Innovative Reaction Techniques with Fluoroantimonic Acid
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Background and Research Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has been a subject of intense scientific interest since its discovery in the mid-20th century. This compound holds the distinction of being the strongest known superacid, with a Hammett acidity function (H0) estimated to be as low as -31.3. Its extreme acidity surpasses that of pure sulfuric acid by more than a trillion times, making it a unique and powerful tool in chemical research and industrial applications.
The development of fluoroantimonic acid can be traced back to the pioneering work of Ronald Gillespie in the 1960s, who extensively studied superacids and their properties. Since then, the field of superacid chemistry has expanded significantly, with fluoroantimonic acid at the forefront of many groundbreaking discoveries and applications.
The primary objective of research into innovative reaction techniques with fluoroantimonic acid is to harness its exceptional proton-donating ability and explore novel chemical transformations that are otherwise impossible or impractical with conventional acids. This superacid's ability to protonate even extremely weak bases opens up new possibilities in organic synthesis, catalysis, and materials science.
One of the key areas of investigation is the use of fluoroantimonic acid in the activation of inert hydrocarbons, particularly in the petroleum industry. Its potential to facilitate the cracking and isomerization of alkanes under milder conditions than traditional methods could lead to more efficient and environmentally friendly processes in oil refining.
Another significant research direction involves the application of fluoroantimonic acid in the synthesis of novel organic compounds. Its ability to generate highly reactive carbocations from a wide range of organic substrates enables unique transformations and the creation of complex molecular structures that are challenging to achieve through conventional methods.
The extreme reactivity of fluoroantimonic acid also presents opportunities in materials science, particularly in the development of super-strong materials and advanced polymers. Researchers are exploring its potential to initiate polymerization reactions under conditions that were previously considered unfeasible, potentially leading to materials with unprecedented properties.
However, the corrosive nature and extreme reactivity of fluoroantimonic acid pose significant challenges in terms of handling, storage, and application. A crucial aspect of ongoing research is the development of safer and more practical methods for utilizing this superacid in both laboratory and industrial settings. This includes the design of specialized containment systems, the exploration of supported or immobilized forms of the acid, and the investigation of novel reaction media that can modulate its reactivity while maintaining its unique properties.
The development of fluoroantimonic acid can be traced back to the pioneering work of Ronald Gillespie in the 1960s, who extensively studied superacids and their properties. Since then, the field of superacid chemistry has expanded significantly, with fluoroantimonic acid at the forefront of many groundbreaking discoveries and applications.
The primary objective of research into innovative reaction techniques with fluoroantimonic acid is to harness its exceptional proton-donating ability and explore novel chemical transformations that are otherwise impossible or impractical with conventional acids. This superacid's ability to protonate even extremely weak bases opens up new possibilities in organic synthesis, catalysis, and materials science.
One of the key areas of investigation is the use of fluoroantimonic acid in the activation of inert hydrocarbons, particularly in the petroleum industry. Its potential to facilitate the cracking and isomerization of alkanes under milder conditions than traditional methods could lead to more efficient and environmentally friendly processes in oil refining.
Another significant research direction involves the application of fluoroantimonic acid in the synthesis of novel organic compounds. Its ability to generate highly reactive carbocations from a wide range of organic substrates enables unique transformations and the creation of complex molecular structures that are challenging to achieve through conventional methods.
The extreme reactivity of fluoroantimonic acid also presents opportunities in materials science, particularly in the development of super-strong materials and advanced polymers. Researchers are exploring its potential to initiate polymerization reactions under conditions that were previously considered unfeasible, potentially leading to materials with unprecedented properties.
However, the corrosive nature and extreme reactivity of fluoroantimonic acid pose significant challenges in terms of handling, storage, and application. A crucial aspect of ongoing research is the development of safer and more practical methods for utilizing this superacid in both laboratory and industrial settings. This includes the design of specialized containment systems, the exploration of supported or immobilized forms of the acid, and the investigation of novel reaction media that can modulate its reactivity while maintaining its unique properties.
Industrial Applications and Market Demand Analysis
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in various industrial sectors due to its unique chemical properties and potential applications. The market demand for innovative reaction techniques utilizing this powerful compound has been steadily growing, driven by the need for more efficient and cost-effective processes in chemical manufacturing, petrochemistry, and materials science.
