How to Propel Catalytic Science with Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Catalysis Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has emerged as a powerful catalyst in organic synthesis and petrochemical processes. Its exceptional acidity, surpassing that of 100% sulfuric acid by over a trillion times, has revolutionized our understanding of acid-catalyzed reactions and opened new avenues for chemical transformations.
The development of fluoroantimonic acid catalysis can be traced back to the mid-20th century, with significant advancements in the field of superacid chemistry. Pioneering work by chemists such as George A. Olah and Ronald Gillespie laid the foundation for understanding the unique properties and potential applications of superacids, including fluoroantimonic acid.
As research in this area progressed, the catalytic potential of fluoroantimonic acid became increasingly apparent. Its ability to protonate even weak bases and activate traditionally inert compounds has made it an invaluable tool in various chemical processes, particularly in the petroleum industry for isomerization and alkylation reactions.
The primary objective in propelling catalytic science with fluoroantimonic acid is to harness its exceptional acidity for more efficient and selective chemical transformations. Researchers aim to develop novel synthetic methodologies, improve existing industrial processes, and explore new reaction pathways that were previously inaccessible with conventional acid catalysts.
One of the key goals is to expand the scope of fluoroantimonic acid catalysis beyond its current applications. This includes investigating its potential in fine chemical synthesis, pharmaceutical production, and materials science. By leveraging its unique properties, scientists hope to enable the formation of complex molecular structures and facilitate challenging chemical transformations with improved yields and selectivity.
Another important objective is to address the limitations and challenges associated with fluoroantimonic acid catalysis. These include its extreme corrosiveness, sensitivity to moisture, and the need for specialized handling techniques. Developing more stable and user-friendly formulations of fluoroantimonic acid, or designing novel reaction systems that can harness its catalytic power while mitigating its drawbacks, are crucial areas of research.
Furthermore, understanding the fundamental mechanisms of fluoroantimonic acid catalysis at the molecular level remains a significant goal. Advanced spectroscopic techniques and computational studies are being employed to elucidate the nature of reactive intermediates and transition states in fluoroantimonic acid-catalyzed reactions. This knowledge is essential for rational catalyst design and the development of more efficient and selective catalytic systems.
The development of fluoroantimonic acid catalysis can be traced back to the mid-20th century, with significant advancements in the field of superacid chemistry. Pioneering work by chemists such as George A. Olah and Ronald Gillespie laid the foundation for understanding the unique properties and potential applications of superacids, including fluoroantimonic acid.
As research in this area progressed, the catalytic potential of fluoroantimonic acid became increasingly apparent. Its ability to protonate even weak bases and activate traditionally inert compounds has made it an invaluable tool in various chemical processes, particularly in the petroleum industry for isomerization and alkylation reactions.
The primary objective in propelling catalytic science with fluoroantimonic acid is to harness its exceptional acidity for more efficient and selective chemical transformations. Researchers aim to develop novel synthetic methodologies, improve existing industrial processes, and explore new reaction pathways that were previously inaccessible with conventional acid catalysts.
One of the key goals is to expand the scope of fluoroantimonic acid catalysis beyond its current applications. This includes investigating its potential in fine chemical synthesis, pharmaceutical production, and materials science. By leveraging its unique properties, scientists hope to enable the formation of complex molecular structures and facilitate challenging chemical transformations with improved yields and selectivity.
Another important objective is to address the limitations and challenges associated with fluoroantimonic acid catalysis. These include its extreme corrosiveness, sensitivity to moisture, and the need for specialized handling techniques. Developing more stable and user-friendly formulations of fluoroantimonic acid, or designing novel reaction systems that can harness its catalytic power while mitigating its drawbacks, are crucial areas of research.
Furthermore, understanding the fundamental mechanisms of fluoroantimonic acid catalysis at the molecular level remains a significant goal. Advanced spectroscopic techniques and computational studies are being employed to elucidate the nature of reactive intermediates and transition states in fluoroantimonic acid-catalyzed reactions. This knowledge is essential for rational catalyst design and the development of more efficient and selective catalytic systems.
