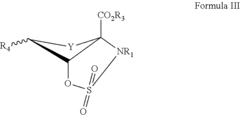
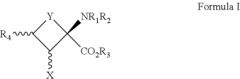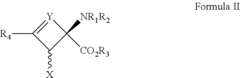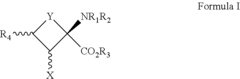How to Target Specific Chemical Reactions with Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has garnered significant attention in the field of chemistry due to its exceptional acidity and unique properties. This powerful compound has been the subject of extensive research and development efforts since its discovery in the mid-20th century.
The evolution of fluoroantimonic acid technology can be traced back to the pioneering work of George A. Olah in the 1960s, who explored the potential of superacids in organic chemistry. Olah's groundbreaking research laid the foundation for understanding the behavior and applications of these extremely acidic substances, including fluoroantimonic acid.
As the strongest known superacid, fluoroantimonic acid has a Hammett acidity function (H0) value of -31.3, making it approximately 20 quintillion times stronger than 100% sulfuric acid. This extraordinary acidity is attributed to the formation of highly electrophilic protons in solution, which can protonate even extremely weak bases.
The primary objective of research into fluoroantimonic acid has been to harness its unique properties for targeted chemical reactions. Scientists and industry professionals have sought to exploit its exceptional protonating ability to facilitate various transformations that are challenging or impossible with conventional acids.
One of the key goals in fluoroantimonic acid research is to develop methods for controlling and directing its reactivity towards specific molecular targets. This involves understanding the mechanisms of proton transfer, the behavior of reactive intermediates, and the factors influencing selectivity in complex chemical systems.
Another important objective is to explore the potential applications of fluoroantimonic acid in industrial processes, particularly in the petrochemical and pharmaceutical sectors. Researchers aim to leverage its strong acidic properties to catalyze reactions, isomerize hydrocarbons, and synthesize novel compounds with improved efficiency and selectivity.
Safety and handling considerations have also been a crucial aspect of fluoroantimonic acid research. Due to its extreme corrosiveness and reactivity, developing protocols for safe storage, transportation, and utilization of this superacid has been a priority for both academic and industrial researchers.
As technology advances, there is a growing interest in combining fluoroantimonic acid with other cutting-edge techniques, such as flow chemistry and microreactor technology. These approaches aim to enhance control over reaction conditions, improve safety, and enable more precise targeting of specific chemical transformations.
The evolution of fluoroantimonic acid technology can be traced back to the pioneering work of George A. Olah in the 1960s, who explored the potential of superacids in organic chemistry. Olah's groundbreaking research laid the foundation for understanding the behavior and applications of these extremely acidic substances, including fluoroantimonic acid.
As the strongest known superacid, fluoroantimonic acid has a Hammett acidity function (H0) value of -31.3, making it approximately 20 quintillion times stronger than 100% sulfuric acid. This extraordinary acidity is attributed to the formation of highly electrophilic protons in solution, which can protonate even extremely weak bases.
The primary objective of research into fluoroantimonic acid has been to harness its unique properties for targeted chemical reactions. Scientists and industry professionals have sought to exploit its exceptional protonating ability to facilitate various transformations that are challenging or impossible with conventional acids.
One of the key goals in fluoroantimonic acid research is to develop methods for controlling and directing its reactivity towards specific molecular targets. This involves understanding the mechanisms of proton transfer, the behavior of reactive intermediates, and the factors influencing selectivity in complex chemical systems.
Another important objective is to explore the potential applications of fluoroantimonic acid in industrial processes, particularly in the petrochemical and pharmaceutical sectors. Researchers aim to leverage its strong acidic properties to catalyze reactions, isomerize hydrocarbons, and synthesize novel compounds with improved efficiency and selectivity.
Safety and handling considerations have also been a crucial aspect of fluoroantimonic acid research. Due to its extreme corrosiveness and reactivity, developing protocols for safe storage, transportation, and utilization of this superacid has been a priority for both academic and industrial researchers.
As technology advances, there is a growing interest in combining fluoroantimonic acid with other cutting-edge techniques, such as flow chemistry and microreactor technology. These approaches aim to enhance control over reaction conditions, improve safety, and enable more precise targeting of specific chemical transformations.
