Fluoroantimonic Acid at the Helm of Chemistry Developments
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid: Background and Objectives
Fluoroantimonic acid, often hailed as the world's strongest superacid, has been at the forefront of chemical research and development for decades. This compound, with its extraordinary acidity and unique chemical properties, has played a pivotal role in advancing various fields of chemistry and industrial applications. The evolution of fluoroantimonic acid research can be traced back to the mid-20th century when scientists began exploring the limits of acidity and the potential of superacids.
The primary objective in the development of fluoroantimonic acid has been to harness its extreme acidity for catalyzing reactions that were previously thought impossible or impractical. This superacid, formed by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5), exhibits an acidity level millions of times stronger than pure sulfuric acid, opening up new possibilities in organic synthesis, petrochemical processing, and materials science.
Throughout its history, the study of fluoroantimonic acid has been driven by both theoretical interest and practical applications. Researchers have sought to understand the fundamental nature of superacidity, pushing the boundaries of the Brønsted-Lowry acid theory and exploring the concept of proton donation in extreme environments. This theoretical pursuit has led to significant advancements in our understanding of acid-base chemistry and reaction mechanisms.
From an application perspective, the goals of fluoroantimonic acid research have evolved to address various industrial challenges. In the petrochemical industry, it has been investigated for its potential in catalyzing the cracking and isomerization of hydrocarbons, offering more efficient and selective processes compared to traditional acid catalysts. In organic synthesis, chemists have aimed to utilize its strong protonating ability to activate inert molecules and facilitate novel reaction pathways.
The development of fluoroantimonic acid has also been closely tied to advancements in materials science and nanotechnology. Researchers have explored its use in the synthesis of novel materials, including nanostructures and advanced polymers, leveraging its ability to generate highly reactive intermediates under controlled conditions.
As the field progresses, current objectives in fluoroantimonic acid research include enhancing its stability and handling safety, developing more environmentally friendly applications, and exploring its potential in emerging fields such as energy storage and advanced materials processing. The ongoing investigation into this superacid continues to push the boundaries of chemical reactivity and catalysis, promising new breakthroughs in both fundamental chemistry and industrial innovation.
The primary objective in the development of fluoroantimonic acid has been to harness its extreme acidity for catalyzing reactions that were previously thought impossible or impractical. This superacid, formed by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5), exhibits an acidity level millions of times stronger than pure sulfuric acid, opening up new possibilities in organic synthesis, petrochemical processing, and materials science.
Throughout its history, the study of fluoroantimonic acid has been driven by both theoretical interest and practical applications. Researchers have sought to understand the fundamental nature of superacidity, pushing the boundaries of the Brønsted-Lowry acid theory and exploring the concept of proton donation in extreme environments. This theoretical pursuit has led to significant advancements in our understanding of acid-base chemistry and reaction mechanisms.
From an application perspective, the goals of fluoroantimonic acid research have evolved to address various industrial challenges. In the petrochemical industry, it has been investigated for its potential in catalyzing the cracking and isomerization of hydrocarbons, offering more efficient and selective processes compared to traditional acid catalysts. In organic synthesis, chemists have aimed to utilize its strong protonating ability to activate inert molecules and facilitate novel reaction pathways.
The development of fluoroantimonic acid has also been closely tied to advancements in materials science and nanotechnology. Researchers have explored its use in the synthesis of novel materials, including nanostructures and advanced polymers, leveraging its ability to generate highly reactive intermediates under controlled conditions.
As the field progresses, current objectives in fluoroantimonic acid research include enhancing its stability and handling safety, developing more environmentally friendly applications, and exploring its potential in emerging fields such as energy storage and advanced materials processing. The ongoing investigation into this superacid continues to push the boundaries of chemical reactivity and catalysis, promising new breakthroughs in both fundamental chemistry and industrial innovation.
Market Analysis for Superacid Applications
The market for superacid applications, particularly those involving fluoroantimonic acid, has shown significant growth and diversification in recent years. This powerful superacid, known for its extreme acidity and unique chemical properties, has found applications across various industries, driving market expansion and technological advancements.
