Transformative Effects of Fluoroantimonic Acid in Chemistry
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Evolution and Research Objectives
Fluoroantimonic acid, a superacid with extraordinary chemical properties, has been a subject of intense research and development in the field of chemistry for several decades. Its evolution as a powerful chemical agent and the objectives of ongoing research reflect the continuous pursuit of understanding and harnessing its unique capabilities.
The journey of fluoroantimonic acid began in the mid-20th century when chemists started exploring superacids. The breakthrough came in 1967 when George A. Olah synthesized fluoroantimonic acid, marking a significant milestone in superacid chemistry. This discovery opened up new possibilities in organic synthesis and catalysis, prompting researchers to delve deeper into its potential applications.
Throughout the 1970s and 1980s, the focus was primarily on understanding the fundamental properties of fluoroantimonic acid. Scientists investigated its structure, reactivity, and behavior under various conditions. This period was crucial in establishing the theoretical foundation for its future applications.
The 1990s saw a shift towards exploring practical applications of fluoroantimonic acid. Researchers began to investigate its potential in industrial processes, particularly in the petrochemical industry. Its ability to catalyze reactions that were previously difficult or impossible became a key area of interest.
In the early 2000s, the research objectives expanded to include environmental considerations. As awareness of green chemistry grew, scientists started exploring ways to use fluoroantimonic acid more efficiently and with minimal environmental impact. This led to studies on containment methods, recovery processes, and alternative reaction media.
Current research objectives focus on several key areas. One primary goal is to enhance the stability and handling of fluoroantimonic acid, making it safer and more practical for large-scale industrial use. Another objective is to expand its application in organic synthesis, particularly in the creation of novel pharmaceutical compounds and advanced materials.
Researchers are also exploring the potential of fluoroantimonic acid in nanotechnology. Its extreme acidity and unique chemical properties make it a promising candidate for manipulating materials at the nanoscale, potentially leading to breakthroughs in electronics and materials science.
Looking ahead, the future research objectives for fluoroantimonic acid are likely to include developing more sustainable production methods, investigating its role in emerging fields such as quantum chemistry, and exploring its potential in solving complex chemical challenges in areas like energy storage and environmental remediation.
The journey of fluoroantimonic acid began in the mid-20th century when chemists started exploring superacids. The breakthrough came in 1967 when George A. Olah synthesized fluoroantimonic acid, marking a significant milestone in superacid chemistry. This discovery opened up new possibilities in organic synthesis and catalysis, prompting researchers to delve deeper into its potential applications.
Throughout the 1970s and 1980s, the focus was primarily on understanding the fundamental properties of fluoroantimonic acid. Scientists investigated its structure, reactivity, and behavior under various conditions. This period was crucial in establishing the theoretical foundation for its future applications.
The 1990s saw a shift towards exploring practical applications of fluoroantimonic acid. Researchers began to investigate its potential in industrial processes, particularly in the petrochemical industry. Its ability to catalyze reactions that were previously difficult or impossible became a key area of interest.
In the early 2000s, the research objectives expanded to include environmental considerations. As awareness of green chemistry grew, scientists started exploring ways to use fluoroantimonic acid more efficiently and with minimal environmental impact. This led to studies on containment methods, recovery processes, and alternative reaction media.
Current research objectives focus on several key areas. One primary goal is to enhance the stability and handling of fluoroantimonic acid, making it safer and more practical for large-scale industrial use. Another objective is to expand its application in organic synthesis, particularly in the creation of novel pharmaceutical compounds and advanced materials.
Researchers are also exploring the potential of fluoroantimonic acid in nanotechnology. Its extreme acidity and unique chemical properties make it a promising candidate for manipulating materials at the nanoscale, potentially leading to breakthroughs in electronics and materials science.
Looking ahead, the future research objectives for fluoroantimonic acid are likely to include developing more sustainable production methods, investigating its role in emerging fields such as quantum chemistry, and exploring its potential in solving complex chemical challenges in areas like energy storage and environmental remediation.
Industrial Applications and Market Potential
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in the chemical industry due to its exceptional proton-donating ability and extreme reactivity. This powerful compound has the potential to revolutionize various industrial processes and open up new avenues for chemical synthesis and material production.
In the petrochemical industry, fluoroantimonic acid shows promise as a highly efficient catalyst for hydrocarbon cracking and isomerization reactions. Its ability to protonate even weak bases makes it an ideal candidate for enhancing the production of high-octane gasoline components and valuable chemical intermediates. The increased yield and selectivity offered by this superacid could lead to substantial cost savings and improved process efficiency for refineries and petrochemical plants.