In the petrochemical industry, fluoroantimonic acid-based techniques have shown promising results in catalytic cracking and isomerization processes. These applications have the potential to revolutionize fuel production by increasing yields and reducing energy consumption. As global energy demands continue to rise, the market for such innovative technologies is expected to expand substantially in the coming years.
The semiconductor industry has also expressed keen interest in fluoroantimonic acid-based etching techniques. With the ever-increasing demand for smaller and more powerful electronic devices, manufacturers are constantly seeking ways to improve the precision and efficiency of their production processes. Fluoroantimonic acid offers unique capabilities in ultra-fine etching and surface modification, potentially enabling the development of next-generation microchips and advanced electronic components.
In the field of materials science, fluoroantimonic acid has demonstrated remarkable potential in the synthesis of novel compounds and materials with exceptional properties. This has sparked interest from various sectors, including aerospace, automotive, and advanced manufacturing. The ability to create materials with enhanced strength, conductivity, or other desirable characteristics could lead to breakthroughs in product design and performance across multiple industries.
The pharmaceutical industry has also begun exploring the use of fluoroantimonic acid in drug synthesis and purification processes. Its strong acidic properties make it an attractive option for certain challenging reactions and separations that are difficult to achieve with conventional methods. As the demand for more complex and targeted therapeutics grows, the market for innovative synthesis techniques is likely to expand.
Environmental concerns and sustainability initiatives have further fueled the demand for more efficient chemical processes. Fluoroantimonic acid-based techniques have the potential to reduce waste generation and energy consumption in various industrial applications, aligning with global efforts to minimize environmental impact. This aspect has attracted attention from both industry players and regulatory bodies, potentially driving further research and development in this field.
While the market potential for innovative reaction techniques with fluoroantimonic acid is significant, it is important to note that safety considerations and handling challenges associated with such a powerful acid may impact its widespread adoption. However, ongoing research into safer handling methods and containment systems is expected to address these concerns and further expand the market opportunities for this technology.
In the petrochemical industry, fluoroantimonic acid-based techniques have shown promising results in catalytic cracking and isomerization processes. These applications have the potential to revolutionize fuel production by increasing yields and reducing energy consumption. As global energy demands continue to rise, the market for such innovative technologies is expected to expand substantially in the coming years.
The semiconductor industry has also expressed keen interest in fluoroantimonic acid-based etching techniques. With the ever-increasing demand for smaller and more powerful electronic devices, manufacturers are constantly seeking ways to improve the precision and efficiency of their production processes. Fluoroantimonic acid offers unique capabilities in ultra-fine etching and surface modification, potentially enabling the development of next-generation microchips and advanced electronic components.
In the field of materials science, fluoroantimonic acid has demonstrated remarkable potential in the synthesis of novel compounds and materials with exceptional properties. This has sparked interest from various sectors, including aerospace, automotive, and advanced manufacturing. The ability to create materials with enhanced strength, conductivity, or other desirable characteristics could lead to breakthroughs in product design and performance across multiple industries.
The pharmaceutical industry has also begun exploring the use of fluoroantimonic acid in drug synthesis and purification processes. Its strong acidic properties make it an attractive option for certain challenging reactions and separations that are difficult to achieve with conventional methods. As the demand for more complex and targeted therapeutics grows, the market for innovative synthesis techniques is likely to expand.
Environmental concerns and sustainability initiatives have further fueled the demand for more efficient chemical processes. Fluoroantimonic acid-based techniques have the potential to reduce waste generation and energy consumption in various industrial applications, aligning with global efforts to minimize environmental impact. This aspect has attracted attention from both industry players and regulatory bodies, potentially driving further research and development in this field.
While the market potential for innovative reaction techniques with fluoroantimonic acid is significant, it is important to note that safety considerations and handling challenges associated with such a powerful acid may impact its widespread adoption. However, ongoing research into safer handling methods and containment systems is expected to address these concerns and further expand the market opportunities for this technology.
Current Challenges in Fluoroantimonic Acid Reactions
Fluoroantimonic acid, known as the world's strongest superacid, presents significant challenges in its handling and application for innovative reaction techniques. The extreme reactivity of this compound, while beneficial for certain chemical processes, poses substantial difficulties in terms of containment, safety, and controlled reactivity.
One of the primary challenges lies in the material compatibility for reaction vessels and equipment. Fluoroantimonic acid rapidly corrodes most conventional materials, including glass and many metals. This necessitates the use of specialized containers made from highly resistant materials such as Teflon or certain alloys, which can significantly increase the cost and complexity of experimental setups.