Industrial Applications and Market Analysis
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in various industrial sectors due to its exceptional proton-donating ability and catalytic properties. The market for fluoroantimonic acid and its applications is primarily driven by the petrochemical industry, where it plays a crucial role in hydrocarbon cracking and isomerization processes.
In the oil and gas sector, fluoroantimonic acid is utilized for enhancing the octane rating of gasoline through alkylation reactions. This application has seen steady growth due to the increasing demand for high-performance fuels in automotive and aviation industries. The ability to target specific chemical reactions with fluoroantimonic acid has led to improved efficiency in refining processes, resulting in higher-quality fuel products and reduced production costs.
The pharmaceutical industry has also shown interest in fluoroantimonic acid for its potential in synthesizing complex organic compounds. Its strong acidic properties enable the activation of certain molecular structures, facilitating the creation of novel drug candidates. This application is expected to expand as drug discovery processes become more sophisticated and demand for targeted therapies increases.
In the field of materials science, fluoroantimonic acid has found applications in the production of advanced polymers and specialty chemicals. Its ability to catalyze specific reactions has led to the development of high-performance materials with unique properties, such as enhanced thermal stability and chemical resistance. This has opened up new opportunities in industries like aerospace, electronics, and automotive manufacturing.
The global market for fluoroantimonic acid and related superacid technologies is projected to grow steadily over the next decade. Key factors driving this growth include increasing investments in research and development, expanding applications in emerging industries, and the ongoing pursuit of more efficient and sustainable chemical processes.
However, the market faces challenges related to the handling and storage of fluoroantimonic acid due to its extreme corrosiveness and reactivity. This has led to the development of specialized equipment and safety protocols, which can increase operational costs for end-users. Additionally, environmental concerns and stringent regulations regarding the use and disposal of highly acidic substances may impact market growth in certain regions.
Despite these challenges, the unique capabilities of fluoroantimonic acid in targeting specific chemical reactions continue to drive innovation across multiple industries. As research progresses, new applications are likely to emerge, particularly in areas such as nanotechnology, energy storage, and advanced materials processing. The market is expected to evolve with a focus on developing safer handling methods and exploring more environmentally friendly alternatives that can offer similar catalytic properties.
In the oil and gas sector, fluoroantimonic acid is utilized for enhancing the octane rating of gasoline through alkylation reactions. This application has seen steady growth due to the increasing demand for high-performance fuels in automotive and aviation industries. The ability to target specific chemical reactions with fluoroantimonic acid has led to improved efficiency in refining processes, resulting in higher-quality fuel products and reduced production costs.
The pharmaceutical industry has also shown interest in fluoroantimonic acid for its potential in synthesizing complex organic compounds. Its strong acidic properties enable the activation of certain molecular structures, facilitating the creation of novel drug candidates. This application is expected to expand as drug discovery processes become more sophisticated and demand for targeted therapies increases.
In the field of materials science, fluoroantimonic acid has found applications in the production of advanced polymers and specialty chemicals. Its ability to catalyze specific reactions has led to the development of high-performance materials with unique properties, such as enhanced thermal stability and chemical resistance. This has opened up new opportunities in industries like aerospace, electronics, and automotive manufacturing.
The global market for fluoroantimonic acid and related superacid technologies is projected to grow steadily over the next decade. Key factors driving this growth include increasing investments in research and development, expanding applications in emerging industries, and the ongoing pursuit of more efficient and sustainable chemical processes.
However, the market faces challenges related to the handling and storage of fluoroantimonic acid due to its extreme corrosiveness and reactivity. This has led to the development of specialized equipment and safety protocols, which can increase operational costs for end-users. Additionally, environmental concerns and stringent regulations regarding the use and disposal of highly acidic substances may impact market growth in certain regions.
Despite these challenges, the unique capabilities of fluoroantimonic acid in targeting specific chemical reactions continue to drive innovation across multiple industries. As research progresses, new applications are likely to emerge, particularly in areas such as nanotechnology, energy storage, and advanced materials processing. The market is expected to evolve with a focus on developing safer handling methods and exploring more environmentally friendly alternatives that can offer similar catalytic properties.
Current Challenges in Selective Chemical Targeting
The selective targeting of specific chemical reactions using fluoroantimonic acid presents several significant challenges in the field of chemical synthesis and catalysis. One of the primary obstacles is the extreme reactivity and corrosiveness of fluoroantimonic acid, which makes it difficult to control and handle safely in laboratory settings. This superacid's ability to protonate even very weak bases poses risks to both researchers and equipment, necessitating specialized containment and handling protocols.
