Fluoroantimonic Acid: Tools for Next‑Gen Reaction Protocols
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Background and Objectives
Fluoroantimonic acid, often referred to as the world's strongest superacid, has been a subject of intense scientific interest since its discovery in the mid-20th century. This compound, with its extraordinary acidity and unique chemical properties, has emerged as a potential game-changer in the field of reaction protocols. The evolution of fluoroantimonic acid research has been marked by significant milestones, from its initial synthesis to its current applications in various industrial and research settings.
The development of fluoroantimonic acid can be traced back to the broader field of superacid chemistry, which gained momentum in the 1960s and 1970s. Pioneering work by chemists such as George A. Olah laid the foundation for understanding and harnessing the power of these extremely acidic substances. Fluoroantimonic acid, a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), stands out due to its unprecedented acidity, far surpassing that of conventional strong acids like sulfuric acid.
Over the years, research into fluoroantimonic acid has been driven by its potential to catalyze reactions that were previously thought impossible or impractical. Its ability to protonate even very weak bases and activate inert compounds has opened up new avenues in organic synthesis, petrochemistry, and materials science. The technological trajectory of fluoroantimonic acid has been characterized by continuous efforts to improve its synthesis, handling, and application methods.
The primary objective in the field of fluoroantimonic acid research is to fully exploit its unique properties for next-generation reaction protocols. This encompasses several key areas of focus. Firstly, there is a push to develop safer and more efficient methods for synthesizing and handling this extremely corrosive substance. Secondly, researchers aim to expand the range of reactions that can be catalyzed or facilitated by fluoroantimonic acid, particularly in the realm of carbon-carbon bond formation and hydrocarbon transformations.
Another critical objective is to understand and control the mechanistic aspects of reactions involving fluoroantimonic acid. This includes elucidating the precise nature of intermediates formed in these highly acidic environments and how they influence reaction pathways. Additionally, there is growing interest in exploring the potential of fluoroantimonic acid in materials processing, such as in the etching of semiconductors or the modification of surface properties of various materials.
As we look towards the future, the technological goals for fluoroantimonic acid extend to its integration with other cutting-edge technologies. This includes its potential role in green chemistry, where its high efficiency could lead to more environmentally friendly processes by reducing the need for large quantities of less effective catalysts. There is also ongoing research into combining fluoroantimonic acid with novel reactor designs and flow chemistry techniques to enhance its applicability in industrial settings.
The development of fluoroantimonic acid can be traced back to the broader field of superacid chemistry, which gained momentum in the 1960s and 1970s. Pioneering work by chemists such as George A. Olah laid the foundation for understanding and harnessing the power of these extremely acidic substances. Fluoroantimonic acid, a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), stands out due to its unprecedented acidity, far surpassing that of conventional strong acids like sulfuric acid.
Over the years, research into fluoroantimonic acid has been driven by its potential to catalyze reactions that were previously thought impossible or impractical. Its ability to protonate even very weak bases and activate inert compounds has opened up new avenues in organic synthesis, petrochemistry, and materials science. The technological trajectory of fluoroantimonic acid has been characterized by continuous efforts to improve its synthesis, handling, and application methods.
The primary objective in the field of fluoroantimonic acid research is to fully exploit its unique properties for next-generation reaction protocols. This encompasses several key areas of focus. Firstly, there is a push to develop safer and more efficient methods for synthesizing and handling this extremely corrosive substance. Secondly, researchers aim to expand the range of reactions that can be catalyzed or facilitated by fluoroantimonic acid, particularly in the realm of carbon-carbon bond formation and hydrocarbon transformations.
Another critical objective is to understand and control the mechanistic aspects of reactions involving fluoroantimonic acid. This includes elucidating the precise nature of intermediates formed in these highly acidic environments and how they influence reaction pathways. Additionally, there is growing interest in exploring the potential of fluoroantimonic acid in materials processing, such as in the etching of semiconductors or the modification of surface properties of various materials.
As we look towards the future, the technological goals for fluoroantimonic acid extend to its integration with other cutting-edge technologies. This includes its potential role in green chemistry, where its high efficiency could lead to more environmentally friendly processes by reducing the need for large quantities of less effective catalysts. There is also ongoing research into combining fluoroantimonic acid with novel reactor designs and flow chemistry techniques to enhance its applicability in industrial settings.
Market Demand Analysis for Advanced Reaction Catalysts
The market demand for advanced reaction catalysts, particularly those involving fluoroantimonic acid, has been steadily growing in recent years. This surge is primarily driven by the increasing need for more efficient and selective chemical processes across various industries. Fluoroantimonic acid, being one of the strongest known superacids, has garnered significant attention for its potential in catalyzing challenging reactions that were previously difficult or impossible to achieve.
In the pharmaceutical sector, there is a growing demand for catalysts that can facilitate the synthesis of complex drug molecules with higher yields and fewer side products. Fluoroantimonic acid-based catalysts have shown promise in enabling novel reaction pathways, potentially revolutionizing drug discovery and development processes. This has led to increased investment in research and development activities focused on harnessing the power of superacids in pharmaceutical manufacturing.
The petrochemical industry has also expressed keen interest in advanced reaction catalysts, particularly for processes involving hydrocarbon transformations. Fluoroantimonic acid's ability to catalyze isomerization and cracking reactions at lower temperatures and pressures than conventional catalysts has sparked interest in its potential to improve energy efficiency and reduce operational costs in refineries.