In the petrochemical sector, fluoroantimonic acid has become a crucial catalyst in alkylation processes, enhancing the production of high-octane gasoline components. This application has seen steady growth, propelled by the increasing demand for cleaner-burning and more efficient fuels. The automotive industry's shift towards higher performance and lower emission standards has further bolstered this market segment.
The electronics industry has also embraced fluoroantimonic acid for its role in the etching of silicon wafers and the production of advanced semiconductors. As the demand for smaller, more powerful electronic devices continues to rise, the market for superacids in this sector is expected to expand significantly. The development of 5G technology and the Internet of Things (IoT) has further accelerated this trend.
In materials science, fluoroantimonic acid has proven invaluable in the synthesis of novel polymers and advanced materials. Its ability to catalyze reactions that were previously challenging or impossible has opened new avenues for material development, particularly in aerospace and defense applications. This has led to a growing market for specialized, high-performance materials.
The pharmaceutical industry has also found applications for fluoroantimonic acid in the synthesis of complex organic compounds and drug precursors. As the demand for new and more effective medications increases, the market for superacids in pharmaceutical research and development is expected to grow steadily.
Environmental applications of fluoroantimonic acid, such as in the treatment of hazardous waste and the removal of pollutants from industrial effluents, represent an emerging market segment. As environmental regulations become more stringent globally, this application area is poised for significant growth.
Despite its versatility, the market for fluoroantimonic acid faces challenges related to its extreme corrosiveness and the need for specialized handling and storage. These factors have led to increased research into safer superacid alternatives and more efficient containment technologies, creating a parallel market for safety equipment and specialized materials.
The global market for superacid applications is geographically diverse, with major demand centers in North America, Europe, and Asia-Pacific. Emerging economies, particularly in Asia, are expected to drive future growth as their industrial and technological sectors expand.
In the petrochemical sector, fluoroantimonic acid has become a crucial catalyst in alkylation processes, enhancing the production of high-octane gasoline components. This application has seen steady growth, propelled by the increasing demand for cleaner-burning and more efficient fuels. The automotive industry's shift towards higher performance and lower emission standards has further bolstered this market segment.
The electronics industry has also embraced fluoroantimonic acid for its role in the etching of silicon wafers and the production of advanced semiconductors. As the demand for smaller, more powerful electronic devices continues to rise, the market for superacids in this sector is expected to expand significantly. The development of 5G technology and the Internet of Things (IoT) has further accelerated this trend.
In materials science, fluoroantimonic acid has proven invaluable in the synthesis of novel polymers and advanced materials. Its ability to catalyze reactions that were previously challenging or impossible has opened new avenues for material development, particularly in aerospace and defense applications. This has led to a growing market for specialized, high-performance materials.
The pharmaceutical industry has also found applications for fluoroantimonic acid in the synthesis of complex organic compounds and drug precursors. As the demand for new and more effective medications increases, the market for superacids in pharmaceutical research and development is expected to grow steadily.
Environmental applications of fluoroantimonic acid, such as in the treatment of hazardous waste and the removal of pollutants from industrial effluents, represent an emerging market segment. As environmental regulations become more stringent globally, this application area is poised for significant growth.
Despite its versatility, the market for fluoroantimonic acid faces challenges related to its extreme corrosiveness and the need for specialized handling and storage. These factors have led to increased research into safer superacid alternatives and more efficient containment technologies, creating a parallel market for safety equipment and specialized materials.
The global market for superacid applications is geographically diverse, with major demand centers in North America, Europe, and Asia-Pacific. Emerging economies, particularly in Asia, are expected to drive future growth as their industrial and technological sectors expand.
Current State and Challenges in Superacid Chemistry
Superacid chemistry has witnessed significant advancements in recent years, with fluoroantimonic acid (HSbF6) emerging as a key player in pushing the boundaries of chemical reactivity. The current state of superacid research is characterized by intense exploration of novel synthetic methodologies and applications across various fields of chemistry.