The extreme corrosiveness of fluoroantimonic acid also presents severe safety hazards for researchers and laboratory personnel. Stringent safety protocols and specialized protective equipment are essential, limiting the accessibility of this reagent in many research environments. The potential for accidental exposure or spills poses significant risks to human health and the environment.
Control and precision in reactions involving fluoroantimonic acid are particularly challenging due to its high reactivity. The acid can initiate unintended side reactions or decompose target molecules, making it difficult to achieve selective transformations. This unpredictability complicates the development of new synthetic methodologies and limits the acid's applicability in fine chemical synthesis.
The extreme moisture sensitivity of fluoroantimonic acid presents another significant hurdle. Even trace amounts of water can lead to violent reactions and the generation of dangerous hydrogen fluoride gas. This necessitates rigorously anhydrous conditions, which are often difficult to maintain consistently in practical laboratory settings.
Scalability remains a major challenge for industrial applications of fluoroantimonic acid-based reactions. The difficulties in handling large quantities of the acid, coupled with safety concerns and material compatibility issues, make it problematic to translate laboratory-scale processes to industrial production.
Environmental concerns also pose significant challenges. The disposal of fluoroantimonic acid and its reaction products requires specialized procedures to prevent environmental contamination. This adds complexity and cost to any process utilizing this superacid, particularly in industrial settings where large volumes may be involved.
Lastly, the limited commercial availability and high cost of fluoroantimonic acid restrict its widespread use in research and development. This economic factor, combined with the technical challenges, often leads researchers to seek alternative reagents or methodologies, potentially limiting innovation in this area of chemistry.
One of the primary challenges lies in the material compatibility for reaction vessels and equipment. Fluoroantimonic acid rapidly corrodes most conventional materials, including glass and many metals. This necessitates the use of specialized containers made from highly resistant materials such as Teflon or certain alloys, which can significantly increase the cost and complexity of experimental setups.
The extreme corrosiveness of fluoroantimonic acid also presents severe safety hazards for researchers and laboratory personnel. Stringent safety protocols and specialized protective equipment are essential, limiting the accessibility of this reagent in many research environments. The potential for accidental exposure or spills poses significant risks to human health and the environment.
Control and precision in reactions involving fluoroantimonic acid are particularly challenging due to its high reactivity. The acid can initiate unintended side reactions or decompose target molecules, making it difficult to achieve selective transformations. This unpredictability complicates the development of new synthetic methodologies and limits the acid's applicability in fine chemical synthesis.
The extreme moisture sensitivity of fluoroantimonic acid presents another significant hurdle. Even trace amounts of water can lead to violent reactions and the generation of dangerous hydrogen fluoride gas. This necessitates rigorously anhydrous conditions, which are often difficult to maintain consistently in practical laboratory settings.
Scalability remains a major challenge for industrial applications of fluoroantimonic acid-based reactions. The difficulties in handling large quantities of the acid, coupled with safety concerns and material compatibility issues, make it problematic to translate laboratory-scale processes to industrial production.
Environmental concerns also pose significant challenges. The disposal of fluoroantimonic acid and its reaction products requires specialized procedures to prevent environmental contamination. This adds complexity and cost to any process utilizing this superacid, particularly in industrial settings where large volumes may be involved.
Lastly, the limited commercial availability and high cost of fluoroantimonic acid restrict its widespread use in research and development. This economic factor, combined with the technical challenges, often leads researchers to seek alternative reagents or methodologies, potentially limiting innovation in this area of chemistry.
Existing Reaction Techniques with Fluoroantimonic Acid
01 Synthesis and production methods
Various methods for synthesizing and producing fluoroantimonic acid are described. These methods may involve the reaction of hydrogen fluoride with antimony pentafluoride or other precursors under specific conditions. The synthesis process often requires careful control of temperature, pressure, and reactant ratios to achieve the desired product.- Synthesis and preparation methods: Various methods for synthesizing and preparing fluoroantimonic acid are described. These methods may involve the reaction of antimony pentafluoride with hydrogen fluoride or other fluorine-containing compounds under specific conditions. The synthesis process often requires careful control of temperature, pressure, and reactant ratios to achieve the desired product.
