How to Excel in Catalysis Research Using Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Catalysis Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has emerged as a powerful catalyst in various chemical reactions. Its exceptional acidity, surpassing that of 100% sulfuric acid by over a trillion times, has revolutionized the field of catalysis research. The development of fluoroantimonic acid catalysis can be traced back to the mid-20th century, with significant advancements in understanding its properties and applications occurring in recent decades.
The evolution of fluoroantimonic acid catalysis has been driven by the increasing demand for more efficient and selective chemical transformations in industries such as petrochemicals, pharmaceuticals, and materials science. As researchers delved deeper into its unique properties, they discovered its ability to catalyze reactions that were previously challenging or impossible with conventional acids.
One of the key trends in fluoroantimonic acid catalysis research has been the exploration of its potential in activating C-H bonds, a fundamental challenge in organic synthesis. This has opened up new possibilities for the functionalization of hydrocarbons and the development of novel synthetic routes for complex molecules.
Another significant trend is the investigation of fluoroantimonic acid's role in promoting carbocation rearrangements, which has implications for the petroleum industry, particularly in the isomerization of hydrocarbons for fuel production.
The primary objective of excelling in catalysis research using fluoroantimonic acid is to harness its exceptional acidity for developing more efficient, selective, and environmentally friendly chemical processes. This includes optimizing reaction conditions, understanding the mechanistic aspects of fluoroantimonic acid-catalyzed reactions, and exploring new applications across various fields of chemistry.
Researchers aim to address several key challenges in fluoroantimonic acid catalysis. These include improving the handling and containment of this highly corrosive substance, developing methods for catalyst recovery and reuse, and minimizing potential environmental impacts. Additionally, there is a focus on expanding the substrate scope of fluoroantimonic acid-catalyzed reactions and enhancing the stereoselectivity of transformations.
The future of fluoroantimonic acid catalysis research is likely to involve the development of novel reaction methodologies, the design of specialized reactor systems, and the integration of computational modeling to predict and optimize reaction outcomes. As the field progresses, researchers will continue to push the boundaries of what is possible in organic synthesis and industrial catalysis, leveraging the unique properties of this remarkable superacid to drive innovation in chemical research and technology.
The evolution of fluoroantimonic acid catalysis has been driven by the increasing demand for more efficient and selective chemical transformations in industries such as petrochemicals, pharmaceuticals, and materials science. As researchers delved deeper into its unique properties, they discovered its ability to catalyze reactions that were previously challenging or impossible with conventional acids.
One of the key trends in fluoroantimonic acid catalysis research has been the exploration of its potential in activating C-H bonds, a fundamental challenge in organic synthesis. This has opened up new possibilities for the functionalization of hydrocarbons and the development of novel synthetic routes for complex molecules.
Another significant trend is the investigation of fluoroantimonic acid's role in promoting carbocation rearrangements, which has implications for the petroleum industry, particularly in the isomerization of hydrocarbons for fuel production.
The primary objective of excelling in catalysis research using fluoroantimonic acid is to harness its exceptional acidity for developing more efficient, selective, and environmentally friendly chemical processes. This includes optimizing reaction conditions, understanding the mechanistic aspects of fluoroantimonic acid-catalyzed reactions, and exploring new applications across various fields of chemistry.
Researchers aim to address several key challenges in fluoroantimonic acid catalysis. These include improving the handling and containment of this highly corrosive substance, developing methods for catalyst recovery and reuse, and minimizing potential environmental impacts. Additionally, there is a focus on expanding the substrate scope of fluoroantimonic acid-catalyzed reactions and enhancing the stereoselectivity of transformations.
The future of fluoroantimonic acid catalysis research is likely to involve the development of novel reaction methodologies, the design of specialized reactor systems, and the integration of computational modeling to predict and optimize reaction outcomes. As the field progresses, researchers will continue to push the boundaries of what is possible in organic synthesis and industrial catalysis, leveraging the unique properties of this remarkable superacid to drive innovation in chemical research and technology.
Market Demand for Superacid Catalysts
The market demand for superacid catalysts, particularly those utilizing fluoroantimonic acid, has been steadily growing in recent years. This growth is primarily driven by the increasing need for more efficient and selective catalytic processes in various industries, including petrochemicals, pharmaceuticals, and fine chemicals manufacturing.