Industrial Demand for Superacid Catalysts
The demand for superacid catalysts in industrial applications has been steadily growing, driven by the need for more efficient and environmentally friendly chemical processes. Fluoroantimonic acid, as one of the strongest known superacids, has garnered significant attention in catalytic science due to its exceptional proton-donating ability and potential to catalyze a wide range of reactions.
In the petrochemical industry, superacid catalysts play a crucial role in various processes, including isomerization, alkylation, and cracking of hydrocarbons. The use of fluoroantimonic acid-based catalysts has shown promise in enhancing the efficiency of these processes, potentially leading to higher yields and reduced energy consumption. This is particularly important in the production of high-octane gasoline components and other value-added petrochemical products.
The pharmaceutical sector has also expressed growing interest in superacid catalysis, especially for the synthesis of complex organic molecules. Fluoroantimonic acid's ability to activate inert C-H bonds and facilitate challenging transformations makes it an attractive option for developing new synthetic routes to pharmaceutical intermediates and active pharmaceutical ingredients (APIs). This could potentially streamline drug discovery processes and enable the production of previously inaccessible compounds.
In the field of materials science, superacid catalysts have found applications in the synthesis of advanced polymers and nanomaterials. Fluoroantimonic acid's extreme acidity can initiate polymerization reactions under mild conditions, leading to the development of novel materials with unique properties. This has implications for industries such as electronics, aerospace, and automotive, where high-performance materials are in constant demand.
The chemical industry's push towards greener and more sustainable processes has also contributed to the increased interest in superacid catalysis. While fluoroantimonic acid itself is highly corrosive and requires careful handling, its potential to enable reactions under milder conditions and with higher selectivity could lead to overall more environmentally friendly processes. This aligns with the industry's goals of reducing waste, improving atom economy, and minimizing the use of harsh reaction conditions.
Despite the promising applications, the industrial adoption of fluoroantimonic acid-based catalysts faces several challenges. The extreme corrosiveness of the acid necessitates specialized equipment and handling procedures, which can be costly to implement on an industrial scale. Additionally, safety concerns and regulatory requirements associated with the use of such strong acids pose hurdles to widespread adoption.
As a result, there is a growing demand for research into the development of more stable and easily handled forms of fluoroantimonic acid-based catalysts, such as supported catalysts or ionic liquids. These efforts aim to harness the catalytic power of fluoroantimonic acid while mitigating its handling and safety issues, making it more accessible for industrial applications.
In the petrochemical industry, superacid catalysts play a crucial role in various processes, including isomerization, alkylation, and cracking of hydrocarbons. The use of fluoroantimonic acid-based catalysts has shown promise in enhancing the efficiency of these processes, potentially leading to higher yields and reduced energy consumption. This is particularly important in the production of high-octane gasoline components and other value-added petrochemical products.
The pharmaceutical sector has also expressed growing interest in superacid catalysis, especially for the synthesis of complex organic molecules. Fluoroantimonic acid's ability to activate inert C-H bonds and facilitate challenging transformations makes it an attractive option for developing new synthetic routes to pharmaceutical intermediates and active pharmaceutical ingredients (APIs). This could potentially streamline drug discovery processes and enable the production of previously inaccessible compounds.
In the field of materials science, superacid catalysts have found applications in the synthesis of advanced polymers and nanomaterials. Fluoroantimonic acid's extreme acidity can initiate polymerization reactions under mild conditions, leading to the development of novel materials with unique properties. This has implications for industries such as electronics, aerospace, and automotive, where high-performance materials are in constant demand.
The chemical industry's push towards greener and more sustainable processes has also contributed to the increased interest in superacid catalysis. While fluoroantimonic acid itself is highly corrosive and requires careful handling, its potential to enable reactions under milder conditions and with higher selectivity could lead to overall more environmentally friendly processes. This aligns with the industry's goals of reducing waste, improving atom economy, and minimizing the use of harsh reaction conditions.