- Applications in catalysis and chemical reactions: Fluoroantimonic acid is utilized as a powerful catalyst in various chemical reactions due to its strong acidity. It can catalyze alkylation, isomerization, and polymerization reactions. The acid's catalytic properties are particularly useful in the petrochemical industry and in the synthesis of certain organic compounds.
- Use in material science and surface treatments: Fluoroantimonic acid finds applications in material science, particularly in surface treatments of metals and other materials. It can be used for etching, cleaning, or modifying surfaces to enhance their properties or prepare them for further processing. The acid's strong reactivity allows for efficient surface modifications in various industrial processes.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, special safety measures and handling procedures are required when working with fluoroantimonic acid. This includes the use of specialized containment materials, personal protective equipment, and proper disposal methods. Safety protocols and risk assessments are crucial for laboratories and industrial facilities using this compound.
- Analytical and characterization techniques: Various analytical and characterization techniques are employed to study fluoroantimonic acid and its reactions. These may include spectroscopic methods, chromatography, and other advanced analytical tools. Such techniques are essential for understanding the acid's properties, monitoring reactions, and ensuring product quality in research and industrial applications.
02 Applications in catalysis
Fluoroantimonic acid is utilized as a powerful catalyst in various chemical reactions. Its superacidic properties make it effective for promoting reactions such as isomerization, alkylation, and polymerization. The acid's catalytic activity is often superior to other strong acids, enabling more efficient and selective transformations in organic synthesis and industrial processes.Expand Specific Solutions03 Material compatibility and handling
Due to its extreme acidity and reactivity, fluoroantimonic acid requires special handling and storage considerations. Research focuses on developing materials and containers that can withstand its corrosive nature. This includes the use of fluoropolymers, specialized alloys, and protective coatings to ensure safe handling and prevent contamination of the acid.Expand Specific Solutions04 Analytical and characterization techniques
Various analytical methods are employed to characterize fluoroantimonic acid and its derivatives. These techniques may include spectroscopic methods, electrochemical analysis, and advanced chromatography. Researchers develop and optimize these methods to accurately determine the composition, purity, and properties of fluoroantimonic acid samples.Expand Specific Solutions05 Applications in materials processing
Fluoroantimonic acid finds applications in materials processing and surface treatment. Its strong acidic properties are utilized for etching, cleaning, and modifying surfaces of various materials, including metals, semiconductors, and ceramics. The acid's unique properties enable the creation of specific surface structures and the removal of contaminants in specialized manufacturing processes.Expand Specific Solutions
Key Players in Superacid Research and Industry
The field of innovative reaction techniques with fluoroantimonic acid is in its early development stage, with a growing market potential due to its applications in various industries. The global market for superacids, including fluoroantimonic acid, is expanding, driven by increasing demand in petrochemicals, pharmaceuticals, and materials science. Technologically, the field is moderately mature, with ongoing research and development efforts. Key players like Central South University, DAIKIN INDUSTRIES Ltd., and Merck Sharp & Dohme Corp. are actively contributing to advancements. Universities such as The Regents of the University of California and William Marsh Rice University are also significant contributors, indicating a strong academic-industry collaboration in pushing the boundaries of this technology.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN has created a novel fluoroantimonic acid reaction platform leveraging their expertise in fluorine chemistry. Their system employs a dual-chamber reactor design, separating the acid reservoir from the reaction zone with a specialized permeable membrane. This approach allows for controlled acid introduction and precise stoichiometry management. DAIKIN's innovation includes a unique vapor-phase reaction technique, minimizing direct acid contact with reactor surfaces. They have also developed a proprietary quenching system that rapidly neutralizes reaction products, enhancing safety and facilitating easier product isolation.
Strengths: Innovative membrane-based acid control, vapor-phase reaction capability, efficient product quenching system. Weaknesses: May be limited to specific types of reactions, potential challenges in scaling up the membrane-based system.
William Marsh Rice University
Technical Solution: Rice University has developed a novel approach to fluoroantimonic acid reactions using a specialized containment system. Their technique involves a custom-designed reactor with fluoropolymer lining to withstand the extreme corrosiveness of the acid. The process incorporates a cryogenic cooling system to maintain optimal reaction temperatures and a sophisticated pressure regulation mechanism to control the volatile nature of the reactions. Additionally, they have implemented advanced in-situ spectroscopic monitoring to analyze reaction kinetics in real-time, allowing for precise control and optimization of reaction conditions.
