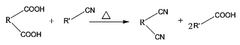How to Unleash Reaction Efficiency Using Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has garnered significant attention in the field of chemistry due to its exceptional acidity and potential applications. This powerful compound, with a Hammett acidity function estimated at -21.6, far surpasses the acidity of pure sulfuric acid and other common strong acids.
The development of fluoroantimonic acid can be traced back to the early 20th century, with significant advancements made in the 1960s and 1970s. Its discovery and subsequent refinement have opened up new possibilities in organic synthesis, catalysis, and materials science. The unique properties of this superacid have led researchers to explore its potential in enhancing reaction efficiency across various chemical processes.
The primary objective of investigating fluoroantimonic acid in the context of reaction efficiency is to harness its exceptional proton-donating capabilities. By leveraging its extreme acidity, researchers aim to overcome limitations in traditional acid-catalyzed reactions, potentially enabling transformations that were previously challenging or impossible to achieve.
One of the key areas of interest is the activation of typically unreactive substrates. Fluoroantimonic acid's ability to protonate even weak bases and generate highly reactive carbocations opens up new pathways for synthetic organic chemistry. This could lead to more efficient routes for producing complex molecules, including pharmaceuticals and advanced materials.
Another important goal is to explore the potential of fluoroantimonic acid in catalytic processes. Its strong acidic nature may allow for lower catalyst loadings and milder reaction conditions, potentially reducing energy consumption and improving overall process efficiency. This aligns with the growing emphasis on green chemistry and sustainable industrial practices.
However, the use of fluoroantimonic acid also presents significant challenges. Its extreme corrosiveness and sensitivity to moisture necessitate specialized handling techniques and equipment. Therefore, a crucial objective is to develop safe and practical methodologies for incorporating this superacid into industrial-scale processes.
As research in this field progresses, there is a growing interest in understanding the fundamental mechanisms by which fluoroantimonic acid enhances reaction efficiency. This includes investigating its behavior at the molecular level, its interactions with various substrates, and its role in stabilizing reactive intermediates.
The development of fluoroantimonic acid can be traced back to the early 20th century, with significant advancements made in the 1960s and 1970s. Its discovery and subsequent refinement have opened up new possibilities in organic synthesis, catalysis, and materials science. The unique properties of this superacid have led researchers to explore its potential in enhancing reaction efficiency across various chemical processes.
The primary objective of investigating fluoroantimonic acid in the context of reaction efficiency is to harness its exceptional proton-donating capabilities. By leveraging its extreme acidity, researchers aim to overcome limitations in traditional acid-catalyzed reactions, potentially enabling transformations that were previously challenging or impossible to achieve.
One of the key areas of interest is the activation of typically unreactive substrates. Fluoroantimonic acid's ability to protonate even weak bases and generate highly reactive carbocations opens up new pathways for synthetic organic chemistry. This could lead to more efficient routes for producing complex molecules, including pharmaceuticals and advanced materials.
Another important goal is to explore the potential of fluoroantimonic acid in catalytic processes. Its strong acidic nature may allow for lower catalyst loadings and milder reaction conditions, potentially reducing energy consumption and improving overall process efficiency. This aligns with the growing emphasis on green chemistry and sustainable industrial practices.
However, the use of fluoroantimonic acid also presents significant challenges. Its extreme corrosiveness and sensitivity to moisture necessitate specialized handling techniques and equipment. Therefore, a crucial objective is to develop safe and practical methodologies for incorporating this superacid into industrial-scale processes.
As research in this field progresses, there is a growing interest in understanding the fundamental mechanisms by which fluoroantimonic acid enhances reaction efficiency. This includes investigating its behavior at the molecular level, its interactions with various substrates, and its role in stabilizing reactive intermediates.
Industrial Demand for Superacid Catalysts
The demand for superacid catalysts in industrial applications has been steadily increasing due to their exceptional catalytic properties and ability to enhance reaction efficiency. Fluoroantimonic acid, being one of the strongest known superacids, has garnered significant attention in various sectors of the chemical industry. Its unique ability to protonate even weak bases and catalyze reactions under mild conditions has made it a valuable tool in numerous industrial processes.
In the petrochemical industry, superacid catalysts like fluoroantimonic acid are crucial for processes such as isomerization, alkylation, and cracking of hydrocarbons. These reactions are fundamental in the production of high-octane gasoline and other valuable petrochemical products. The demand for more efficient and environmentally friendly catalytic processes has driven research into the application of superacids in this sector.