One of the primary challenges in superacid chemistry revolves around the handling and containment of these highly corrosive substances. Fluoroantimonic acid, being one of the strongest known superacids, poses particular difficulties due to its extreme reactivity with most materials. Researchers are actively developing specialized equipment and protocols to safely manipulate and study these powerful acids without compromising experimental integrity or researcher safety.
Another significant challenge lies in the precise measurement and quantification of superacid strength. Traditional acidity scales, such as the pH scale, are inadequate for describing the proton-donating ability of superacids. The development of more accurate and standardized methods for determining superacid strength remains an ongoing area of research, with implications for both theoretical understanding and practical applications.
The stability and longevity of superacids under various conditions present additional hurdles. Fluoroantimonic acid, for instance, is highly sensitive to moisture and can rapidly decompose in the presence of water. This necessitates the use of rigorously anhydrous conditions and specialized storage techniques, which can be challenging to maintain consistently in research and industrial settings.
Furthermore, the environmental impact and disposal of superacids pose significant challenges. The extreme reactivity of these substances makes them potentially hazardous to ecosystems if not properly managed. Developing eco-friendly synthesis methods and finding safer alternatives to traditional superacids are active areas of research aimed at addressing these concerns.
In the realm of catalysis, superacids like fluoroantimonic acid have shown immense potential. However, harnessing their catalytic prowess while mitigating their corrosive nature remains a delicate balancing act. Researchers are exploring novel catalyst designs and support materials that can withstand the harsh acidic environment while maintaining catalytic efficiency.
The scalability of superacid-based processes from laboratory to industrial scale presents another set of challenges. Issues such as heat management, material compatibility, and process safety become increasingly critical as reaction volumes increase. Overcoming these obstacles is crucial for the widespread adoption of superacid chemistry in large-scale manufacturing and chemical production.
One of the primary challenges in superacid chemistry revolves around the handling and containment of these highly corrosive substances. Fluoroantimonic acid, being one of the strongest known superacids, poses particular difficulties due to its extreme reactivity with most materials. Researchers are actively developing specialized equipment and protocols to safely manipulate and study these powerful acids without compromising experimental integrity or researcher safety.
Another significant challenge lies in the precise measurement and quantification of superacid strength. Traditional acidity scales, such as the pH scale, are inadequate for describing the proton-donating ability of superacids. The development of more accurate and standardized methods for determining superacid strength remains an ongoing area of research, with implications for both theoretical understanding and practical applications.
The stability and longevity of superacids under various conditions present additional hurdles. Fluoroantimonic acid, for instance, is highly sensitive to moisture and can rapidly decompose in the presence of water. This necessitates the use of rigorously anhydrous conditions and specialized storage techniques, which can be challenging to maintain consistently in research and industrial settings.
Furthermore, the environmental impact and disposal of superacids pose significant challenges. The extreme reactivity of these substances makes them potentially hazardous to ecosystems if not properly managed. Developing eco-friendly synthesis methods and finding safer alternatives to traditional superacids are active areas of research aimed at addressing these concerns.
In the realm of catalysis, superacids like fluoroantimonic acid have shown immense potential. However, harnessing their catalytic prowess while mitigating their corrosive nature remains a delicate balancing act. Researchers are exploring novel catalyst designs and support materials that can withstand the harsh acidic environment while maintaining catalytic efficiency.
The scalability of superacid-based processes from laboratory to industrial scale presents another set of challenges. Issues such as heat management, material compatibility, and process safety become increasingly critical as reaction volumes increase. Overcoming these obstacles is crucial for the widespread adoption of superacid chemistry in large-scale manufacturing and chemical production.
Existing Synthesis and Handling Methods
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride with antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in catalysis and chemical reactions: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting hydrocarbon transformations, such as isomerization, alkylation, and cracking reactions. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acid catalysts.
- Use in materials science and surface treatments: Fluoroantimonic acid finds applications in materials science, particularly in surface treatments and modifications. It can be used to etch or modify surfaces of various materials, including metals, semiconductors, and ceramics. The acid's strong etching properties make it useful in creating specific surface structures or patterns.