Strengths: Exceptional corrosion resistance, precise temperature and pressure control, real-time reaction monitoring. Weaknesses: High cost of specialized equipment, limited scalability for industrial applications.
Breakthrough Innovations in Fluoroantimonic Acid Chemistry
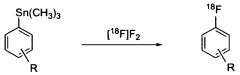
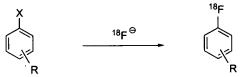
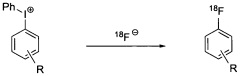
Nucleophilic fluorination of aromatic compounds
PatentWO2010008522A2
Innovation
- A novel nucleophilic radiohalogenation reaction that proceeds without a stable carrier ion, using no-carrier-added [F-18] fluoride ion produced by proton irradiation, which regiospecifically substitutes the iodyl group in iodylbenzene derivatives to produce high-specific-activity F-18 labeled aromatic compounds, enabling efficient fluorination of aromatic rings with various substituents.
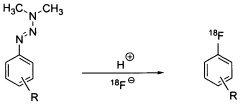
Method for the production of [18f] fluoride-marked aromatic l-amino acids
PatentInactiveEP1663915A1
Innovation
- A process involving nucleophilic substitution of a negatively charged [18F]fluoride ion with a suitable L-enantiomeric compound, followed by cleavage of protective groups, allowing for direct labeling and production of [18F]fluoro-L-phenylalanine derivatives in few steps, eliminating the need for enantiomer separation and achieving high stereochemical purity.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant safety and environmental challenges that must be carefully addressed in any innovative reaction techniques. The extreme corrosiveness and reactivity of this compound necessitate stringent safety protocols and specialized handling equipment. Personal protective equipment (PPE) for workers must include fully encapsulating chemical-resistant suits, gloves, and respiratory protection to prevent any exposure.
Containment systems for fluoroantimonic acid reactions require materials that can withstand its highly corrosive nature, such as fluoropolymers or specially treated metals. Double containment and leak detection systems are essential to prevent accidental releases. Ventilation systems must be designed to capture and neutralize any vapors or fumes generated during reactions.
Environmental considerations are paramount when working with fluoroantimonic acid. Its potential for severe environmental damage if released necessitates comprehensive spill prevention and response plans. Waste management protocols must be developed to safely neutralize and dispose of any acid-containing materials. Water-free environments are crucial, as the acid reacts violently with water, potentially leading to explosive situations.
The use of fluoroantimonic acid also raises concerns about the production and handling of its precursors, particularly hydrogen fluoride and antimony pentafluoride. These compounds themselves pose significant safety and environmental risks, requiring careful management throughout the supply chain.
Long-term environmental impacts of fluoroantimonic acid production and use must be assessed, including potential effects on air and water quality, soil contamination, and ecosystem health. Lifecycle analysis of reaction processes involving this superacid should be conducted to identify opportunities for minimizing environmental footprint and improving sustainability.
Regulatory compliance is a critical aspect of working with fluoroantimonic acid. Researchers and industries must navigate complex hazardous material regulations, emissions standards, and workplace safety requirements. Continuous monitoring and documentation of safety practices and environmental performance are essential for maintaining regulatory compliance and public trust.
Containment systems for fluoroantimonic acid reactions require materials that can withstand its highly corrosive nature, such as fluoropolymers or specially treated metals. Double containment and leak detection systems are essential to prevent accidental releases. Ventilation systems must be designed to capture and neutralize any vapors or fumes generated during reactions.
Environmental considerations are paramount when working with fluoroantimonic acid. Its potential for severe environmental damage if released necessitates comprehensive spill prevention and response plans. Waste management protocols must be developed to safely neutralize and dispose of any acid-containing materials. Water-free environments are crucial, as the acid reacts violently with water, potentially leading to explosive situations.
The use of fluoroantimonic acid also raises concerns about the production and handling of its precursors, particularly hydrogen fluoride and antimony pentafluoride. These compounds themselves pose significant safety and environmental risks, requiring careful management throughout the supply chain.
Long-term environmental impacts of fluoroantimonic acid production and use must be assessed, including potential effects on air and water quality, soil contamination, and ecosystem health. Lifecycle analysis of reaction processes involving this superacid should be conducted to identify opportunities for minimizing environmental footprint and improving sustainability.
Regulatory compliance is a critical aspect of working with fluoroantimonic acid. Researchers and industries must navigate complex hazardous material regulations, emissions standards, and workplace safety requirements. Continuous monitoring and documentation of safety practices and environmental performance are essential for maintaining regulatory compliance and public trust.