The pharmaceutical industry has also shown increasing interest in superacid catalysts for the synthesis of complex organic molecules. Fluoroantimonic acid's ability to facilitate challenging transformations, such as C-C bond formations and rearrangements, has made it an attractive option for developing new synthetic routes to active pharmaceutical ingredients. This has led to a growing demand for superacid catalysts in drug discovery and development processes.
In the field of polymer chemistry, superacid catalysts have found applications in the production of high-performance materials. Fluoroantimonic acid's strong acidic properties can initiate polymerization reactions and modify polymer structures, leading to materials with enhanced properties. This has resulted in increased demand from industries producing specialty plastics, coatings, and advanced composite materials.
The electronics industry has also recognized the potential of superacid catalysts in the production of advanced materials for semiconductor devices. Fluoroantimonic acid's ability to etch and modify surfaces at the nanoscale has made it valuable in the fabrication of microelectronic components and advanced display technologies.
Environmental applications have emerged as another area driving the demand for superacid catalysts. Their use in the treatment of industrial waste and the removal of pollutants from exhaust gases has gained traction as industries seek to comply with stricter environmental regulations.
As industries continue to push the boundaries of chemical transformations and seek more efficient processes, the demand for superacid catalysts is expected to grow further. Research into new applications and the development of more stable and recyclable superacid catalyst systems will likely drive innovation in this field, opening up new possibilities for industrial applications and further increasing demand.
In the petrochemical industry, superacid catalysts like fluoroantimonic acid are crucial for processes such as isomerization, alkylation, and cracking of hydrocarbons. These reactions are fundamental in the production of high-octane gasoline and other valuable petrochemical products. The demand for more efficient and environmentally friendly catalytic processes has driven research into the application of superacids in this sector.
The pharmaceutical industry has also shown increasing interest in superacid catalysts for the synthesis of complex organic molecules. Fluoroantimonic acid's ability to facilitate challenging transformations, such as C-C bond formations and rearrangements, has made it an attractive option for developing new synthetic routes to active pharmaceutical ingredients. This has led to a growing demand for superacid catalysts in drug discovery and development processes.
In the field of polymer chemistry, superacid catalysts have found applications in the production of high-performance materials. Fluoroantimonic acid's strong acidic properties can initiate polymerization reactions and modify polymer structures, leading to materials with enhanced properties. This has resulted in increased demand from industries producing specialty plastics, coatings, and advanced composite materials.
The electronics industry has also recognized the potential of superacid catalysts in the production of advanced materials for semiconductor devices. Fluoroantimonic acid's ability to etch and modify surfaces at the nanoscale has made it valuable in the fabrication of microelectronic components and advanced display technologies.
Environmental applications have emerged as another area driving the demand for superacid catalysts. Their use in the treatment of industrial waste and the removal of pollutants from exhaust gases has gained traction as industries seek to comply with stricter environmental regulations.
As industries continue to push the boundaries of chemical transformations and seek more efficient processes, the demand for superacid catalysts is expected to grow further. Research into new applications and the development of more stable and recyclable superacid catalyst systems will likely drive innovation in this field, opening up new possibilities for industrial applications and further increasing demand.
Current State and Challenges in Superacid Chemistry
Superacid chemistry has witnessed significant advancements in recent years, with fluoroantimonic acid (HSbF6) emerging as a powerful tool for enhancing reaction efficiency. The current state of superacid research is characterized by intense focus on developing novel synthetic methodologies and exploring unique reactivity patterns.
One of the primary challenges in superacid chemistry is the safe handling and containment of these highly corrosive substances. Researchers are actively developing specialized equipment and protocols to mitigate risks associated with superacid use. Additionally, the extreme reactivity of superacids often leads to unexpected side reactions, necessitating careful control of reaction conditions and thorough product analysis.
The application of fluoroantimonic acid in organic synthesis has opened new avenues for creating complex molecules. Its exceptional acidity allows for the activation of traditionally unreactive substrates, enabling transformations that were previously unfeasible. However, the full potential of fluoroantimonic acid remains largely untapped due to limitations in understanding its reaction mechanisms at the molecular level.