Despite the promising applications, the industrial adoption of fluoroantimonic acid-based catalysts faces several challenges. The extreme corrosiveness of the acid necessitates specialized equipment and handling procedures, which can be costly to implement on an industrial scale. Additionally, safety concerns and regulatory requirements associated with the use of such strong acids pose hurdles to widespread adoption.
As a result, there is a growing demand for research into the development of more stable and easily handled forms of fluoroantimonic acid-based catalysts, such as supported catalysts or ionic liquids. These efforts aim to harness the catalytic power of fluoroantimonic acid while mitigating its handling and safety issues, making it more accessible for industrial applications.
Current State and Challenges in Fluoroantimonic Acid Application
Fluoroantimonic acid, recognized as one of the strongest superacids, has garnered significant attention in catalytic science due to its exceptional proton-donating ability. The current state of its application in catalysis is characterized by both promising advancements and substantial challenges.
In recent years, researchers have made notable progress in harnessing the catalytic potential of fluoroantimonic acid. Its extreme acidity has proven particularly effective in promoting various organic transformations, including alkylations, isomerizations, and cracking reactions. The acid's ability to activate C-H bonds and facilitate carbocation formation has opened new avenues for synthesizing complex organic molecules and petrochemicals.
However, the widespread adoption of fluoroantimonic acid in industrial catalysis faces several significant hurdles. Foremost among these is its extreme corrosiveness, which necessitates specialized handling equipment and safety protocols. This not only increases operational costs but also limits the scalability of processes involving fluoroantimonic acid.
Another challenge lies in controlling the reactivity of fluoroantimonic acid. Its exceptional strength can lead to undesired side reactions and product degradation, making it difficult to achieve high selectivity in certain catalytic processes. Researchers are actively exploring methods to modulate its acidity and develop more selective catalytic systems.
The environmental impact of fluoroantimonic acid is also a major concern. Its production and use involve highly toxic and environmentally harmful substances, particularly hydrofluoric acid. This has led to increased scrutiny from regulatory bodies and a push towards developing greener alternatives or more sustainable production methods.
Despite these challenges, the scientific community continues to investigate novel applications of fluoroantimonic acid in catalysis. Recent studies have focused on immobilizing the acid on solid supports to enhance its stability and recyclability. This approach shows promise in mitigating some of the handling and environmental issues associated with the liquid acid.
Advancements in computational chemistry have also contributed to a better understanding of fluoroantimonic acid's behavior at the molecular level. These insights are crucial for designing more efficient and selective catalytic systems that leverage the acid's unique properties while minimizing its drawbacks.
In conclusion, while fluoroantimonic acid holds immense potential for propelling catalytic science forward, its current application is limited by significant technical, safety, and environmental challenges. Overcoming these obstacles through innovative research and engineering solutions will be key to fully realizing its catalytic potential and expanding its use in both academic and industrial settings.
In recent years, researchers have made notable progress in harnessing the catalytic potential of fluoroantimonic acid. Its extreme acidity has proven particularly effective in promoting various organic transformations, including alkylations, isomerizations, and cracking reactions. The acid's ability to activate C-H bonds and facilitate carbocation formation has opened new avenues for synthesizing complex organic molecules and petrochemicals.
However, the widespread adoption of fluoroantimonic acid in industrial catalysis faces several significant hurdles. Foremost among these is its extreme corrosiveness, which necessitates specialized handling equipment and safety protocols. This not only increases operational costs but also limits the scalability of processes involving fluoroantimonic acid.
Another challenge lies in controlling the reactivity of fluoroantimonic acid. Its exceptional strength can lead to undesired side reactions and product degradation, making it difficult to achieve high selectivity in certain catalytic processes. Researchers are actively exploring methods to modulate its acidity and develop more selective catalytic systems.
The environmental impact of fluoroantimonic acid is also a major concern. Its production and use involve highly toxic and environmentally harmful substances, particularly hydrofluoric acid. This has led to increased scrutiny from regulatory bodies and a push towards developing greener alternatives or more sustainable production methods.