The electronics sector is another area where fluoroantimonic acid could make a significant impact. Its extreme acidity and unique chemical properties make it suitable for etching and cleaning semiconductor materials, potentially improving the manufacturing processes of microchips and other electronic components. As the demand for smaller and more powerful electronic devices continues to grow, the market for advanced etching agents like fluoroantimonic acid is expected to expand.
In the field of materials science, fluoroantimonic acid's ability to generate highly reactive carbocations opens up possibilities for synthesizing novel polymers and advanced materials. This could lead to the development of stronger, more durable, or functionally enhanced materials for applications in aerospace, automotive, and construction industries.
The pharmaceutical industry may also benefit from the unique properties of fluoroantimonic acid. Its potential use in the synthesis of complex organic molecules could streamline drug manufacturing processes, potentially reducing production costs and accelerating the development of new therapeutic compounds.
However, the market potential of fluoroantimonic acid is tempered by significant challenges. Its extreme corrosiveness and reactivity necessitate specialized handling and storage equipment, which may limit its widespread adoption. Additionally, safety concerns and environmental regulations pose hurdles to its large-scale industrial use.
Despite these challenges, the global market for superacids, including fluoroantimonic acid, is projected to grow as industries seek more efficient and powerful chemical tools. Research and development efforts are likely to focus on developing safer handling methods and exploring new applications, which could further expand the market potential of this remarkable compound.
As industries continue to push the boundaries of chemical processes and material properties, fluoroantimonic acid stands poised to play a transformative role in various sectors. Its unique capabilities may drive innovation in chemical synthesis, catalysis, and materials engineering, potentially reshaping industrial landscapes and opening up new market opportunities in the coming years.
In the petrochemical industry, fluoroantimonic acid shows promise as a highly efficient catalyst for hydrocarbon cracking and isomerization reactions. Its ability to protonate even weak bases makes it an ideal candidate for enhancing the production of high-octane gasoline components and valuable chemical intermediates. The increased yield and selectivity offered by this superacid could lead to substantial cost savings and improved process efficiency for refineries and petrochemical plants.
The electronics sector is another area where fluoroantimonic acid could make a significant impact. Its extreme acidity and unique chemical properties make it suitable for etching and cleaning semiconductor materials, potentially improving the manufacturing processes of microchips and other electronic components. As the demand for smaller and more powerful electronic devices continues to grow, the market for advanced etching agents like fluoroantimonic acid is expected to expand.
In the field of materials science, fluoroantimonic acid's ability to generate highly reactive carbocations opens up possibilities for synthesizing novel polymers and advanced materials. This could lead to the development of stronger, more durable, or functionally enhanced materials for applications in aerospace, automotive, and construction industries.
The pharmaceutical industry may also benefit from the unique properties of fluoroantimonic acid. Its potential use in the synthesis of complex organic molecules could streamline drug manufacturing processes, potentially reducing production costs and accelerating the development of new therapeutic compounds.
However, the market potential of fluoroantimonic acid is tempered by significant challenges. Its extreme corrosiveness and reactivity necessitate specialized handling and storage equipment, which may limit its widespread adoption. Additionally, safety concerns and environmental regulations pose hurdles to its large-scale industrial use.
Despite these challenges, the global market for superacids, including fluoroantimonic acid, is projected to grow as industries seek more efficient and powerful chemical tools. Research and development efforts are likely to focus on developing safer handling methods and exploring new applications, which could further expand the market potential of this remarkable compound.
As industries continue to push the boundaries of chemical processes and material properties, fluoroantimonic acid stands poised to play a transformative role in various sectors. Its unique capabilities may drive innovation in chemical synthesis, catalysis, and materials engineering, potentially reshaping industrial landscapes and opening up new market opportunities in the coming years.
Current Challenges in Superacid Chemistry
Superacid chemistry, particularly in the realm of fluoroantimonic acid, faces several significant challenges that hinder its full potential in transformative chemical processes. One of the primary obstacles is the extreme reactivity and corrosiveness of superacids, which pose substantial safety risks and handling difficulties. This necessitates specialized equipment and rigorous safety protocols, limiting widespread adoption in industrial settings.
The stability of superacids under various conditions remains a critical issue. Fluoroantimonic acid, being highly hygroscopic, readily reacts with moisture in the air, making storage and long-term use problematic. This instability not only affects the acid's effectiveness but also complicates experimental reproducibility and scalability of reactions involving superacids.