Another major challenge lies in achieving precise selectivity when using such a powerful acid. Fluoroantimonic acid's exceptional strength can lead to unintended side reactions or over-activation of multiple functional groups within complex molecules. This lack of specificity can result in reduced yields of desired products and increased formation of unwanted by-products, complicating purification processes and diminishing overall reaction efficiency.
The stability of reaction intermediates and products in the presence of fluoroantimonic acid also presents a significant hurdle. Many organic compounds are susceptible to decomposition or structural rearrangement under strongly acidic conditions, potentially limiting the range of substrates and reaction types that can be effectively targeted using this superacid.
Furthermore, the compatibility of fluoroantimonic acid with various solvents and reaction media is limited, restricting the range of reaction conditions that can be employed. This constraint often necessitates the use of specialized, acid-resistant reaction vessels and equipment, which can increase the complexity and cost of experimental setups.
The environmental impact and disposal of fluoroantimonic acid and its reaction products pose additional challenges. The acid's extreme corrosiveness and potential for generating toxic by-products require careful consideration of waste management and neutralization procedures, adding layers of complexity to experimental design and execution.
Scaling up reactions involving fluoroantimonic acid from laboratory to industrial scale presents its own set of challenges. The need for specialized equipment, safety measures, and waste handling procedures can make large-scale applications economically unfeasible or technically impractical for many processes.
Lastly, the development of milder, more selective alternatives to fluoroantimonic acid remains an ongoing challenge in the field. Researchers are continually seeking ways to achieve similar levels of activation and reactivity using less hazardous and more easily handled reagents, driving innovation in catalysis and synthetic methodology.
Another major challenge lies in achieving precise selectivity when using such a powerful acid. Fluoroantimonic acid's exceptional strength can lead to unintended side reactions or over-activation of multiple functional groups within complex molecules. This lack of specificity can result in reduced yields of desired products and increased formation of unwanted by-products, complicating purification processes and diminishing overall reaction efficiency.
The stability of reaction intermediates and products in the presence of fluoroantimonic acid also presents a significant hurdle. Many organic compounds are susceptible to decomposition or structural rearrangement under strongly acidic conditions, potentially limiting the range of substrates and reaction types that can be effectively targeted using this superacid.
Furthermore, the compatibility of fluoroantimonic acid with various solvents and reaction media is limited, restricting the range of reaction conditions that can be employed. This constraint often necessitates the use of specialized, acid-resistant reaction vessels and equipment, which can increase the complexity and cost of experimental setups.
The environmental impact and disposal of fluoroantimonic acid and its reaction products pose additional challenges. The acid's extreme corrosiveness and potential for generating toxic by-products require careful consideration of waste management and neutralization procedures, adding layers of complexity to experimental design and execution.
Scaling up reactions involving fluoroantimonic acid from laboratory to industrial scale presents its own set of challenges. The need for specialized equipment, safety measures, and waste handling procedures can make large-scale applications economically unfeasible or technically impractical for many processes.
Lastly, the development of milder, more selective alternatives to fluoroantimonic acid remains an ongoing challenge in the field. Researchers are continually seeking ways to achieve similar levels of activation and reactivity using less hazardous and more easily handled reagents, driving innovation in catalysis and synthetic methodology.
Existing Methodologies for Reaction Selectivity
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in chemical reactions and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acid catalysts.
- Use in materials science and surface treatments: Fluoroantimonic acid finds applications in materials science, particularly in surface treatments and modifications. It can be used to etch or modify surfaces of various materials, including metals and semiconductors. The acid's unique properties allow for precise control of surface characteristics in advanced materials and electronic components.
- Safety and handling considerations: Due to its extreme reactivity and corrosiveness, fluoroantimonic acid requires specialized safety measures and handling procedures. This includes the use of specific containment materials, personal protective equipment, and controlled environments. Proper storage, transportation, and disposal methods are crucial to prevent accidents and environmental contamination.
- Analytical and research applications: Fluoroantimonic acid is employed in various analytical and research applications. Its unique properties make it valuable in spectroscopic studies, chemical analysis, and as a reference material for superacidity. It is also used in the development of new materials and in studying extreme acid-base interactions.