Despite these challenges, the scientific community continues to investigate novel applications of fluoroantimonic acid in catalysis. Recent studies have focused on immobilizing the acid on solid supports to enhance its stability and recyclability. This approach shows promise in mitigating some of the handling and environmental issues associated with the liquid acid.
Advancements in computational chemistry have also contributed to a better understanding of fluoroantimonic acid's behavior at the molecular level. These insights are crucial for designing more efficient and selective catalytic systems that leverage the acid's unique properties while minimizing its drawbacks.
In conclusion, while fluoroantimonic acid holds immense potential for propelling catalytic science forward, its current application is limited by significant technical, safety, and environmental challenges. Overcoming these obstacles through innovative research and engineering solutions will be key to fully realizing its catalytic potential and expanding its use in both academic and industrial settings.
Existing Fluoroantimonic Acid Catalytic Processes
01 Catalytic activity in organic reactions
Fluoroantimonic acid exhibits strong catalytic activity in various organic reactions, particularly in hydrocarbon transformations. Its super-acidic properties make it effective for isomerization, alkylation, and cracking processes. The acid's ability to generate carbocations facilitates these reactions, making it valuable in petrochemical industries.- Catalytic activity in organic reactions: Fluoroantimonic acid exhibits strong catalytic activity in various organic reactions, particularly in hydrocarbon transformations. Its super-acidic properties make it effective for isomerization, alkylation, and cracking processes. The acid's ability to generate carbocations facilitates these reactions, making it valuable in petrochemical industries.
- Application in polymerization processes: Fluoroantimonic acid serves as a potent catalyst in polymerization reactions. It can initiate cationic polymerization of olefins and other monomers, leading to the formation of high molecular weight polymers. The acid's strong electron-withdrawing properties contribute to its effectiveness in controlling polymer chain growth and molecular weight distribution.
- Use in electrochemical applications: The unique properties of fluoroantimonic acid make it suitable for various electrochemical applications. It can be used in the development of high-performance batteries, fuel cells, and other energy storage devices. The acid's ability to facilitate ion transport and electron transfer processes contributes to improved electrochemical performance.
- Synthesis and preparation methods: Various methods for synthesizing and preparing fluoroantimonic acid have been developed. These include the reaction of hydrogen fluoride with antimony pentafluoride, as well as other novel approaches involving different precursors and reaction conditions. The synthesis methods aim to optimize the purity and stability of the acid for specific applications.
- Safety and handling considerations: Due to its extreme acidity and reactivity, special safety measures and handling procedures are required when working with fluoroantimonic acid. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage and disposal. Research focuses on developing safer formulations and handling techniques to mitigate risks associated with its use.
02 Application in polymerization processes
Fluoroantimonic acid serves as a potent catalyst in polymerization reactions. It can initiate cationic polymerization of olefins and other monomers, leading to the formation of high molecular weight polymers. The acid's strong electron-withdrawing properties contribute to its effectiveness in these processes.Expand Specific Solutions03 Use in electrochemical applications
The catalytic activity of fluoroantimonic acid extends to electrochemical processes. It can be employed in electrolyte solutions for batteries and fuel cells, enhancing their performance. The acid's unique properties allow for improved electron transfer and ionic conductivity in these systems.Expand Specific Solutions04 Catalysis in inorganic synthesis
Fluoroantimonic acid demonstrates catalytic activity in the synthesis of various inorganic compounds. It can facilitate reactions involving metal complexes, oxide formations, and other inorganic transformations. The acid's strong protonating ability plays a crucial role in these processes.Expand Specific Solutions05 Environmental and safety considerations
While fluoroantimonic acid shows remarkable catalytic activity, its use comes with significant environmental and safety challenges. Research focuses on developing safer handling methods, containment strategies, and potential alternatives that can match its catalytic efficiency while minimizing risks associated with its extreme corrosiveness and reactivity.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Research and Production
The catalytic science field utilizing fluoroantimonic acid is in a nascent stage, with significant potential for growth. The market size is expanding as researchers explore its applications in various industries. While the technology is still developing, several key players are advancing its maturity. Companies like BASF SE, 3M Innovative Properties Co., and ExxonMobil Technology & Engineering Co. are leading research efforts. Academic institutions such as The Regents of the University of California, Yale University, and Hunan University are contributing to fundamental research. The competitive landscape is characterized by a mix of established chemical companies and innovative research institutions, each striving to unlock the full potential of fluoroantimonic acid in catalytic processes.