Another challenge lies in the limited substrate scope for superacid-mediated reactions. While fluoroantimonic acid exhibits exceptional catalytic activity, its extreme acidity can lead to undesired side reactions or decomposition of sensitive organic compounds. This restricts its applicability in certain areas of synthetic chemistry, particularly in the synthesis of complex natural products or pharmaceuticals.
The environmental impact of superacids, especially fluoroantimonic acid, is a growing concern. The production and disposal of these highly corrosive substances pose significant environmental risks, necessitating the development of more sustainable and eco-friendly alternatives or improved containment and recycling methods.
Mechanistic understanding of superacid-mediated reactions remains incomplete, particularly in complex systems. The extreme acidity of fluoroantimonic acid often leads to rapid and difficult-to-characterize intermediates, making it challenging to elucidate reaction pathways and design more efficient processes.
Scale-up and industrial application of superacid chemistry present formidable engineering challenges. The corrosive nature of fluoroantimonic acid requires specialized reactor designs and materials, which can be prohibitively expensive for large-scale operations. Additionally, ensuring uniform mixing and temperature control in larger reactors becomes increasingly difficult due to the highly exothermic nature of many superacid-catalyzed reactions.
Lastly, the development of novel superacid systems with tunable properties and enhanced selectivity remains an ongoing challenge. While fluoroantimonic acid is exceptionally strong, there is a need for superacids that can offer similar reactivity with improved stability, easier handling, and greater functional group tolerance. This would significantly expand the utility of superacid chemistry across various domains of chemical synthesis and materials science.
The stability of superacids under various conditions remains a critical issue. Fluoroantimonic acid, being highly hygroscopic, readily reacts with moisture in the air, making storage and long-term use problematic. This instability not only affects the acid's effectiveness but also complicates experimental reproducibility and scalability of reactions involving superacids.
Another challenge lies in the limited substrate scope for superacid-mediated reactions. While fluoroantimonic acid exhibits exceptional catalytic activity, its extreme acidity can lead to undesired side reactions or decomposition of sensitive organic compounds. This restricts its applicability in certain areas of synthetic chemistry, particularly in the synthesis of complex natural products or pharmaceuticals.
The environmental impact of superacids, especially fluoroantimonic acid, is a growing concern. The production and disposal of these highly corrosive substances pose significant environmental risks, necessitating the development of more sustainable and eco-friendly alternatives or improved containment and recycling methods.
Mechanistic understanding of superacid-mediated reactions remains incomplete, particularly in complex systems. The extreme acidity of fluoroantimonic acid often leads to rapid and difficult-to-characterize intermediates, making it challenging to elucidate reaction pathways and design more efficient processes.
Scale-up and industrial application of superacid chemistry present formidable engineering challenges. The corrosive nature of fluoroantimonic acid requires specialized reactor designs and materials, which can be prohibitively expensive for large-scale operations. Additionally, ensuring uniform mixing and temperature control in larger reactors becomes increasingly difficult due to the highly exothermic nature of many superacid-catalyzed reactions.
Lastly, the development of novel superacid systems with tunable properties and enhanced selectivity remains an ongoing challenge. While fluoroantimonic acid is exceptionally strong, there is a need for superacids that can offer similar reactivity with improved stability, easier handling, and greater functional group tolerance. This would significantly expand the utility of superacid chemistry across various domains of chemical synthesis and materials science.
Synthesis and Handling Techniques
01 Chemical transformations and reactions
Fluoroantimonic acid, being a superacid, can catalyze various chemical transformations and reactions. Its extreme acidity enables it to protonate even very weak bases, leading to unique reaction pathways and the formation of novel compounds. This property makes it valuable in organic synthesis and petrochemical processes.- Chemical transformations and reactions: Fluoroantimonic acid, being a superacid, can catalyze various chemical transformations and reactions. Its extreme acidity enables it to protonate even very weak bases, leading to unique reaction pathways and the formation of novel compounds. This property makes it valuable in organic synthesis and industrial processes where strong acid catalysts are required.
- Material modification and surface treatment: The transformative effects of fluoroantimonic acid extend to material modification and surface treatment applications. Its strong acidic nature can be utilized to etch or modify surfaces of various materials, including metals and semiconductors. This property is particularly useful in the manufacturing of electronic components and in the preparation of specialized materials with enhanced properties.