Recent studies have focused on elucidating the precise nature of superacid-substrate interactions. Advanced spectroscopic techniques, such as in situ NMR and time-resolved IR spectroscopy, are being employed to capture fleeting intermediates and transition states. These investigations aim to provide a more comprehensive understanding of superacid-mediated reactions, potentially leading to more predictable and controllable processes.
Another significant challenge lies in the scalability of superacid-mediated reactions. While laboratory-scale experiments have demonstrated impressive results, translating these findings to industrial-scale processes presents numerous obstacles. Engineers and chemists are collaborating to design robust reactor systems capable of withstanding the harsh conditions imposed by superacids while maintaining reaction efficiency.
The environmental impact of superacid chemistry is also a growing concern. Efforts are underway to develop greener alternatives and recycling methods for superacids. Some researchers are exploring the potential of supported superacids, which could offer easier handling and reduced waste generation while retaining high catalytic activity.
In the realm of materials science, superacids are finding applications in the synthesis of advanced polymers and nanomaterials. The ability of fluoroantimonic acid to generate highly electrophilic species is being harnessed to create materials with unique properties. However, controlling the morphology and composition of these materials remains a significant challenge, requiring further research into the fundamental aspects of superacid-induced polymerization and nanostructure formation.
One of the primary challenges in superacid chemistry is the safe handling and containment of these highly corrosive substances. Researchers are actively developing specialized equipment and protocols to mitigate risks associated with superacid use. Additionally, the extreme reactivity of superacids often leads to unexpected side reactions, necessitating careful control of reaction conditions and thorough product analysis.
The application of fluoroantimonic acid in organic synthesis has opened new avenues for creating complex molecules. Its exceptional acidity allows for the activation of traditionally unreactive substrates, enabling transformations that were previously unfeasible. However, the full potential of fluoroantimonic acid remains largely untapped due to limitations in understanding its reaction mechanisms at the molecular level.
Recent studies have focused on elucidating the precise nature of superacid-substrate interactions. Advanced spectroscopic techniques, such as in situ NMR and time-resolved IR spectroscopy, are being employed to capture fleeting intermediates and transition states. These investigations aim to provide a more comprehensive understanding of superacid-mediated reactions, potentially leading to more predictable and controllable processes.
Another significant challenge lies in the scalability of superacid-mediated reactions. While laboratory-scale experiments have demonstrated impressive results, translating these findings to industrial-scale processes presents numerous obstacles. Engineers and chemists are collaborating to design robust reactor systems capable of withstanding the harsh conditions imposed by superacids while maintaining reaction efficiency.
The environmental impact of superacid chemistry is also a growing concern. Efforts are underway to develop greener alternatives and recycling methods for superacids. Some researchers are exploring the potential of supported superacids, which could offer easier handling and reduced waste generation while retaining high catalytic activity.
In the realm of materials science, superacids are finding applications in the synthesis of advanced polymers and nanomaterials. The ability of fluoroantimonic acid to generate highly electrophilic species is being harnessed to create materials with unique properties. However, controlling the morphology and composition of these materials remains a significant challenge, requiring further research into the fundamental aspects of superacid-induced polymerization and nanostructure formation.
Existing Fluoroantimonic Acid Reaction Methodologies
01 Reaction efficiency enhancement in chemical processes
Various methods and apparatus are employed to improve the reaction efficiency of fluoroantimonic acid in chemical processes. These include optimizing reaction conditions, using specialized catalysts, and developing novel reactor designs to enhance mixing and heat transfer.- Reaction vessel design for fluoroantimonic acid: Specialized reaction vessels are designed to handle the highly corrosive nature of fluoroantimonic acid. These vessels incorporate materials resistant to acid attack and may include features for controlled addition of reactants, temperature regulation, and safe handling of the acid to improve reaction efficiency.
- Catalytic applications of fluoroantimonic acid: Fluoroantimonic acid is utilized as a super-acid catalyst in various chemical reactions. Its extreme acidity enhances reaction rates and efficiencies in processes such as isomerization, alkylation, and polymerization. Proper catalyst loading and reaction conditions are crucial for optimizing its catalytic performance.
- Temperature control in fluoroantimonic acid reactions: Precise temperature control is essential for maximizing the reaction efficiency of fluoroantimonic acid. Specialized cooling systems and heat exchangers are employed to maintain optimal reaction temperatures, as the acid's reactivity and stability are highly temperature-dependent.