BASF Corp.
Technical Solution: BASF has developed a proprietary process for the production and application of fluoroantimonic acid in catalytic science. Their approach involves the controlled synthesis of fluoroantimonic acid using high-purity antimony pentafluoride and hydrogen fluoride under strictly anhydrous conditions. BASF's method ensures the production of ultra-pure fluoroantimonic acid, which is crucial for its effectiveness as a superacid catalyst. They have also developed specialized handling and storage techniques to maintain the acid's potency and prevent degradation. BASF's application of fluoroantimonic acid in catalytic processes focuses on enhancing reaction rates and selectivity in various organic transformations, particularly in the petrochemical industry.
Strengths: High-purity production, specialized handling techniques, and extensive application knowledge in industrial processes. Weaknesses: High production costs and extreme safety precautions required due to the acid's corrosive nature.
BASF SE
Technical Solution: BASF SE has pioneered advanced applications of fluoroantimonic acid in catalytic science, focusing on its use in the production of high-value chemicals and materials. Their research has led to the development of novel reaction pathways that leverage the superacidic properties of fluoroantimonic acid to catalyze challenging transformations. BASF SE has also invested in creating specialized reactor designs and materials that can withstand the extreme corrosiveness of the acid, enabling its use in continuous flow processes. Their approach includes the integration of in-situ acid regeneration systems to maintain catalytic activity and reduce waste. Additionally, BASF SE has explored the use of supported fluoroantimonic acid catalysts to enhance recyclability and ease of handling in industrial applications.
Strengths: Innovative reactor designs, continuous flow processes, and supported catalyst systems. Weaknesses: High initial investment costs and potential environmental concerns related to fluorine-containing compounds.
Breakthrough Innovations in Fluoroantimonic Acid Catalysis
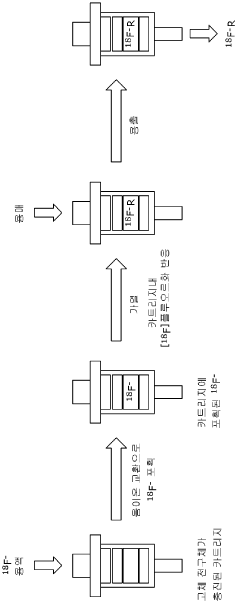
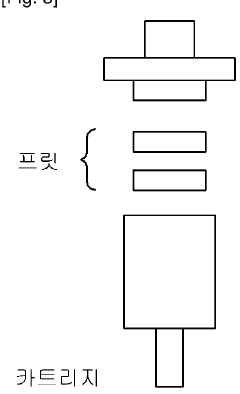
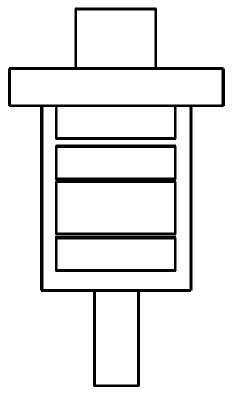
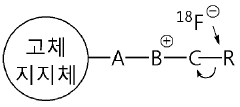
Precursor compound connected to solid support for manufacturing 18f radiopharmaceutical, method for manufacturing same, and application thereof
PatentWO2012081880A2
Innovation
- Development of a solid precursor with an organic salt linked to a solid support that acts as both an anion exchange resin and phase transfer catalyst, allowing for intramolecular nucleophilic substitution reactions, thereby simplifying the fluorination process and reducing the need for separate purification steps.