- Energy storage and conversion: Fluoroantimonic acid has potential applications in energy storage and conversion technologies. Its unique properties can be exploited in the development of advanced battery systems, fuel cells, and other energy-related devices. The acid's ability to facilitate certain electrochemical reactions makes it a subject of interest in research aimed at improving energy efficiency and storage capacity.
- Analytical and detection methods: The extreme acidity of fluoroantimonic acid can be utilized in analytical chemistry and detection methods. Its ability to protonate a wide range of compounds makes it useful in spectroscopic techniques and chemical analysis. This property can be exploited in the development of sensitive detection methods for various substances, including trace contaminants and chemical warfare agents.
- Environmental and safety considerations: While fluoroantimonic acid has many potential applications, its transformative effects also necessitate careful consideration of environmental and safety aspects. The extreme reactivity of the acid requires specialized handling procedures and safety measures. Research in this area focuses on developing safer methods for using and storing the acid, as well as exploring less hazardous alternatives that can provide similar transformative effects in various applications.
02 Material modification and surface treatment
The transformative effects of fluoroantimonic acid extend to material modification and surface treatment. It can be used to etch or modify surfaces of various materials, including metals and semiconductors. This property is particularly useful in the manufacturing of electronic components and in the development of advanced materials with specific surface properties.Expand Specific Solutions03 Energy storage and conversion applications
Fluoroantimonic acid has potential applications in energy storage and conversion technologies. Its unique properties can be exploited in the development of advanced battery systems, fuel cells, and other energy-related devices. The acid's ability to facilitate certain electrochemical reactions makes it a subject of interest in renewable energy research.Expand Specific Solutions04 Analytical and characterization techniques
The transformative effects of fluoroantimonic acid can be utilized in analytical and characterization techniques. Its strong protonating ability allows for the study of molecular structures and reaction mechanisms that are difficult to observe with conventional acids. This makes it valuable in spectroscopic studies and in the development of new analytical methods.Expand Specific Solutions05 Environmental and safety considerations
While fluoroantimonic acid has many transformative effects, its extreme reactivity also poses significant environmental and safety challenges. Research into containment, handling, and disposal methods is crucial. Understanding these aspects is important for the responsible use of this superacid in industrial and research settings, and for developing safer alternatives where possible.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Research
The field of fluoroantimonic acid in chemistry is in a nascent stage of development, with significant potential for growth. The market size is relatively small but expanding, driven by increasing research and industrial applications. Technologically, it's still in the early stages of maturity, with ongoing research at prestigious institutions like California Institute of Technology, Massachusetts Institute of Technology, and Case Western Reserve University. Companies such as 3M Innovative Properties Co. and DuPont de Nemours, Inc. are also actively involved in advancing this technology. The competitive landscape is characterized by a mix of academic institutions and industrial players, each contributing to the development of novel applications and improved synthesis methods for fluoroantimonic acid.
California Institute of Technology
Technical Solution: Caltech has been at the forefront of research on fluoroantimonic acid, focusing on its applications in organic synthesis and catalysis. Their approach involves using fluoroantimonic acid as a superacid catalyst for various chemical transformations, including isomerization, alkylation, and polymerization reactions. They have developed specialized containment and handling techniques to work with this highly corrosive substance, enabling them to explore its full potential in chemical synthesis. Caltech's research also extends to studying the acid's unique protonating abilities and its potential in generating novel reactive intermediates.
Strengths: Cutting-edge research facilities, expertise in handling superacids, strong focus on fundamental chemical principles. Weaknesses: Limited industrial-scale application experience, potential safety concerns due to the extreme reactivity of fluoroantimonic acid.
Massachusetts Institute of Technology
Technical Solution: MIT's research on fluoroantimonic acid focuses on its potential in materials science and nanotechnology. They have developed methods to use the acid for surface etching and modification of various materials, including semiconductors and metals. MIT researchers have also explored the acid's potential in creating novel nanostructures and thin films. Their approach involves precise control of acid concentration and exposure time to achieve desired surface properties. Additionally, MIT has investigated the use of fluoroantimonic acid in the synthesis of new classes of materials with unique electronic and optical properties.
Strengths: Interdisciplinary approach combining chemistry, materials science, and engineering; state-of-the-art characterization techniques. Weaknesses: High cost of research due to specialized equipment requirements; challenges in scaling up processes for industrial applications.