In the petrochemical sector, superacid catalysts are gaining traction due to their ability to facilitate complex hydrocarbon transformations at lower temperatures and pressures. This results in significant energy savings and improved process economics. The demand for cleaner fuels and higher-value petrochemical products has further boosted the adoption of superacid catalysts in refining and petrochemical operations.
The pharmaceutical industry has also shown a growing interest in superacid catalysts, particularly for the synthesis of complex drug molecules. Fluoroantimonic acid-based catalysts offer unique advantages in terms of reaction selectivity and yield, making them attractive for the production of high-value pharmaceutical intermediates and active pharmaceutical ingredients (APIs).
Fine chemicals manufacturers are increasingly exploring the potential of superacid catalysts to develop more sustainable and cost-effective production routes. The ability of these catalysts to promote reactions under milder conditions and with higher atom economy aligns well with the industry's push towards greener chemistry practices.
The electronics industry represents another emerging market for superacid catalysts. In semiconductor manufacturing, these catalysts are being investigated for their potential in etching and surface modification processes, offering possibilities for creating more advanced and miniaturized electronic components.
Despite the growing demand, the market for superacid catalysts faces certain challenges. The extreme corrosiveness of fluoroantimonic acid necessitates specialized handling and equipment, which can increase implementation costs. Additionally, safety and environmental concerns associated with the use of such strong acids pose regulatory hurdles in some regions.
However, ongoing research and development efforts are focused on addressing these challenges. Innovations in catalyst design, such as supported and immobilized superacid catalysts, are aimed at improving safety profiles and ease of handling. These advancements are expected to further expand the market potential of superacid catalysts across various industries.
The global market for superacid catalysts is projected to experience robust growth in the coming years. This growth is expected to be particularly strong in emerging economies, where rapid industrialization and increasing investments in chemical and pharmaceutical manufacturing are creating new opportunities for advanced catalytic technologies.
In the petrochemical sector, superacid catalysts are gaining traction due to their ability to facilitate complex hydrocarbon transformations at lower temperatures and pressures. This results in significant energy savings and improved process economics. The demand for cleaner fuels and higher-value petrochemical products has further boosted the adoption of superacid catalysts in refining and petrochemical operations.
The pharmaceutical industry has also shown a growing interest in superacid catalysts, particularly for the synthesis of complex drug molecules. Fluoroantimonic acid-based catalysts offer unique advantages in terms of reaction selectivity and yield, making them attractive for the production of high-value pharmaceutical intermediates and active pharmaceutical ingredients (APIs).
Fine chemicals manufacturers are increasingly exploring the potential of superacid catalysts to develop more sustainable and cost-effective production routes. The ability of these catalysts to promote reactions under milder conditions and with higher atom economy aligns well with the industry's push towards greener chemistry practices.
The electronics industry represents another emerging market for superacid catalysts. In semiconductor manufacturing, these catalysts are being investigated for their potential in etching and surface modification processes, offering possibilities for creating more advanced and miniaturized electronic components.
Despite the growing demand, the market for superacid catalysts faces certain challenges. The extreme corrosiveness of fluoroantimonic acid necessitates specialized handling and equipment, which can increase implementation costs. Additionally, safety and environmental concerns associated with the use of such strong acids pose regulatory hurdles in some regions.
However, ongoing research and development efforts are focused on addressing these challenges. Innovations in catalyst design, such as supported and immobilized superacid catalysts, are aimed at improving safety profiles and ease of handling. These advancements are expected to further expand the market potential of superacid catalysts across various industries.
The global market for superacid catalysts is projected to experience robust growth in the coming years. This growth is expected to be particularly strong in emerging economies, where rapid industrialization and increasing investments in chemical and pharmaceutical manufacturing are creating new opportunities for advanced catalytic technologies.
Current State and Challenges in Fluoroantimonic Acid Research
Fluoroantimonic acid, recognized as one of the strongest superacids, has garnered significant attention in catalysis research due to its exceptional proton-donating ability. The current state of research in this field is characterized by both promising advancements and formidable challenges.
Recent studies have demonstrated the remarkable catalytic potential of fluoroantimonic acid in various organic transformations, particularly in hydrocarbon processing and petrochemical industries. Its ability to catalyze reactions under mild conditions has led to increased efficiency and selectivity in numerous processes. However, the widespread adoption of this superacid in industrial applications remains limited due to several critical challenges.