Curing agents for cationically curable compositions
PatentWO2004060959A1
Innovation
- The use of a catalyst system comprising a combination of photochemically active and non-photochemically active salts, where the anions are classified based on their DSC exotherm energy values to achieve a balance between curing efficiency and colorlessness, resulting in high-performance cationically cured compositions with increased thermal stability and reduced color.
Safety and Handling Protocols for Fluoroantimonic Acid
Fluoroantimonic acid is one of the strongest known superacids, with a Hammett acidity function estimated at -21.6. Its extreme corrosiveness and reactivity necessitate rigorous safety measures and handling protocols. When working with this compound, researchers must prioritize personal protection equipment (PPE) including chemical-resistant suits, gloves, and full-face respirators with appropriate filters. All operations involving fluoroantimonic acid should be conducted in a well-ventilated fume hood equipped with acid-resistant materials.
Storage of fluoroantimonic acid requires specialized containers made of materials such as polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA), as it reacts violently with glass, metals, and most other common laboratory materials. These containers must be kept in a cool, dry area away from any potential reactants or ignition sources. Regular inspections of storage areas and containers are crucial to detect any signs of degradation or leakage.
Transportation of fluoroantimonic acid demands strict adherence to international regulations for hazardous materials. It must be packaged in UN-approved containers with appropriate labeling and documentation. Only trained personnel should handle the transport, and emergency response plans must be in place along the entire transportation route.
In the event of a spill or exposure, immediate action is critical. Spill kits specifically designed for superacids should be readily available in all areas where fluoroantimonic acid is used or stored. These kits typically include neutralizing agents, absorbent materials, and disposal containers. Personnel must be trained in spill response procedures and the use of emergency eyewash stations and safety showers.
Waste management for fluoroantimonic acid requires specialized protocols. Neutralization should be performed carefully, often using a base such as sodium hydroxide, followed by proper disposal according to local and national regulations. Dilution with water is extremely dangerous due to the violent exothermic reaction and should never be attempted as a disposal method.
Regular safety training and drills are essential for all personnel working with or around fluoroantimonic acid. This includes proper handling techniques, emergency procedures, and recognition of potential hazards. Documentation of all safety protocols, incident reports, and training records must be meticulously maintained and regularly updated to ensure compliance with safety standards and regulations.
Given the extreme nature of fluoroantimonic acid, collaboration with safety experts and regulatory bodies is crucial when establishing and updating handling protocols. Continuous monitoring of industry best practices and emerging safety technologies can help improve the overall safety profile when working with this powerful but dangerous compound in catalytic science applications.
Storage of fluoroantimonic acid requires specialized containers made of materials such as polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA), as it reacts violently with glass, metals, and most other common laboratory materials. These containers must be kept in a cool, dry area away from any potential reactants or ignition sources. Regular inspections of storage areas and containers are crucial to detect any signs of degradation or leakage.
Transportation of fluoroantimonic acid demands strict adherence to international regulations for hazardous materials. It must be packaged in UN-approved containers with appropriate labeling and documentation. Only trained personnel should handle the transport, and emergency response plans must be in place along the entire transportation route.
In the event of a spill or exposure, immediate action is critical. Spill kits specifically designed for superacids should be readily available in all areas where fluoroantimonic acid is used or stored. These kits typically include neutralizing agents, absorbent materials, and disposal containers. Personnel must be trained in spill response procedures and the use of emergency eyewash stations and safety showers.
Waste management for fluoroantimonic acid requires specialized protocols. Neutralization should be performed carefully, often using a base such as sodium hydroxide, followed by proper disposal according to local and national regulations. Dilution with water is extremely dangerous due to the violent exothermic reaction and should never be attempted as a disposal method.
Regular safety training and drills are essential for all personnel working with or around fluoroantimonic acid. This includes proper handling techniques, emergency procedures, and recognition of potential hazards. Documentation of all safety protocols, incident reports, and training records must be meticulously maintained and regularly updated to ensure compliance with safety standards and regulations.
Given the extreme nature of fluoroantimonic acid, collaboration with safety experts and regulatory bodies is crucial when establishing and updating handling protocols. Continuous monitoring of industry best practices and emerging safety technologies can help improve the overall safety profile when working with this powerful but dangerous compound in catalytic science applications.