Breakthrough Reactions Enabled by Fluoroantimonic Acid
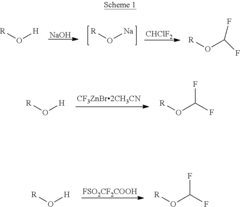
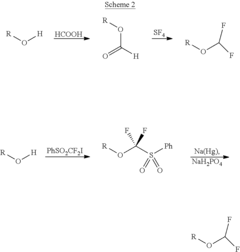
Process for the synthesis of difluoromethyl ether-based compounds
PatentInactiveUS20180016227A1
Innovation
- A commercially viable process involving the reaction of alcohols with a Vilsmeier reagent followed by a sulfurating agent to form thioformyl esters, which are then converted with 2,2-difluoro-1,3-dimethylimidazolidine to produce difluoromethyl ethers, offering high yield and purity.
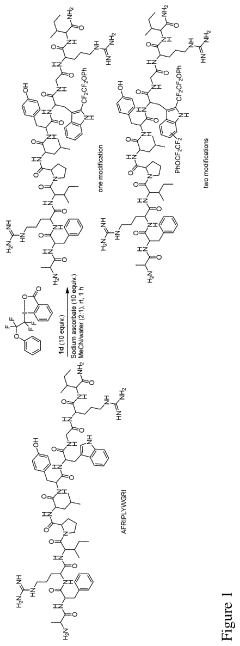
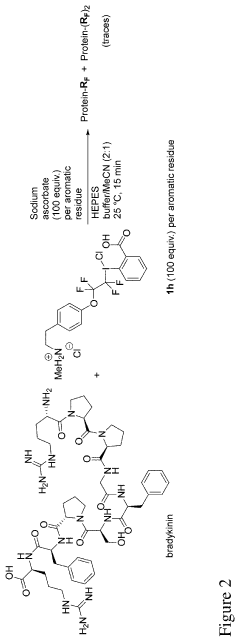
A method for functionalization of an aromatic amino acid or a nucleobase
PatentActiveUS20220177514A1
Innovation
- A transition metal-free method using hypervalent iodine fluoroalkyl reagents with reductants to rapidly functionalize aromatic amino acids and nucleobases, forming fluoroalkylating radicals that selectively react with target residues, enabling fast and versatile bioconjugation.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant safety and environmental challenges that demand careful consideration in its handling and application. The extreme corrosiveness and reactivity of this compound necessitate stringent safety protocols to protect personnel and equipment. Specialized containment systems, typically constructed from materials like polytetrafluoroethylene (PTFE) or fluorinated ethylene propylene (FEP), are essential to prevent catastrophic reactions with common substances.
Personal protective equipment (PPE) for handling fluoroantimonic acid must be of the highest grade, including fully encapsulating chemical-resistant suits, multiple layers of gloves, and self-contained breathing apparatus. Emergency response plans and decontamination procedures must be meticulously designed and regularly practiced to mitigate potential incidents.
The environmental impact of fluoroantimonic acid is a critical concern. Its ability to react violently with water and organic matter poses severe risks to ecosystems if released. Proper disposal methods are crucial, often involving neutralization with carefully selected bases under controlled conditions. The potential for atmospheric contamination through vapors or aerosols necessitates advanced air filtration and scrubbing systems in facilities where it is used or produced.
Long-term environmental effects of fluoroantimonic acid exposure are not fully understood, highlighting the need for ongoing research into its ecological impact. Bioaccumulation and persistence in the environment are areas of particular concern, given the compound's extreme reactivity and potential for widespread contamination.
Regulatory frameworks governing the use, transport, and disposal of fluoroantimonic acid vary globally but are generally stringent. Compliance with these regulations requires comprehensive documentation, specialized training for personnel, and regular audits. The development of safer alternatives or process modifications to reduce reliance on fluoroantimonic acid is an active area of research, driven by both safety concerns and environmental sustainability goals.
In industrial applications, the implementation of closed-loop systems and rigorous containment strategies is essential to minimize the risk of environmental release. Continuous monitoring of air and water quality in and around facilities using fluoroantimonic acid is crucial for early detection of potential leaks or contamination events.
Personal protective equipment (PPE) for handling fluoroantimonic acid must be of the highest grade, including fully encapsulating chemical-resistant suits, multiple layers of gloves, and self-contained breathing apparatus. Emergency response plans and decontamination procedures must be meticulously designed and regularly practiced to mitigate potential incidents.
The environmental impact of fluoroantimonic acid is a critical concern. Its ability to react violently with water and organic matter poses severe risks to ecosystems if released. Proper disposal methods are crucial, often involving neutralization with carefully selected bases under controlled conditions. The potential for atmospheric contamination through vapors or aerosols necessitates advanced air filtration and scrubbing systems in facilities where it is used or produced.