- Safety considerations and handling procedures: Due to its extreme corrosiveness and reactivity, special safety measures and handling procedures are required when working with fluoroantimonic acid. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage, transport, and disposal. Proper training and safety measures are essential to prevent accidents and ensure safe handling.
- Analytical and characterization techniques: Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its properties. These include spectroscopic methods, electrochemical analyses, and computational studies. Such techniques are crucial for understanding the acid's structure, reactivity, and behavior in different chemical environments, as well as for quality control in its production and application.
02 Applications in chemical reactions and catalysis
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids, making it valuable in organic synthesis and petrochemical industries.Expand Specific Solutions03 Use in materials science and surface treatments
Fluoroantimonic acid finds applications in materials science, particularly in surface treatments and modifications. It can be used to etch or activate surfaces, create specialized coatings, and modify the properties of various materials. The acid's unique properties make it suitable for developing advanced materials with specific characteristics.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, handling fluoroantimonic acid requires stringent safety measures. Specialized equipment, containment systems, and personal protective gear are essential when working with this superacid. Proper storage, transportation, and disposal protocols must be followed to prevent accidents and environmental contamination.Expand Specific Solutions05 Analytical and research applications
Fluoroantimonic acid is used in various analytical and research applications. Its unique properties make it valuable in spectroscopy, chromatography, and other analytical techniques. The acid can be employed to study reaction mechanisms, investigate molecular structures, and develop new analytical methods in chemistry and related fields.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Research
The development of Fluoroantimonic Acid in chemistry is at a mature stage, with significant market potential across various industries. The global market for superacids, including Fluoroantimonic Acid, is experiencing steady growth due to increasing applications in pharmaceuticals, petrochemicals, and materials science. Major players like DuPont de Nemours, Inc., Merck Sharp & Dohme Corp., and Pfizer Inc. are investing heavily in research and development to enhance the acid's applications. The technology's maturity is evident from the involvement of diverse entities, including academic institutions like Hunan University and Shanghai University, as well as industrial giants such as China Petroleum & Chemical Corp. and Shell Oil Co. This competitive landscape indicates a robust ecosystem of innovation and commercialization in Fluoroantimonic Acid technology.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for synthesizing and handling fluoroantimonic acid using specialized fluoropolymer-lined reactors and storage vessels. Their method involves carefully controlled addition of antimony pentafluoride to anhydrous hydrogen fluoride under inert atmosphere. DuPont has also pioneered applications of fluoroantimonic acid as a superacid catalyst in petrochemical processes like alkylation and isomerization of hydrocarbons. Their technology allows for more efficient and selective chemical transformations compared to conventional acid catalysts.
Strengths: Extensive experience with fluorine chemistry, advanced materials expertise for containment. Weaknesses: High costs, limited large-scale production capabilities.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp (Sinopec) has invested in research on fluoroantimonic acid catalysis for petroleum refining applications. They have developed pilot-scale processes using fluoroantimonic acid to catalyze alkylation reactions for producing high-octane gasoline blending components. Sinopec's approach involves in-situ generation of the superacid catalyst by combining HF and SbF5 precursors in specially designed reactors. They are working to optimize catalyst composition and process conditions to maximize selectivity while minimizing corrosion issues.
Strengths: Large-scale petrochemical expertise, significant R&D resources. Weaknesses: Less experience with extreme acids, potential safety/environmental concerns.
Core Innovations in Fluoroantimonic Acid Chemistry
Novel heterocyclic probe, preparation method and application thereof
PatentActiveIN201611029461A
Innovation
- Development of a novel benzophenone-quinoline probe that exhibits significant changes in absorption and emission bands under acidic conditions, enabling rapid and reversible detection of acid vapors and chemical warfare agents, integrated into a sensor device with a circuit comprising light emitting diodes, resistors, and a relay for alarm activation.
