveness. Methods for its preparation, storage, and safe handling are crucial for its application in research and industry. This includes the use of fluorine-resistant materials and strict safety protocols.Expand Specific Solutions04 Reactions with organic compounds
Fluoroantimonic acid exhibits unique reactivity with organic compounds. It can facilitate carbocation formation, leading to rearrangements and fragmentations not observed with weaker acids. This property is exploited in organic synthesis for creating novel compounds and in studying reaction mechanisms.Expand Specific Solutions05 Industrial applications and process improvements
The use of fluoroantimonic acid in industrial processes has led to improvements in efficiency and yield. It is particularly valuable in the production of high-octane gasoline components, specialty polymers, and fine chemicals. Research continues to explore new applications and optimize existing processes involving this superacid.Expand Specific Solutions
Key Players in Superacid Research and Production
The competitive landscape for achieving unparalleled reactions with fluoroantimonic acid is characterized by a mature market with established players and ongoing research. The industry is in a consolidation phase, with major chemical companies like DuPont de Nemours, Inc. and Bayer AG leading commercial applications. Academic institutions such as Yale University, Kyoto University, and Oxford University Innovation Ltd. are driving fundamental research. The market size is relatively niche but growing, driven by applications in petrochemicals and materials science. Technologically, the field is moderately mature, with companies like Mitsubishi Materials Corp. and Nissan Chemical Corp. focusing on improving synthesis methods and exploring new applications. Collaboration between industry and academia, exemplified by partnerships involving institutions like the University of California, is accelerating innovation in this specialized field.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN has leveraged its expertise in fluorine chemistry to develop a novel method for the controlled generation and use of fluoroantimonic acid in situ. Their approach involves the real-time production of the superacid through a patented gas-phase reaction system, eliminating the need for storage and transportation of large quantities of the hazardous substance. DAIKIN's technology incorporates advanced sensors and control systems to maintain precise reaction conditions, ensuring optimal acid strength and reactivity. This in situ generation method has been successfully applied in the production of high-purity fluorinated compounds used in various industries, including pharmaceuticals and advanced materials.
Strengths: In situ acid generation reduces handling risks, expertise in fluorine chemistry, and broad applicability across industries. Weaknesses: Complex system requirements and potential limitations in acid concentration achievable through gas-phase reactions.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for the synthesis and handling of fluoroantimonic acid, utilizing advanced containment systems and specialized materials. Their method involves the controlled reaction of hydrogen fluoride with antimony pentafluoride under precise temperature and pressure conditions. DuPont's approach incorporates a unique stabilization technique that allows for safer storage and transportation of this superacid. They have also engineered custom reaction vessels lined with fluoropolymers to withstand the extreme corrosiveness of fluoroantimonic acid, enabling its use in various industrial applications.
Strengths: Extensive experience in handling hazardous materials, advanced containment technology, and proprietary stabilization methods. Weaknesses: High production costs and limited scalability due to safety concerns.
Innovative Approaches in Fluoroantimonic Acid Chemistry
Method for preparing phthalonitrile-based compound
PatentWO2023003058A1
Innovation
- A method involving the direct reaction of a phthalic acid-based compound with a nitrile-based compound under supercritical conditions, eliminating the need for ammonia and catalysts, and allowing for the reuse of remaining compounds, thereby producing high-purity phthalonitrile-based compounds in an environmentally friendly manner.
Composition and method for treating a subterranean formation
PatentInactiveUS6806236B2
Innovation
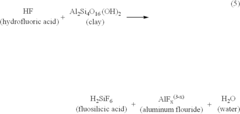
- The development of acidic compositions comprising fluoboric acid, boron sources, and chelating agents like citric acid, which slowly generate hydrofluoric acid in situ, effectively increasing permeability by stabilizing clays and preventing precipitation of aluminum fluorides and hydrated silica, thereby extending the treatment's effectiveness deeper into the formation.