02 Applications in chemical reactions and catalysis
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids, making it valuable in organic synthesis and petrochemical industries.Expand Specific Solutions03 Material compatibility and storage
Due to its highly corrosive nature, fluoroantimonic acid requires specialized materials for handling and storage. Research has been conducted on developing resistant materials, such as certain fluoropolymers and specially treated metals, that can withstand the acid's corrosive properties. Proper storage and containment systems are crucial for safe handling and prevention of environmental contamination.Expand Specific Solutions04 Safety measures and environmental considerations
Handling fluoroantimonic acid requires stringent safety protocols due to its extreme reactivity and corrosiveness. Research has focused on developing improved safety measures, including specialized personal protective equipment, containment systems, and neutralization techniques. Environmental impact assessments and waste management strategies have also been studied to minimize potential hazards associated with its use and disposal.Expand Specific Solutions05 Analytical applications and detection methods
Fluoroantimonic acid has found use in analytical chemistry for specialized applications. Research has been conducted on developing methods for detecting and quantifying the acid, as well as its potential use in analytical techniques. These methods are important for quality control in production, environmental monitoring, and safety assessments in laboratories and industrial settings.Expand Specific Solutions
Key Players in Superacid Research and Industry
The development of targeted chemical reactions using fluoroantimonic acid is in its early stages, with a growing market driven by applications in pharmaceuticals and materials science. The technology's maturity varies across industries, with companies like DAIKIN INDUSTRIES Ltd. and Mitsubishi Gas Chemical Co., Inc. leading in industrial applications. Pharmaceutical giants such as F. Hoffmann-La Roche Ltd. and Regeneron Pharmaceuticals, Inc. are exploring its potential in drug development. Academic institutions like Emory University and Kyushu University are contributing to fundamental research. The competitive landscape is diverse, with specialized chemical companies like Central Glass Co., Ltd. and Lianhe Chemical Technology Co., Ltd. focusing on production and refinement of fluoroantimonic acid for various applications.
Mitsubishi Gas Chemical Co., Inc.
Technical Solution: Mitsubishi Gas Chemical Co., Inc. has pioneered a unique approach to targeting specific chemical reactions with fluoroantimonic acid. Their method involves the use of specially designed ionic liquid systems as reaction media. These ionic liquids act as stabilizers for the fluoroantimonic acid, allowing for more controlled and selective reactions. The company has also developed a range of novel catalysts that work synergistically with fluoroantimonic acid in these ionic liquid systems, enabling highly specific transformations that were previously challenging or impossible.
Strengths: Innovative use of ionic liquids, enhanced reaction selectivity, potential for new chemical transformations. Weaknesses: Complex reaction systems, potential high costs of ionic liquids and specialized catalysts.
Central Glass Co., Ltd.
Technical Solution: Central Glass Co., Ltd. has developed a proprietary process for the production and handling of fluoroantimonic acid, one of the strongest known superacids. Their method involves the controlled reaction of hydrogen fluoride with antimony pentafluoride under strictly anhydrous conditions. The company has engineered specialized corrosion-resistant reactors and storage vessels made from fluoropolymer materials to contain this highly reactive substance. They have also implemented advanced safety protocols and remote handling systems to mitigate the extreme hazards associated with fluoroantimonic acid.
Strengths: Expertise in superacid chemistry, advanced containment technology, and safety protocols. Weaknesses: High production costs, limited applications, and extreme safety requirements.
Innovations in Fluoroantimonic Acid Catalysis
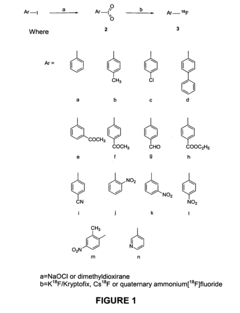
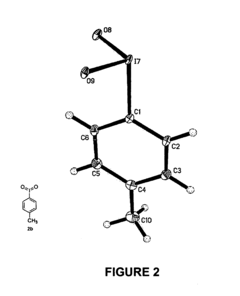
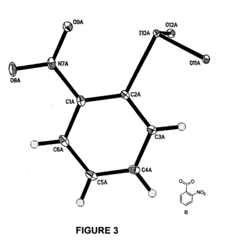
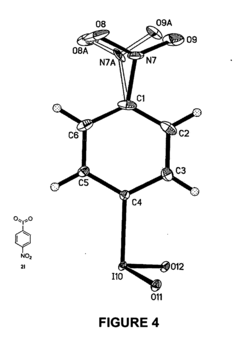
Nucleophilic fluorination of aromatic compounds
PatentInactiveUS20110178302A1
Innovation
- A novel aromatic nucleophilic radiohalogenation reaction is developed without the addition of a stable carrier ion, using no-carrier-added [F-18] fluoride ion produced by proton irradiation, which nucleophilically substitutes the iodyl group in iodylbenzene derivatives to produce regiospecific F-18 labeled compounds.