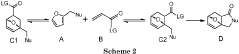
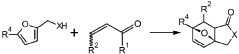
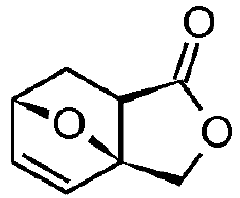
Diels-alder reaction with furanics to obtain aromatics
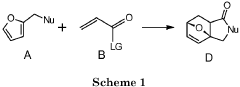
PatentInactiveEP3663297A1
Innovation
- A method involving the reaction of furfuryl alcohol or its analogues with a dienophile containing an α,β-unsaturated carbonyl group, using a specific reaction pathway that forms a single phthalide compound through intermediates, facilitating a clean and efficient production process.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, presents significant safety and environmental challenges that demand careful consideration in its handling, storage, and disposal. The extreme corrosiveness and reactivity of this compound necessitate stringent safety protocols to protect personnel and prevent accidents in laboratory and industrial settings.
Personal protective equipment (PPE) is crucial when working with fluoroantimonic acid. This includes chemical-resistant suits, gloves, and face shields, as well as respiratory protection to guard against potential fumes. Specialized containment systems, such as perfluorinated plastic containers, are essential for storage and transport due to the acid's ability to react with most materials.
Emergency response procedures must be well-established and regularly practiced. This includes having appropriate neutralizing agents on hand, such as calcium carbonate or sodium bicarbonate, to mitigate spills. Proper ventilation systems are critical to prevent the accumulation of toxic fumes, and safety showers and eyewash stations should be readily accessible in case of exposure.
Environmental considerations are equally important. The release of fluoroantimonic acid into the environment can have severe consequences on ecosystems and water sources. Strict waste management protocols must be implemented to ensure proper neutralization and disposal of acid residues and contaminated materials. This may involve specialized treatment facilities capable of handling such hazardous waste.
Long-term environmental impact assessments are necessary to understand the potential effects of accidental releases or chronic low-level exposure. This includes studying the acid's behavior in various environmental matrices and its potential for bioaccumulation in food chains.
Research into safer alternatives and improved handling techniques is ongoing. This includes the development of less hazardous superacids with similar reactivity, as well as advanced containment technologies that can withstand the extreme corrosiveness of fluoroantimonic acid.
Regulatory compliance is a critical aspect of working with fluoroantimonic acid. Organizations must adhere to strict guidelines set by environmental protection agencies and occupational safety authorities. This often involves detailed record-keeping, regular safety audits, and continuous training programs for personnel involved in handling the acid.
As chemistry developments continue to push the boundaries of what is possible with superacids like fluoroantimonic acid, the importance of robust safety and environmental protocols cannot be overstated. Balancing the potential benefits of these powerful chemical tools with the need to protect human health and the environment remains a key challenge in the field.
Personal protective equipment (PPE) is crucial when working with fluoroantimonic acid. This includes chemical-resistant suits, gloves, and face shields, as well as respiratory protection to guard against potential fumes. Specialized containment systems, such as perfluorinated plastic containers, are essential for storage and transport due to the acid's ability to react with most materials.
Emergency response procedures must be well-established and regularly practiced. This includes having appropriate neutralizing agents on hand, such as calcium carbonate or sodium bicarbonate, to mitigate spills. Proper ventilation systems are critical to prevent the accumulation of toxic fumes, and safety showers and eyewash stations should be readily accessible in case of exposure.
Environmental considerations are equally important. The release of fluoroantimonic acid into the environment can have severe consequences on ecosystems and water sources. Strict waste management protocols must be implemented to ensure proper neutralization and disposal of acid residues and contaminated materials. This may involve specialized treatment facilities capable of handling such hazardous waste.
Long-term environmental impact assessments are necessary to understand the potential effects of accidental releases or chronic low-level exposure. This includes studying the acid's behavior in various environmental matrices and its potential for bioaccumulation in food chains.
Research into safer alternatives and improved handling techniques is ongoing. This includes the development of less hazardous superacids with similar reactivity, as well as advanced containment technologies that can withstand the extreme corrosiveness of fluoroantimonic acid.
Regulatory compliance is a critical aspect of working with fluoroantimonic acid. Organizations must adhere to strict guidelines set by environmental protection agencies and occupational safety authorities. This often involves detailed record-keeping, regular safety audits, and continuous training programs for personnel involved in handling the acid.