- Purification and handling of fluoroantimonic acid: Efficient purification and handling techniques are crucial for maintaining the potency and reaction efficiency of fluoroantimonic acid. This includes methods for removing impurities, storing the acid under controlled conditions, and developing safe transfer systems to minimize exposure and contamination risks.
- Reaction monitoring and analysis for fluoroantimonic acid: Advanced monitoring and analysis techniques are employed to assess and optimize the reaction efficiency of fluoroantimonic acid. This includes in-situ spectroscopic methods, real-time reaction progress tracking, and post-reaction analysis to determine yield and purity of products.
02 Fluoroantimonic acid handling and storage systems
Specialized equipment and systems are developed for the safe handling and storage of fluoroantimonic acid, which is highly corrosive and reactive. These systems are designed to maintain the acid's purity and reactivity while ensuring safety and efficiency in industrial applications.Expand Specific Solutions03 Analytical methods for fluoroantimonic acid reactions
Advanced analytical techniques and instruments are utilized to monitor and analyze fluoroantimonic acid reactions. These methods help in understanding reaction kinetics, identifying intermediates, and optimizing reaction conditions to improve overall efficiency.Expand Specific Solutions04 Applications of fluoroantimonic acid in material processing
Fluoroantimonic acid is employed in various material processing applications, such as surface treatment, etching, and catalysis. The efficiency of these processes is enhanced through the development of specialized equipment and optimized reaction parameters.Expand Specific Solutions05 Waste management and environmental considerations
Efficient waste management systems and environmentally friendly processes are developed for handling fluoroantimonic acid reactions. These include recycling methods, neutralization techniques, and the use of alternative, less hazardous reagents to improve overall process sustainability.Expand Specific Solutions
Key Players in Superacid Research and Applications
The development of fluoroantimonic acid technology is in a mature stage, with a growing market driven by its applications in various industries. The global market size for superacids, including fluoroantimonic acid, is expanding due to increasing demand in petrochemicals, pharmaceuticals, and materials science. Companies like DAIKIN INDUSTRIES Ltd., Mitsubishi Gas Chemical Co., Inc., and AGC, Inc. are at the forefront of this technology, leveraging their expertise in fluorine chemistry. DuPont de Nemours, Inc. and Bayer AG are also significant players, contributing to the advancement of synthesis methods and applications. The technology's maturity is evident in the diverse range of companies involved, from established chemical giants to specialized firms like Quanzhou YUJI Advanced MATERIALS Co., Ltd., indicating a competitive and innovative landscape.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for the synthesis and handling of fluoroantimonic acid, utilizing specialized corrosion-resistant materials and advanced containment systems. Their method involves the controlled reaction of hydrogen fluoride with antimony pentafluoride under precise temperature and pressure conditions. DuPont's approach also incorporates a novel purification step to remove trace impurities that could potentially reduce the acid's reactivity. Additionally, they have engineered a unique delivery system that allows for the safe and efficient application of the superacid in various industrial processes, particularly in the petrochemical and pharmaceutical sectors.
Strengths: Extensive experience in handling highly corrosive materials, advanced safety protocols, and established industrial-scale production capabilities. Weaknesses: High production costs and limited applications outside of specialized industries.
Bayer AG
Technical Solution: Bayer has developed an innovative approach to utilizing fluoroantimonic acid in organic synthesis reactions. Their method involves the use of a specially designed reactor system that allows for the controlled addition of fluoroantimonic acid to organic substrates under inert conditions. Bayer's technology incorporates a novel quenching mechanism that rapidly neutralizes the superacid after the desired reaction has taken place, enhancing safety and product isolation. Furthermore, they have developed a series of fluoroantimonic acid-compatible catalysts that significantly enhance reaction rates and selectivity in certain transformations, particularly in the synthesis of complex pharmaceutical intermediates.
Strengths: Strong expertise in organic synthesis and pharmaceutical applications, advanced reactor design, and catalyst development. Weaknesses: Limited experience in large-scale handling of superacids compared to some competitors.
Core Innovations in Fluoroantimonic Acid Catalysis
Method for the production of [<18>f] fluoride-marked aromatic l-amino acids
PatentWO2005037737A1
Innovation
- A method involving nucleophilic substitution of a negatively charged 18F fluoride ion with a suitable L-enantiomeric compound, followed by cleavage of protective groups, to produce 18F fluorine-labeled aromatic L-amino acids in a few steps, ensuring high reproducibility and stereochemical purity.