Long-term environmental effects of fluoroantimonic acid exposure are not fully understood, highlighting the need for ongoing research into its ecological impact. Bioaccumulation and persistence in the environment are areas of particular concern, given the compound's extreme reactivity and potential for widespread contamination.
Regulatory frameworks governing the use, transport, and disposal of fluoroantimonic acid vary globally but are generally stringent. Compliance with these regulations requires comprehensive documentation, specialized training for personnel, and regular audits. The development of safer alternatives or process modifications to reduce reliance on fluoroantimonic acid is an active area of research, driven by both safety concerns and environmental sustainability goals.
In industrial applications, the implementation of closed-loop systems and rigorous containment strategies is essential to minimize the risk of environmental release. Continuous monitoring of air and water quality in and around facilities using fluoroantimonic acid is crucial for early detection of potential leaks or contamination events.
Regulatory Framework for Superacid Usage
The regulatory framework for superacid usage, particularly concerning fluoroantimonic acid, is a critical aspect of its application in chemistry. Given the extreme reactivity and potential hazards associated with this powerful superacid, stringent regulations have been established to ensure safe handling, storage, and disposal.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a foundation for the classification and communication of chemical hazards. Under this system, fluoroantimonic acid is classified as a highly corrosive substance, requiring specific labeling and safety data sheets.
In the United States, the Occupational Safety and Health Administration (OSHA) has set strict guidelines for the use of superacids in industrial and research settings. These regulations mandate the use of appropriate personal protective equipment (PPE), including chemical-resistant suits, gloves, and respiratory protection. Additionally, the Environmental Protection Agency (EPA) regulates the disposal of superacids under the Resource Conservation and Recovery Act (RCRA), classifying them as hazardous waste.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes rigorous requirements on the registration and assessment of chemicals, including superacids. Manufacturers and importers must provide detailed safety information and risk assessments before these substances can be used within the EU.
In academic and research environments, institutional review boards and safety committees play a crucial role in overseeing the use of fluoroantimonic acid. These bodies typically require detailed experimental protocols, risk assessments, and emergency response plans before approving research involving superacids.
Transportation of fluoroantimonic acid is subject to strict regulations under the International Air Transport Association (IATA) Dangerous Goods Regulations and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify packaging requirements, labeling standards, and documentation necessary for the safe transport of superacids.
Given the potential for misuse, many countries have implemented controlled substance regulations that restrict access to superacids. These regulations often require special licenses or permits for purchase and use, with detailed record-keeping requirements to track the movement and application of these substances.
As research into the transformative effects of fluoroantimonic acid in chemistry continues to advance, regulatory frameworks are likely to evolve. Ongoing dialogue between researchers, industry representatives, and regulatory bodies is essential to ensure that safety standards keep pace with scientific developments while facilitating responsible innovation in this field.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a foundation for the classification and communication of chemical hazards. Under this system, fluoroantimonic acid is classified as a highly corrosive substance, requiring specific labeling and safety data sheets.
In the United States, the Occupational Safety and Health Administration (OSHA) has set strict guidelines for the use of superacids in industrial and research settings. These regulations mandate the use of appropriate personal protective equipment (PPE), including chemical-resistant suits, gloves, and respiratory protection. Additionally, the Environmental Protection Agency (EPA) regulates the disposal of superacids under the Resource Conservation and Recovery Act (RCRA), classifying them as hazardous waste.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes rigorous requirements on the registration and assessment of chemicals, including superacids. Manufacturers and importers must provide detailed safety information and risk assessments before these substances can be used within the EU.
In academic and research environments, institutional review boards and safety committees play a crucial role in overseeing the use of fluoroantimonic acid. These bodies typically require detailed experimental protocols, risk assessments, and emergency response plans before approving research involving superacids.
Transportation of fluoroantimonic acid is subject to strict regulations under the International Air Transport Association (IATA) Dangerous Goods Regulations and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify packaging requirements, labeling standards, and documentation necessary for the safe transport of superacids.
Given the potential for misuse, many countries have implemented controlled substance regulations that restrict access to superacids. These regulations often require special licenses or permits for purchase and use, with detailed record-keeping requirements to track the movement and application of these substances.
As research into the transformative effects of fluoroantimonic acid in chemistry continues to advance, regulatory frameworks are likely to evolve. Ongoing dialogue between researchers, industry representatives, and regulatory bodies is essential to ensure that safety standards keep pace with scientific developments while facilitating responsible innovation in this field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!