One of the primary obstacles in fluoroantimonic acid research is its extreme corrosiveness and reactivity. This property, while beneficial for catalysis, poses significant safety concerns and requires specialized handling techniques and equipment. Researchers are actively exploring methods to mitigate these risks, including the development of containment systems and protective coatings for reaction vessels.
Another challenge lies in the controlled application of fluoroantimonic acid in catalytic processes. Its high reactivity can lead to undesired side reactions and product degradation, necessitating precise control over reaction conditions. Current research efforts are focused on optimizing reaction parameters and developing novel reactor designs to enhance selectivity and yield.
The environmental impact of fluoroantimonic acid usage is also a growing concern. Its production and disposal involve hazardous materials, prompting investigations into more sustainable alternatives and recycling methods. Researchers are exploring the potential of ionic liquids and supported acid catalysts as environmentally friendly substitutes that retain the superacid's catalytic prowess.
In terms of characterization and mechanistic studies, the extreme acidity of fluoroantimonic acid presents unique analytical challenges. Traditional spectroscopic techniques often fall short in providing detailed insights into reaction intermediates and pathways. Advanced in situ spectroscopic methods and computational modeling are being developed to bridge this gap and elucidate the fundamental aspects of fluoroantimonic acid catalysis.
The scalability of fluoroantimonic acid-based processes from laboratory to industrial scale remains a significant hurdle. Issues related to heat management, mass transfer limitations, and catalyst recovery need to be addressed for successful scale-up. Ongoing research is focused on developing innovative reactor technologies and process intensification strategies to overcome these challenges.
Despite these obstacles, the field of fluoroantimonic acid research continues to evolve, driven by its potential to revolutionize catalytic processes. Collaborative efforts between academia and industry are paving the way for novel applications and improved methodologies, promising exciting developments in the near future.
Recent studies have demonstrated the remarkable catalytic potential of fluoroantimonic acid in various organic transformations, particularly in hydrocarbon processing and petrochemical industries. Its ability to catalyze reactions under mild conditions has led to increased efficiency and selectivity in numerous processes. However, the widespread adoption of this superacid in industrial applications remains limited due to several critical challenges.
One of the primary obstacles in fluoroantimonic acid research is its extreme corrosiveness and reactivity. This property, while beneficial for catalysis, poses significant safety concerns and requires specialized handling techniques and equipment. Researchers are actively exploring methods to mitigate these risks, including the development of containment systems and protective coatings for reaction vessels.
Another challenge lies in the controlled application of fluoroantimonic acid in catalytic processes. Its high reactivity can lead to undesired side reactions and product degradation, necessitating precise control over reaction conditions. Current research efforts are focused on optimizing reaction parameters and developing novel reactor designs to enhance selectivity and yield.
The environmental impact of fluoroantimonic acid usage is also a growing concern. Its production and disposal involve hazardous materials, prompting investigations into more sustainable alternatives and recycling methods. Researchers are exploring the potential of ionic liquids and supported acid catalysts as environmentally friendly substitutes that retain the superacid's catalytic prowess.
In terms of characterization and mechanistic studies, the extreme acidity of fluoroantimonic acid presents unique analytical challenges. Traditional spectroscopic techniques often fall short in providing detailed insights into reaction intermediates and pathways. Advanced in situ spectroscopic methods and computational modeling are being developed to bridge this gap and elucidate the fundamental aspects of fluoroantimonic acid catalysis.
The scalability of fluoroantimonic acid-based processes from laboratory to industrial scale remains a significant hurdle. Issues related to heat management, mass transfer limitations, and catalyst recovery need to be addressed for successful scale-up. Ongoing research is focused on developing innovative reactor technologies and process intensification strategies to overcome these challenges.
Despite these obstacles, the field of fluoroantimonic acid research continues to evolve, driven by its potential to revolutionize catalytic processes. Collaborative efforts between academia and industry are paving the way for novel applications and improved methodologies, promising exciting developments in the near future.
Existing Applications of Fluoroantimonic Acid in Catalysis
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and preparation of fluoroantimonic acid: Fluoroantimonic acid is synthesized by combining hydrogen fluoride and antimony pentafluoride. The process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the resulting superacid.
- Applications in organic synthesis and catalysis: Fluoroantimonic acid is utilized as a powerful catalyst in various organic synthesis reactions. Its extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids. It has found applications in alkylation, isomerization, and polymerization processes in the chemical and petrochemical industries.