As chemistry developments continue to push the boundaries of what is possible with superacids like fluoroantimonic acid, the importance of robust safety and environmental protocols cannot be overstated. Balancing the potential benefits of these powerful chemical tools with the need to protect human health and the environment remains a key challenge in the field.
Industrial Applications and Potential
Fluoroantimonic acid, recognized as the world's strongest superacid, has emerged as a powerful catalyst in various industrial processes. Its exceptional acidity and unique chemical properties have opened up new possibilities for chemical synthesis and materials processing.
In the petrochemical industry, fluoroantimonic acid has found significant applications in the isomerization of hydrocarbons. This process is crucial for improving the octane rating of gasoline, resulting in more efficient and cleaner-burning fuels. The superacid's ability to catalyze reactions at lower temperatures and pressures compared to traditional catalysts has led to increased energy efficiency and reduced operational costs in refineries.
The electronics industry has also benefited from fluoroantimonic acid's potential. Its use in the etching of silicon wafers has enabled the production of more intricate and smaller semiconductor devices. This has contributed to the ongoing miniaturization trend in electronics, supporting the development of more powerful and compact electronic devices.
In the field of materials science, fluoroantimonic acid has shown promise in the synthesis of novel polymers and advanced materials. Its strong acidic properties allow for the activation of typically unreactive compounds, enabling the creation of materials with unique properties and applications. This has potential implications for the development of high-performance plastics, coatings, and composite materials.
The pharmaceutical industry is exploring the use of fluoroantimonic acid in the synthesis of complex organic molecules. Its ability to catalyze challenging reactions could potentially streamline the production of certain drugs and lead to the discovery of new pharmaceutical compounds. However, the extreme reactivity of the acid necessitates careful handling and specialized equipment, which may limit its widespread adoption in this sector.
Despite its potential, the industrial application of fluoroantimonic acid faces several challenges. Its corrosive nature requires specialized containment and handling procedures, which can increase production costs. Additionally, safety concerns and environmental considerations must be carefully addressed to ensure responsible use of this powerful chemical agent.
Looking ahead, ongoing research is focused on developing more stable and easily handled forms of fluoroantimonic acid, which could expand its industrial applications. The potential for this superacid to enable new chemical transformations and improve existing processes continues to drive interest in its development and application across various industries.
In the petrochemical industry, fluoroantimonic acid has found significant applications in the isomerization of hydrocarbons. This process is crucial for improving the octane rating of gasoline, resulting in more efficient and cleaner-burning fuels. The superacid's ability to catalyze reactions at lower temperatures and pressures compared to traditional catalysts has led to increased energy efficiency and reduced operational costs in refineries.
The electronics industry has also benefited from fluoroantimonic acid's potential. Its use in the etching of silicon wafers has enabled the production of more intricate and smaller semiconductor devices. This has contributed to the ongoing miniaturization trend in electronics, supporting the development of more powerful and compact electronic devices.
In the field of materials science, fluoroantimonic acid has shown promise in the synthesis of novel polymers and advanced materials. Its strong acidic properties allow for the activation of typically unreactive compounds, enabling the creation of materials with unique properties and applications. This has potential implications for the development of high-performance plastics, coatings, and composite materials.
The pharmaceutical industry is exploring the use of fluoroantimonic acid in the synthesis of complex organic molecules. Its ability to catalyze challenging reactions could potentially streamline the production of certain drugs and lead to the discovery of new pharmaceutical compounds. However, the extreme reactivity of the acid necessitates careful handling and specialized equipment, which may limit its widespread adoption in this sector.
Despite its potential, the industrial application of fluoroantimonic acid faces several challenges. Its corrosive nature requires specialized containment and handling procedures, which can increase production costs. Additionally, safety concerns and environmental considerations must be carefully addressed to ensure responsible use of this powerful chemical agent.
Looking ahead, ongoing research is focused on developing more stable and easily handled forms of fluoroantimonic acid, which could expand its industrial applications. The potential for this superacid to enable new chemical transformations and improve existing processes continues to drive interest in its development and application across various industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!