Safety and Handling Protocols for Fluoroantimonic Acid
Fluoroantimonic acid is one of the strongest known superacids, with a Hammett acidity function estimated at -31.3. Its extreme reactivity and corrosiveness necessitate stringent safety measures and handling protocols. Proper training and specialized equipment are essential for working with this highly dangerous substance.
Personal protective equipment (PPE) is crucial when handling fluoroantimonic acid. This includes a fully encapsulating chemical suit, multiple layers of chemical-resistant gloves, and a self-contained breathing apparatus. All PPE must be thoroughly inspected before use and replaced immediately if any signs of degradation are observed.
Storage of fluoroantimonic acid requires specially designed containers made of materials resistant to its corrosive properties, such as PTFE (Teflon) or certain fluoropolymers. These containers must be kept in a cool, dry, well-ventilated area, away from any potential reactants or incompatible materials. Regular inspections of storage facilities are necessary to ensure integrity and prevent leaks.
Handling procedures must be conducted in a designated area with proper ventilation systems, including fume hoods equipped with acid-resistant liners. All work surfaces should be covered with PTFE-coated materials to prevent accidental spills from causing immediate damage. Transfer of the acid should only be performed using specialized pumps and tubing systems designed to withstand its corrosive nature.
Emergency response protocols must be established and regularly practiced. This includes having readily accessible eyewash stations, safety showers, and spill containment kits specifically designed for superacids. A detailed evacuation plan should be in place, and all personnel must be trained in its execution.
Waste disposal of fluoroantimonic acid and related materials requires specialized procedures. Neutralization should only be attempted by trained professionals using appropriate dilution techniques and neutralizing agents. All waste must be collected in designated containers and disposed of through certified hazardous waste management services.
Regular safety audits and equipment checks are essential to maintain a safe working environment. This includes periodic testing of all safety equipment, verification of containment systems, and updates to safety protocols based on the latest research and best practices in handling superacids.
Documentation and record-keeping are critical aspects of safety management when working with fluoroantimonic acid. Detailed logs of all handling procedures, including quantities used, exposure times, and any incidents or near-misses, must be maintained. This information is vital for continuous improvement of safety protocols and for compliance with regulatory requirements.
Personal protective equipment (PPE) is crucial when handling fluoroantimonic acid. This includes a fully encapsulating chemical suit, multiple layers of chemical-resistant gloves, and a self-contained breathing apparatus. All PPE must be thoroughly inspected before use and replaced immediately if any signs of degradation are observed.
Storage of fluoroantimonic acid requires specially designed containers made of materials resistant to its corrosive properties, such as PTFE (Teflon) or certain fluoropolymers. These containers must be kept in a cool, dry, well-ventilated area, away from any potential reactants or incompatible materials. Regular inspections of storage facilities are necessary to ensure integrity and prevent leaks.
Handling procedures must be conducted in a designated area with proper ventilation systems, including fume hoods equipped with acid-resistant liners. All work surfaces should be covered with PTFE-coated materials to prevent accidental spills from causing immediate damage. Transfer of the acid should only be performed using specialized pumps and tubing systems designed to withstand its corrosive nature.
Emergency response protocols must be established and regularly practiced. This includes having readily accessible eyewash stations, safety showers, and spill containment kits specifically designed for superacids. A detailed evacuation plan should be in place, and all personnel must be trained in its execution.
Waste disposal of fluoroantimonic acid and related materials requires specialized procedures. Neutralization should only be attempted by trained professionals using appropriate dilution techniques and neutralizing agents. All waste must be collected in designated containers and disposed of through certified hazardous waste management services.
Regular safety audits and equipment checks are essential to maintain a safe working environment. This includes periodic testing of all safety equipment, verification of containment systems, and updates to safety protocols based on the latest research and best practices in handling superacids.
Documentation and record-keeping are critical aspects of safety management when working with fluoroantimonic acid. Detailed logs of all handling procedures, including quantities used, exposure times, and any incidents or near-misses, must be maintained. This information is vital for continuous improvement of safety protocols and for compliance with regulatory requirements.