- Use in materials science and surface treatments: The superacidic properties of fluoroantimonic acid make it useful in materials science applications. It can be used for surface etching, modification of materials, and in the production of specialized coatings. The acid's ability to interact with various substrates allows for the creation of materials with unique properties.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, special safety measures are required when handling fluoroantimonic acid. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage and disposal. Research has been conducted to develop safer handling methods and to mitigate potential hazards associated with its use.
- Analytical and characterization techniques: Various analytical methods have been developed to characterize fluoroantimonic acid and its reactions. These include spectroscopic techniques, electrochemical methods, and specialized apparatus for measuring its superacidic properties. Such techniques are crucial for understanding the behavior of this superacid and optimizing its applications in research and industry.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are challenging or impossible with conventional acid catalysts, making it valuable in the production of specialty chemicals and advanced materials.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials. The acid's unique properties make it suitable for generating highly reactive surfaces or initiating specific chemical transformations on material surfaces.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and safety protocols. This includes the use of specific containment materials, personal protective equipment, and controlled environments. Proper storage, transportation, and disposal methods are crucial to prevent accidents and environmental contamination. Safety measures also involve neutralization techniques and emergency response procedures.Expand Specific Solutions05 Analytical and characterization methods
Various analytical techniques have been developed to characterize fluoroantimonic acid and its reactions. These methods include spectroscopic analyses, electrochemical measurements, and specialized titration procedures. Advanced instrumentation and methodologies are employed to study the acid's properties, reaction mechanisms, and interactions with other substances, contributing to a better understanding of its behavior and potential applications.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Catalysis Industry
The field of catalysis research using fluoroantimonic acid is in a nascent stage, with significant potential for growth. The market size is relatively small but expanding, driven by increasing demand for efficient catalysts in various industrial processes. Technologically, the field is still developing, with varying levels of maturity among key players. Companies like Sinochem Lantian Co., Ltd. and DAIKIN INDUSTRIES Ltd. are at the forefront, leveraging their expertise in fluorine chemistry. Academic institutions such as Wisconsin Alumni Research Foundation, Yale University, and Monash University are contributing to fundamental research. Industrial players like Honeywell International Technologies Ltd. and F. Hoffmann-La Roche Ltd. are exploring practical applications, while specialized entities like Xi'an Modern Chemistry Research Institute focus on advancing the technology for specific sectors.
Sinochem Lantian Co., Ltd.
Technical Solution: Sinochem Lantian has developed a proprietary process for the synthesis and purification of fluoroantimonic acid, utilizing advanced fluorination technology and high-purity antimony pentafluoride. Their method involves a controlled reaction between hydrogen fluoride and antimony pentafluoride under precise temperature and pressure conditions. The company has also implemented a sophisticated containment and handling system to ensure safe production and storage of this highly corrosive superacid.
Strengths: High-purity product, advanced safety measures, and established production capabilities. Weaknesses: High production costs and limited applications beyond specialized research.
Wisconsin Alumni Research Foundation
Technical Solution: WARF has patented a novel approach to using fluoroantimonic acid in catalysis research. Their method involves immobilizing the superacid on a porous support material, creating a heterogeneous catalyst system. This innovation allows for easier handling and recovery of the catalyst while maintaining its extreme acidity. The foundation has also developed specialized reactor designs that can withstand the corrosive nature of fluoroantimonic acid, enabling longer-duration experiments and more diverse reaction conditions.
Strengths: Innovative immobilization technique, improved safety and handling, potential for broader applications. Weaknesses: Possible reduction in catalytic activity compared to free acid, complexity in catalyst preparation.
Core Innovations in Fluoroantimonic Acid Synthesis and Handling
Method for Catalytically Producing Formic Acid and Regenerating the Catalyst Used in the Process with Little Overpressure
PatentActiveUS20190291093A1
Innovation
- A method involving a vanadyl ion, vanadate ion, or polyoxometalate ion catalyst, contacted with alpha-hydroxyaldehyde, alpha-hydroxycarboxylic acid, or carbohydrates in a liquid solution at elevated temperatures, using a gas mixture with a high oxygen volume fraction to oxidize the catalyst, thereby reducing the pressure requirements and costs.