Imaging agents
PatentActiveUS20130225828A1
Innovation
- Development of novel amino acid compounds labeled with longer-lived isotopes like fluorine-18, iodine, and technetium, which exhibit high specificity and selectivity for tumor tissues, allowing for effective PET and SPECT imaging with improved logistical and economic benefits.
Safety and Handling Protocols
Fluoroantimonic acid is one of the strongest known superacids, capable of protonating even extremely weak bases. Its exceptional reactivity necessitates stringent safety measures and specialized handling protocols. When working with this highly corrosive substance, personnel must wear appropriate personal protective equipment (PPE), including chemical-resistant suits, gloves, and face shields. All operations involving fluoroantimonic acid should be conducted in a fume hood with proper ventilation to prevent exposure to toxic fumes.
The acid must be stored in containers made of materials resistant to its corrosive properties, such as polytetrafluoroethylene (PTFE) or fluorinated ethylene propylene (FEP). Glass and metal containers are unsuitable due to the acid's ability to dissolve these materials. Proper labeling and segregation from incompatible substances are crucial to prevent accidental mixing and potential violent reactions.
Emergency response procedures must be established and regularly reviewed. This includes having readily accessible eyewash stations, safety showers, and spill containment equipment. Personnel should be trained in proper spill cleanup techniques and the use of neutralizing agents, such as sodium bicarbonate or calcium carbonate, to mitigate the effects of accidental releases.
Due to its extreme reactivity, fluoroantimonic acid should only be handled by experienced chemists in well-equipped laboratories. The acid's preparation and use require specialized apparatus, often involving the combination of hydrogen fluoride and antimony pentafluoride under controlled conditions. Strict temperature control is essential, as the acid can become unstable at elevated temperatures.
Waste disposal protocols for fluoroantimonic acid and related materials must comply with local and national regulations for hazardous waste management. Neutralization and dilution procedures should be carefully followed before disposal, and certified waste management services should be employed for final disposal.
Regular safety audits and equipment inspections are necessary to ensure the integrity of containment systems and the effectiveness of safety measures. Detailed records of acid usage, storage, and disposal should be maintained for regulatory compliance and internal safety reviews.
Lastly, a comprehensive emergency response plan should be in place, including procedures for evacuation, containment of large spills, and coordination with local emergency services. Regular drills and training sessions should be conducted to ensure all personnel are prepared to respond effectively in case of an incident involving fluoroantimonic acid.
The acid must be stored in containers made of materials resistant to its corrosive properties, such as polytetrafluoroethylene (PTFE) or fluorinated ethylene propylene (FEP). Glass and metal containers are unsuitable due to the acid's ability to dissolve these materials. Proper labeling and segregation from incompatible substances are crucial to prevent accidental mixing and potential violent reactions.
Emergency response procedures must be established and regularly reviewed. This includes having readily accessible eyewash stations, safety showers, and spill containment equipment. Personnel should be trained in proper spill cleanup techniques and the use of neutralizing agents, such as sodium bicarbonate or calcium carbonate, to mitigate the effects of accidental releases.
Due to its extreme reactivity, fluoroantimonic acid should only be handled by experienced chemists in well-equipped laboratories. The acid's preparation and use require specialized apparatus, often involving the combination of hydrogen fluoride and antimony pentafluoride under controlled conditions. Strict temperature control is essential, as the acid can become unstable at elevated temperatures.
Waste disposal protocols for fluoroantimonic acid and related materials must comply with local and national regulations for hazardous waste management. Neutralization and dilution procedures should be carefully followed before disposal, and certified waste management services should be employed for final disposal.