Environmental Impact and Sustainability Considerations
The use of fluoroantimonic acid in achieving unparalleled reactions raises significant environmental and sustainability concerns that must be carefully considered. This superacid, known for its extreme corrosiveness and reactivity, poses substantial risks to ecosystems and human health if not handled and disposed of properly.
One of the primary environmental concerns is the potential for soil and water contamination. Fluoroantimonic acid can rapidly degrade organic matter and react violently with water, potentially leading to the release of toxic fluoride and antimony compounds. These contaminants can persist in the environment, bioaccumulate in food chains, and cause long-term ecological damage. Aquatic ecosystems are particularly vulnerable, as even small amounts of the acid can drastically alter pH levels and devastate marine life.
Air pollution is another critical issue associated with fluoroantimonic acid usage. The acid can generate hazardous fumes, including hydrogen fluoride and antimony pentafluoride, which are known respiratory irritants and can contribute to the formation of acid rain. Proper ventilation and air filtration systems are essential to mitigate these risks, but the potential for accidental releases remains a concern.
From a sustainability perspective, the production and use of fluoroantimonic acid present challenges in terms of resource consumption and waste management. The synthesis of this superacid requires energy-intensive processes and relies on finite resources, particularly fluorine and antimony. Developing more sustainable production methods and exploring alternative catalysts or reaction pathways could help address these issues.
Waste management is a critical aspect of environmental stewardship when working with fluoroantimonic acid. The acid and its byproducts require specialized handling and disposal procedures to prevent environmental contamination. Developing effective neutralization and treatment protocols is essential, as is implementing closed-loop systems to minimize waste generation and maximize resource recovery.
To address these environmental and sustainability concerns, research efforts should focus on developing greener alternatives that can achieve similar reactivity with reduced environmental impact. This may involve exploring bio-inspired catalysts, ionic liquids, or other novel reaction media that offer comparable performance while minimizing ecological risks.
Additionally, implementing rigorous safety protocols, containment measures, and monitoring systems is crucial to prevent accidental releases and ensure responsible use of fluoroantimonic acid in research and industrial settings. Continuous improvement in handling techniques and equipment design can help mitigate environmental risks and enhance overall sustainability.
One of the primary environmental concerns is the potential for soil and water contamination. Fluoroantimonic acid can rapidly degrade organic matter and react violently with water, potentially leading to the release of toxic fluoride and antimony compounds. These contaminants can persist in the environment, bioaccumulate in food chains, and cause long-term ecological damage. Aquatic ecosystems are particularly vulnerable, as even small amounts of the acid can drastically alter pH levels and devastate marine life.
Air pollution is another critical issue associated with fluoroantimonic acid usage. The acid can generate hazardous fumes, including hydrogen fluoride and antimony pentafluoride, which are known respiratory irritants and can contribute to the formation of acid rain. Proper ventilation and air filtration systems are essential to mitigate these risks, but the potential for accidental releases remains a concern.
From a sustainability perspective, the production and use of fluoroantimonic acid present challenges in terms of resource consumption and waste management. The synthesis of this superacid requires energy-intensive processes and relies on finite resources, particularly fluorine and antimony. Developing more sustainable production methods and exploring alternative catalysts or reaction pathways could help address these issues.
Waste management is a critical aspect of environmental stewardship when working with fluoroantimonic acid. The acid and its byproducts require specialized handling and disposal procedures to prevent environmental contamination. Developing effective neutralization and treatment protocols is essential, as is implementing closed-loop systems to minimize waste generation and maximize resource recovery.
To address these environmental and sustainability concerns, research efforts should focus on developing greener alternatives that can achieve similar reactivity with reduced environmental impact. This may involve exploring bio-inspired catalysts, ionic liquids, or other novel reaction media that offer comparable performance while minimizing ecological risks.
Additionally, implementing rigorous safety protocols, containment measures, and monitoring systems is crucial to prevent accidental releases and ensure responsible use of fluoroantimonic acid in research and industrial settings. Continuous improvement in handling techniques and equipment design can help mitigate environmental risks and enhance overall sustainability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!