Method for preparing phthalonitrile-based compound
PatentWO2023003058A1
Innovation
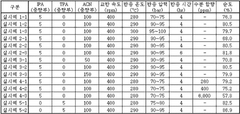
- A method involving the direct reaction of a phthalic acid-based compound with a nitrile-based compound under supercritical conditions, eliminating the need for ammonia and catalysts, and allowing for the reuse of remaining compounds, thereby producing high-purity phthalonitrile-based compounds in an environmentally friendly manner.
Safety and Handling Protocols for Fluoroantimonic Acid
Fluoroantimonic acid is one of the strongest known superacids, with a Hammett acidity function estimated at H0 ≈ -31.3. Its extreme corrosiveness and reactivity necessitate stringent safety measures and handling protocols to mitigate risks associated with its use in research and industrial applications.
Personal protective equipment (PPE) is paramount when working with fluoroantimonic acid. Operators must wear fully encapsulating chemical-resistant suits, including gloves, boots, and face shields. The suit material should be specifically rated for fluorinated compounds and superacids. Double-gloving with fluoropolymer-based gloves is recommended, with frequent changes to prevent permeation.
Proper ventilation is crucial in any area where fluoroantimonic acid is handled. Fume hoods with specialized acid-resistant linings and exhaust systems are required. The workspace should be equipped with emergency eyewash stations and safety showers in close proximity to the handling area.
Storage protocols for fluoroantimonic acid are equally critical. The acid must be kept in specially designed containers made of materials resistant to both hydrofluoric acid and antimony pentafluoride, such as PTFE or PFA. These containers should be stored in secondary containment units within designated, well-ventilated areas away from incompatible substances.
Spill response procedures must be established and rehearsed regularly. Specialized spill kits containing neutralizing agents like calcium carbonate or sodium bicarbonate should be readily available. In the event of a spill, immediate evacuation of the area is necessary, followed by containment and neutralization by trained personnel wearing appropriate PPE.
Waste disposal of fluoroantimonic acid and related materials requires careful consideration. Neutralization followed by proper dilution is typically necessary before disposal. All waste handling must comply with local, state, and federal regulations for hazardous materials.
Training is a critical component of safety protocols. All personnel working with or around fluoroantimonic acid must undergo comprehensive safety training, including proper handling techniques, emergency procedures, and first aid measures. Regular refresher courses and safety drills should be conducted to maintain preparedness.
Documentation and record-keeping are essential aspects of safety protocols. Detailed logs of acid usage, storage conditions, and any incidents or near-misses should be maintained. Safety data sheets (SDS) must be readily accessible, and all procedures should be clearly documented and regularly reviewed for updates or improvements.
Personal protective equipment (PPE) is paramount when working with fluoroantimonic acid. Operators must wear fully encapsulating chemical-resistant suits, including gloves, boots, and face shields. The suit material should be specifically rated for fluorinated compounds and superacids. Double-gloving with fluoropolymer-based gloves is recommended, with frequent changes to prevent permeation.
Proper ventilation is crucial in any area where fluoroantimonic acid is handled. Fume hoods with specialized acid-resistant linings and exhaust systems are required. The workspace should be equipped with emergency eyewash stations and safety showers in close proximity to the handling area.
Storage protocols for fluoroantimonic acid are equally critical. The acid must be kept in specially designed containers made of materials resistant to both hydrofluoric acid and antimony pentafluoride, such as PTFE or PFA. These containers should be stored in secondary containment units within designated, well-ventilated areas away from incompatible substances.
Spill response procedures must be established and rehearsed regularly. Specialized spill kits containing neutralizing agents like calcium carbonate or sodium bicarbonate should be readily available. In the event of a spill, immediate evacuation of the area is necessary, followed by containment and neutralization by trained personnel wearing appropriate PPE.
Waste disposal of fluoroantimonic acid and related materials requires careful consideration. Neutralization followed by proper dilution is typically necessary before disposal. All waste handling must comply with local, state, and federal regulations for hazardous materials.
Training is a critical component of safety protocols. All personnel working with or around fluoroantimonic acid must undergo comprehensive safety training, including proper handling techniques, emergency procedures, and first aid measures. Regular refresher courses and safety drills should be conducted to maintain preparedness.