Regular safety audits and equipment inspections are necessary to ensure the integrity of containment systems and the effectiveness of safety measures. Detailed records of acid usage, storage, and disposal should be maintained for regulatory compliance and internal safety reviews.
Lastly, a comprehensive emergency response plan should be in place, including procedures for evacuation, containment of large spills, and coordination with local emergency services. Regular drills and training sessions should be conducted to ensure all personnel are prepared to respond effectively in case of an incident involving fluoroantimonic acid.
Environmental Impact Assessment
The use of fluoroantimonic acid in targeting specific chemical reactions raises significant environmental concerns due to its highly corrosive and reactive nature. This superacid, composed of a mixture of hydrogen fluoride and antimony pentafluoride, poses severe risks to ecosystems and human health if not properly managed.
When released into the environment, fluoroantimonic acid can cause extensive damage to soil and water systems. Its extreme acidity can lead to rapid soil degradation, altering pH levels and destroying microbial communities essential for ecosystem balance. Aquatic environments are particularly vulnerable, as even small quantities of the acid can cause widespread fish kills and disrupt entire food chains.
The acid's fluorine content presents additional environmental hazards. Fluorine compounds can persist in the environment for extended periods, accumulating in plants and animals. This bioaccumulation can result in long-term ecological impacts, affecting biodiversity and potentially entering the human food chain.
Air pollution is another critical concern. Vapors from fluoroantimonic acid can contribute to the formation of acid rain, impacting vegetation and infrastructure over large areas. The release of antimony compounds into the atmosphere may also have far-reaching consequences, as these substances can be toxic to various organisms and persist in the environment.
The production and disposal of fluoroantimonic acid require stringent safety measures to prevent environmental contamination. Accidental spills or improper disposal can lead to severe localized environmental damage, necessitating costly and complex cleanup operations. The potential for groundwater contamination is particularly alarming, as it could affect drinking water sources for both humans and wildlife.
To mitigate these environmental risks, strict protocols must be implemented for the handling, storage, and disposal of fluoroantimonic acid. This includes using specialized containment systems, implementing robust waste treatment processes, and establishing comprehensive emergency response plans. Regular environmental monitoring in areas where the acid is used or produced is essential to detect and address any potential contamination quickly.
Research into less environmentally harmful alternatives for targeting specific chemical reactions should be prioritized. This could involve developing new catalysts or reaction methodologies that achieve similar selectivity without the associated environmental risks. Additionally, exploring green chemistry principles in the design of chemical processes could lead to more sustainable approaches in industrial applications.
When released into the environment, fluoroantimonic acid can cause extensive damage to soil and water systems. Its extreme acidity can lead to rapid soil degradation, altering pH levels and destroying microbial communities essential for ecosystem balance. Aquatic environments are particularly vulnerable, as even small quantities of the acid can cause widespread fish kills and disrupt entire food chains.
The acid's fluorine content presents additional environmental hazards. Fluorine compounds can persist in the environment for extended periods, accumulating in plants and animals. This bioaccumulation can result in long-term ecological impacts, affecting biodiversity and potentially entering the human food chain.
Air pollution is another critical concern. Vapors from fluoroantimonic acid can contribute to the formation of acid rain, impacting vegetation and infrastructure over large areas. The release of antimony compounds into the atmosphere may also have far-reaching consequences, as these substances can be toxic to various organisms and persist in the environment.
The production and disposal of fluoroantimonic acid require stringent safety measures to prevent environmental contamination. Accidental spills or improper disposal can lead to severe localized environmental damage, necessitating costly and complex cleanup operations. The potential for groundwater contamination is particularly alarming, as it could affect drinking water sources for both humans and wildlife.
To mitigate these environmental risks, strict protocols must be implemented for the handling, storage, and disposal of fluoroantimonic acid. This includes using specialized containment systems, implementing robust waste treatment processes, and establishing comprehensive emergency response plans. Regular environmental monitoring in areas where the acid is used or produced is essential to detect and address any potential contamination quickly.
Research into less environmentally harmful alternatives for targeting specific chemical reactions should be prioritized. This could involve developing new catalysts or reaction methodologies that achieve similar selectivity without the associated environmental risks. Additionally, exploring green chemistry principles in the design of chemical processes could lead to more sustainable approaches in industrial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!