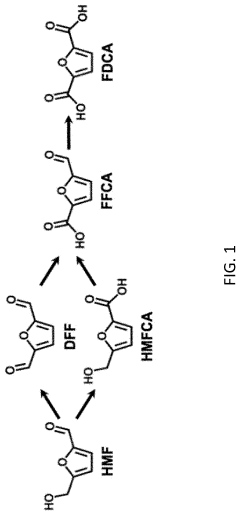
Electrochemical oxidation of 5-hydroxymethylfurfural using copper-based anodes
PatentActiveUS20200248322A1
Innovation
- The use of copper-based anodes in electrochemical cells with an oxygen-donating electrolyte solution allows for the oxidation of HMF to FDCA with a yield of at least 75% and Faradaic efficiency of at least 75%, eliminating the need for precious metal catalysts and operating at ambient conditions.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant safety and environmental challenges in catalysis research. Its extreme corrosiveness and reactivity demand rigorous safety protocols and specialized handling equipment. Researchers must use inert atmospheres, such as dry nitrogen or argon, to prevent moisture contamination and subsequent violent reactions.
Personal protective equipment (PPE) is crucial when working with fluoroantimonic acid. This includes chemical-resistant suits, gloves, and face shields. Proper ventilation systems and fume hoods are essential to mitigate exposure risks. Emergency showers and eyewash stations must be readily accessible in case of accidental contact.
Storage and transportation of fluoroantimonic acid require specialized containers made of materials resistant to its corrosive nature, such as polytetrafluoroethylene (PTFE) or certain fluoropolymers. Strict inventory control and secure storage practices are necessary to prevent unauthorized access or accidental release.
Environmental considerations are paramount when using fluoroantimonic acid in catalysis research. Its potential for environmental contamination is severe, necessitating robust waste management protocols. Neutralization procedures must be carefully designed and implemented to render the acid safe for disposal. Recycling and recovery methods should be explored to minimize waste generation and environmental impact.
Researchers must also consider the broader ecological implications of using such a potent acid. This includes assessing the lifecycle environmental impact of fluoroantimonic acid production, use, and disposal. Developing greener alternatives or optimizing processes to reduce the required quantities of the acid should be a priority in catalysis research.
Regulatory compliance is another critical aspect of safety and environmental considerations. Researchers must adhere to local, national, and international regulations governing the use of hazardous materials. This may include obtaining necessary permits, maintaining detailed records of usage and disposal, and participating in regular safety audits and environmental impact assessments.
Training and education play a vital role in ensuring safe and environmentally responsible use of fluoroantimonic acid. All personnel involved in its handling must receive comprehensive training on safety procedures, emergency response protocols, and proper waste management techniques. Regular refresher courses and safety drills should be conducted to maintain a high level of preparedness and awareness.
Personal protective equipment (PPE) is crucial when working with fluoroantimonic acid. This includes chemical-resistant suits, gloves, and face shields. Proper ventilation systems and fume hoods are essential to mitigate exposure risks. Emergency showers and eyewash stations must be readily accessible in case of accidental contact.
Storage and transportation of fluoroantimonic acid require specialized containers made of materials resistant to its corrosive nature, such as polytetrafluoroethylene (PTFE) or certain fluoropolymers. Strict inventory control and secure storage practices are necessary to prevent unauthorized access or accidental release.
Environmental considerations are paramount when using fluoroantimonic acid in catalysis research. Its potential for environmental contamination is severe, necessitating robust waste management protocols. Neutralization procedures must be carefully designed and implemented to render the acid safe for disposal. Recycling and recovery methods should be explored to minimize waste generation and environmental impact.
Researchers must also consider the broader ecological implications of using such a potent acid. This includes assessing the lifecycle environmental impact of fluoroantimonic acid production, use, and disposal. Developing greener alternatives or optimizing processes to reduce the required quantities of the acid should be a priority in catalysis research.
Regulatory compliance is another critical aspect of safety and environmental considerations. Researchers must adhere to local, national, and international regulations governing the use of hazardous materials. This may include obtaining necessary permits, maintaining detailed records of usage and disposal, and participating in regular safety audits and environmental impact assessments.
Training and education play a vital role in ensuring safe and environmentally responsible use of fluoroantimonic acid. All personnel involved in its handling must receive comprehensive training on safety procedures, emergency response protocols, and proper waste management techniques. Regular refresher courses and safety drills should be conducted to maintain a high level of preparedness and awareness.