Documentation and record-keeping are essential aspects of safety protocols. Detailed logs of acid usage, storage conditions, and any incidents or near-misses should be maintained. Safety data sheets (SDS) must be readily accessible, and all procedures should be clearly documented and regularly reviewed for updates or improvements.
Environmental Impact and Waste Management Strategies
The use of fluoroantimonic acid in chemical reactions presents significant environmental challenges that require careful consideration and management. As one of the strongest known superacids, fluoroantimonic acid can have severe impacts on ecosystems if released into the environment. Its highly corrosive nature and ability to react with a wide range of substances pose risks to soil, water, and air quality.
To mitigate these environmental risks, comprehensive waste management strategies are essential. Neutralization is a primary method for treating fluoroantimonic acid waste. This process involves carefully adding the acid to a large volume of cold water, followed by neutralization with bases such as sodium hydroxide or calcium hydroxide. The resulting salts can then be disposed of more safely. However, this process must be conducted with extreme caution due to the highly exothermic nature of the reaction.
Containment is another critical aspect of managing fluoroantimonic acid. Specialized storage and handling equipment made from materials resistant to superacids, such as PTFE (Teflon), are necessary to prevent leaks and spills. Double containment systems and rigorous inspection protocols should be implemented to ensure the integrity of storage vessels and transfer systems.
In terms of waste reduction, optimizing reaction conditions to minimize the amount of fluoroantimonic acid required can significantly decrease environmental impact. This may involve exploring catalytic processes or alternative reaction pathways that achieve similar efficiencies with less hazardous materials.
Recycling and regeneration of fluoroantimonic acid can also play a role in reducing waste. While challenging due to the acid's extreme reactivity, developing efficient recycling processes could significantly decrease the volume of waste generated and the need for new acid production.
Monitoring and detection systems are crucial for early identification of potential releases. Advanced sensors and real-time monitoring equipment should be employed to detect even trace amounts of fluoroantimonic acid in air, water, or soil. This allows for rapid response and containment in case of accidental release.
Employee training and safety protocols are integral to preventing environmental contamination. Comprehensive education on proper handling, storage, and disposal procedures, as well as emergency response training, can significantly reduce the risk of accidents and environmental exposure.
Lastly, research into less hazardous alternatives that can achieve similar reaction efficiencies should be a long-term goal. While fluoroantimonic acid offers unique catalytic properties, the development of safer superacids or entirely different catalytic systems could provide more environmentally friendly options for unleashing reaction efficiency in the future.
To mitigate these environmental risks, comprehensive waste management strategies are essential. Neutralization is a primary method for treating fluoroantimonic acid waste. This process involves carefully adding the acid to a large volume of cold water, followed by neutralization with bases such as sodium hydroxide or calcium hydroxide. The resulting salts can then be disposed of more safely. However, this process must be conducted with extreme caution due to the highly exothermic nature of the reaction.
Containment is another critical aspect of managing fluoroantimonic acid. Specialized storage and handling equipment made from materials resistant to superacids, such as PTFE (Teflon), are necessary to prevent leaks and spills. Double containment systems and rigorous inspection protocols should be implemented to ensure the integrity of storage vessels and transfer systems.
In terms of waste reduction, optimizing reaction conditions to minimize the amount of fluoroantimonic acid required can significantly decrease environmental impact. This may involve exploring catalytic processes or alternative reaction pathways that achieve similar efficiencies with less hazardous materials.
Recycling and regeneration of fluoroantimonic acid can also play a role in reducing waste. While challenging due to the acid's extreme reactivity, developing efficient recycling processes could significantly decrease the volume of waste generated and the need for new acid production.
Monitoring and detection systems are crucial for early identification of potential releases. Advanced sensors and real-time monitoring equipment should be employed to detect even trace amounts of fluoroantimonic acid in air, water, or soil. This allows for rapid response and containment in case of accidental release.
Employee training and safety protocols are integral to preventing environmental contamination. Comprehensive education on proper handling, storage, and disposal procedures, as well as emergency response training, can significantly reduce the risk of accidents and environmental exposure.
Lastly, research into less hazardous alternatives that can achieve similar reaction efficiencies should be a long-term goal. While fluoroantimonic acid offers unique catalytic properties, the development of safer superacids or entirely different catalytic systems could provide more environmentally friendly options for unleashing reaction efficiency in the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!