Environmental Impact of Fluoroantimonic Acid Usage
The use of fluoroantimonic acid in catalytic science raises significant environmental concerns due to its highly corrosive and reactive nature. As one of the strongest known superacids, its potential impact on ecosystems and human health cannot be overlooked.
Fluoroantimonic acid is extremely hygroscopic and reacts violently with water, producing toxic and corrosive fumes. This property poses a severe risk of air and water pollution if the acid is accidentally released into the environment. The resulting hydrofluoric acid and antimony compounds can cause long-lasting damage to soil and aquatic ecosystems.
The production and handling of fluoroantimonic acid require stringent safety measures and specialized equipment. Any breach in containment could lead to catastrophic environmental consequences. The acid's ability to dissolve glass and many metals further complicates its safe storage and transport, increasing the risk of accidental releases.
Waste management is another critical environmental concern. The disposal of fluoroantimonic acid and its byproducts must be carefully managed to prevent contamination of soil and groundwater. Neutralization processes can generate large volumes of hazardous waste, which require proper treatment and disposal.
The environmental impact extends to the production of the acid's components. The mining and processing of antimony and the production of hydrogen fluoride both have significant environmental footprints, including habitat destruction, energy consumption, and greenhouse gas emissions.
In catalytic applications, while fluoroantimonic acid can potentially improve reaction efficiency and reduce overall energy consumption, the environmental risks associated with its use may outweigh these benefits in many cases. Researchers and industry professionals must carefully evaluate the trade-offs between catalytic performance and environmental safety.
Efforts to mitigate the environmental impact of fluoroantimonic acid usage focus on developing safer handling protocols, improving containment technologies, and exploring less hazardous alternatives. Some research is directed towards immobilizing the acid on solid supports or developing ionic liquid analogues that retain catalytic activity while reducing environmental risks.
Given these environmental concerns, the use of fluoroantimonic acid in catalytic science must be approached with extreme caution. Rigorous risk assessments, stringent safety protocols, and ongoing research into safer alternatives are essential to balance the potential benefits of this powerful catalyst with the imperative of environmental protection.
Fluoroantimonic acid is extremely hygroscopic and reacts violently with water, producing toxic and corrosive fumes. This property poses a severe risk of air and water pollution if the acid is accidentally released into the environment. The resulting hydrofluoric acid and antimony compounds can cause long-lasting damage to soil and aquatic ecosystems.
The production and handling of fluoroantimonic acid require stringent safety measures and specialized equipment. Any breach in containment could lead to catastrophic environmental consequences. The acid's ability to dissolve glass and many metals further complicates its safe storage and transport, increasing the risk of accidental releases.
Waste management is another critical environmental concern. The disposal of fluoroantimonic acid and its byproducts must be carefully managed to prevent contamination of soil and groundwater. Neutralization processes can generate large volumes of hazardous waste, which require proper treatment and disposal.
The environmental impact extends to the production of the acid's components. The mining and processing of antimony and the production of hydrogen fluoride both have significant environmental footprints, including habitat destruction, energy consumption, and greenhouse gas emissions.
In catalytic applications, while fluoroantimonic acid can potentially improve reaction efficiency and reduce overall energy consumption, the environmental risks associated with its use may outweigh these benefits in many cases. Researchers and industry professionals must carefully evaluate the trade-offs between catalytic performance and environmental safety.
Efforts to mitigate the environmental impact of fluoroantimonic acid usage focus on developing safer handling protocols, improving containment technologies, and exploring less hazardous alternatives. Some research is directed towards immobilizing the acid on solid supports or developing ionic liquid analogues that retain catalytic activity while reducing environmental risks.
Given these environmental concerns, the use of fluoroantimonic acid in catalytic science must be approached with extreme caution. Rigorous risk assessments, stringent safety protocols, and ongoing research into safer alternatives are essential to balance the potential benefits of this powerful catalyst with the imperative of environmental protection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!