Economic Viability of Fluoroantimonic Acid Catalysis
The economic viability of fluoroantimonic acid catalysis is a critical consideration for researchers and industries exploring its potential applications. This superacid, known for its extreme acidity and catalytic prowess, presents both opportunities and challenges from an economic standpoint.
Fluoroantimonic acid's exceptional catalytic properties offer the potential for increased reaction rates and improved yields in various chemical processes. This efficiency could translate to significant cost savings in industrial applications, particularly in petrochemical refining and organic synthesis. The ability to catalyze reactions under milder conditions may also reduce energy consumption, further enhancing its economic appeal.
However, the production and handling of fluoroantimonic acid pose substantial economic hurdles. The acid's corrosive nature necessitates specialized equipment and safety measures, significantly increasing initial capital investments. Materials compatible with fluoroantimonic acid, such as PTFE or certain alloys, are often expensive, adding to operational costs.
The precursors for fluoroantimonic acid, particularly antimony pentafluoride, are relatively costly and not widely available. This scarcity can lead to supply chain vulnerabilities and price fluctuations, potentially impacting the long-term economic stability of processes relying on this catalyst.
Environmental and safety regulations surrounding the use of such a potent acid also contribute to increased operational expenses. Stringent waste management protocols and worker safety measures are essential, adding to the overall cost of implementation.
Despite these challenges, the unique catalytic properties of fluoroantimonic acid may justify its use in high-value applications where alternative catalysts fall short. Industries producing specialty chemicals or pharmaceuticals might find the economic trade-offs favorable if the acid enables the synthesis of otherwise difficult-to-produce compounds.
The economic viability also depends on the scale of operation. Large-scale industrial processes might benefit from economies of scale, potentially offsetting some of the high initial and operational costs. Conversely, smaller-scale or research applications may find the economic barriers more challenging to overcome.
Advancements in containment technologies and more efficient synthesis methods for fluoroantimonic acid could improve its economic outlook. Research into recyclable or immobilized forms of the acid may also enhance its long-term economic viability by reducing waste and simplifying handling procedures.
In conclusion, while fluoroantimonic acid catalysis offers promising efficiency gains, its economic viability is currently limited by high costs and practical challenges. Future technological advancements and careful cost-benefit analyses will be crucial in determining its broader adoption across various industries.
Fluoroantimonic acid's exceptional catalytic properties offer the potential for increased reaction rates and improved yields in various chemical processes. This efficiency could translate to significant cost savings in industrial applications, particularly in petrochemical refining and organic synthesis. The ability to catalyze reactions under milder conditions may also reduce energy consumption, further enhancing its economic appeal.
However, the production and handling of fluoroantimonic acid pose substantial economic hurdles. The acid's corrosive nature necessitates specialized equipment and safety measures, significantly increasing initial capital investments. Materials compatible with fluoroantimonic acid, such as PTFE or certain alloys, are often expensive, adding to operational costs.
The precursors for fluoroantimonic acid, particularly antimony pentafluoride, are relatively costly and not widely available. This scarcity can lead to supply chain vulnerabilities and price fluctuations, potentially impacting the long-term economic stability of processes relying on this catalyst.
Environmental and safety regulations surrounding the use of such a potent acid also contribute to increased operational expenses. Stringent waste management protocols and worker safety measures are essential, adding to the overall cost of implementation.
Despite these challenges, the unique catalytic properties of fluoroantimonic acid may justify its use in high-value applications where alternative catalysts fall short. Industries producing specialty chemicals or pharmaceuticals might find the economic trade-offs favorable if the acid enables the synthesis of otherwise difficult-to-produce compounds.
The economic viability also depends on the scale of operation. Large-scale industrial processes might benefit from economies of scale, potentially offsetting some of the high initial and operational costs. Conversely, smaller-scale or research applications may find the economic barriers more challenging to overcome.
Advancements in containment technologies and more efficient synthesis methods for fluoroantimonic acid could improve its economic outlook. Research into recyclable or immobilized forms of the acid may also enhance its long-term economic viability by reducing waste and simplifying handling procedures.
In conclusion, while fluoroantimonic acid catalysis offers promising efficiency gains, its economic viability is currently limited by high costs and practical challenges. Future technological advancements and careful cost-benefit analyses will be crucial in determining its broader adoption across various industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!