Economic Feasibility of Fluoroantimonic Acid Processes
The economic feasibility of fluoroantimonic acid processes is a critical consideration for industrial applications. Fluoroantimonic acid, known as the world's strongest superacid, offers unique catalytic properties that can potentially revolutionize certain chemical reactions. However, its implementation on an industrial scale faces significant economic challenges.
The production costs of fluoroantimonic acid are substantial, primarily due to the expensive raw materials required, such as antimony pentafluoride and hydrogen fluoride. These precursors are not only costly but also hazardous, necessitating specialized handling and storage facilities. The synthesis process itself demands stringent safety measures and sophisticated equipment, further increasing capital expenditures.
Operational expenses for fluoroantimonic acid processes are equally demanding. The extreme corrosiveness of the acid necessitates the use of highly resistant materials, such as fluoropolymers or specially treated alloys, for all equipment in contact with the acid. This requirement significantly raises maintenance and replacement costs. Additionally, the acid's sensitivity to moisture requires strictly controlled, anhydrous conditions, adding to the complexity and cost of process control systems.
Despite these challenges, the economic potential of fluoroantimonic acid processes lies in their ability to catalyze reactions that are otherwise difficult or impossible to achieve. This capability can lead to the production of high-value chemicals or enable more efficient synthetic routes for existing products. For instance, in the petrochemical industry, fluoroantimonic acid catalysts could potentially improve the efficiency of alkylation processes, leading to higher-quality fuels.
The economic viability of these processes also depends on scale. While small-scale laboratory use may be prohibitively expensive, large-scale industrial applications could potentially benefit from economies of scale, reducing per-unit costs. However, achieving such scale requires significant initial investment and carries substantial risk.
Environmental and regulatory factors play a crucial role in the economic assessment. The hazardous nature of fluoroantimonic acid necessitates extensive safety measures and waste management protocols, which add to operational costs. Compliance with increasingly stringent environmental regulations may require additional investments in containment and treatment systems.
In conclusion, while fluoroantimonic acid processes offer unique chemical capabilities, their economic feasibility remains challenging. The high costs associated with production, handling, and safety measures must be carefully weighed against the potential value of the products or process improvements. Future advancements in materials science and process engineering may improve the economic outlook, but current applications are likely to be limited to high-value, specialized chemical processes where alternative methods are not viable.
The production costs of fluoroantimonic acid are substantial, primarily due to the expensive raw materials required, such as antimony pentafluoride and hydrogen fluoride. These precursors are not only costly but also hazardous, necessitating specialized handling and storage facilities. The synthesis process itself demands stringent safety measures and sophisticated equipment, further increasing capital expenditures.
Operational expenses for fluoroantimonic acid processes are equally demanding. The extreme corrosiveness of the acid necessitates the use of highly resistant materials, such as fluoropolymers or specially treated alloys, for all equipment in contact with the acid. This requirement significantly raises maintenance and replacement costs. Additionally, the acid's sensitivity to moisture requires strictly controlled, anhydrous conditions, adding to the complexity and cost of process control systems.
Despite these challenges, the economic potential of fluoroantimonic acid processes lies in their ability to catalyze reactions that are otherwise difficult or impossible to achieve. This capability can lead to the production of high-value chemicals or enable more efficient synthetic routes for existing products. For instance, in the petrochemical industry, fluoroantimonic acid catalysts could potentially improve the efficiency of alkylation processes, leading to higher-quality fuels.
The economic viability of these processes also depends on scale. While small-scale laboratory use may be prohibitively expensive, large-scale industrial applications could potentially benefit from economies of scale, reducing per-unit costs. However, achieving such scale requires significant initial investment and carries substantial risk.
Environmental and regulatory factors play a crucial role in the economic assessment. The hazardous nature of fluoroantimonic acid necessitates extensive safety measures and waste management protocols, which add to operational costs. Compliance with increasingly stringent environmental regulations may require additional investments in containment and treatment systems.
In conclusion, while fluoroantimonic acid processes offer unique chemical capabilities, their economic feasibility remains challenging. The high costs associated with production, handling, and safety measures must be carefully weighed against the potential value of the products or process improvements. Future advancements in materials science and process engineering may improve the economic outlook, but current applications are likely to be limited to high-value, specialized chemical processes where alternative methods are not viable.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!