In the field of materials science, the demand for catalysts capable of synthesizing advanced polymers and nanomaterials is on the rise. Fluoroantimonic acid-based systems have demonstrated potential in facilitating the production of high-performance materials with unique properties, opening up new possibilities in areas such as aerospace, electronics, and energy storage.
The specialty chemicals sector has shown a growing appetite for catalysts that can enable the production of fine chemicals with higher purity and yield. Fluoroantimonic acid's exceptional acidity and catalytic activity make it an attractive option for developing next-generation catalytic systems that can meet these demanding requirements.
Environmental concerns and stringent regulations have also been driving the demand for more sustainable and efficient catalytic processes. While fluoroantimonic acid itself poses significant handling challenges due to its extreme reactivity, research into milder derivatives and supported catalyst systems has gained momentum, aiming to harness its catalytic power while addressing safety and environmental considerations.
Despite the promising potential, the market for fluoroantimonic acid-based catalysts faces challenges related to safety, handling, and scalability. These factors have led to increased research efforts focused on developing more stable and user-friendly catalyst systems that can deliver similar performance benefits. As a result, the market demand extends beyond pure fluoroantimonic acid to encompass a broader range of advanced superacid catalysts and related technologies.
In the pharmaceutical sector, there is a growing demand for catalysts that can facilitate the synthesis of complex drug molecules with higher yields and fewer side products. Fluoroantimonic acid-based catalysts have shown promise in enabling novel reaction pathways, potentially revolutionizing drug discovery and development processes. This has led to increased investment in research and development activities focused on harnessing the power of superacids in pharmaceutical manufacturing.
The petrochemical industry has also expressed keen interest in advanced reaction catalysts, particularly for processes involving hydrocarbon transformations. Fluoroantimonic acid's ability to catalyze isomerization and cracking reactions at lower temperatures and pressures than conventional catalysts has sparked interest in its potential to improve energy efficiency and reduce operational costs in refineries.
In the field of materials science, the demand for catalysts capable of synthesizing advanced polymers and nanomaterials is on the rise. Fluoroantimonic acid-based systems have demonstrated potential in facilitating the production of high-performance materials with unique properties, opening up new possibilities in areas such as aerospace, electronics, and energy storage.
The specialty chemicals sector has shown a growing appetite for catalysts that can enable the production of fine chemicals with higher purity and yield. Fluoroantimonic acid's exceptional acidity and catalytic activity make it an attractive option for developing next-generation catalytic systems that can meet these demanding requirements.
Environmental concerns and stringent regulations have also been driving the demand for more sustainable and efficient catalytic processes. While fluoroantimonic acid itself poses significant handling challenges due to its extreme reactivity, research into milder derivatives and supported catalyst systems has gained momentum, aiming to harness its catalytic power while addressing safety and environmental considerations.
Despite the promising potential, the market for fluoroantimonic acid-based catalysts faces challenges related to safety, handling, and scalability. These factors have led to increased research efforts focused on developing more stable and user-friendly catalyst systems that can deliver similar performance benefits. As a result, the market demand extends beyond pure fluoroantimonic acid to encompass a broader range of advanced superacid catalysts and related technologies.
Current State and Challenges in Superacid Chemistry
Superacid chemistry has witnessed significant advancements in recent years, with fluoroantimonic acid emerging as a powerful tool for next-generation reaction protocols. The current state of superacid chemistry is characterized by intense research into novel applications and improved synthesis methods. Fluoroantimonic acid, a combination of hydrogen fluoride and antimony pentafluoride, stands out as one of the strongest known superacids, capable of protonating even extremely weak bases.
The field faces several key challenges, primarily centered around the handling and containment of these highly corrosive substances. Researchers are actively developing specialized equipment and safety protocols to mitigate risks associated with superacid use. Another significant hurdle is the scalability of superacid-mediated reactions, as many processes that show promise in laboratory settings prove difficult to implement on an industrial scale.
Efforts to expand the applicability of superacids in organic synthesis have led to breakthroughs in carbon-carbon bond formation and the activation of typically unreactive compounds. However, the extreme reactivity of superacids often results in unwanted side reactions, necessitating the development of more selective catalytic systems. This challenge has spurred research into tailored superacid catalysts with improved specificity and reduced environmental impact.
The environmental concerns surrounding superacid chemistry present another significant challenge. The production and use of fluoroantimonic acid and other superacids generate hazardous waste, prompting investigations into greener alternatives and more efficient recycling methods. Some researchers are exploring ionic liquid-based superacids as potentially more environmentally friendly options.
In the realm of materials science, superacids are finding novel applications in the synthesis of advanced materials, including nanomaterials and functional polymers. However, controlling the reactivity of superacids in these processes remains a formidable challenge, requiring precise reaction conditions and innovative quenching techniques.
The analytical aspects of superacid chemistry also present unique challenges. Conventional pH measurement techniques are inadequate for quantifying the acidity of these extreme systems, necessitating the development of new analytical methods. Researchers are exploring advanced spectroscopic techniques and computational models to better understand and characterize superacid behavior.
As the field progresses, there is a growing focus on harnessing the power of superacids for sustainable chemistry applications. This includes their potential use in biomass conversion, CO2 activation, and the development of next-generation energy storage materials. However, realizing these applications requires overcoming significant technical hurdles related to selectivity, stability, and process integration.
The field faces several key challenges, primarily centered around the handling and containment of these highly corrosive substances. Researchers are actively developing specialized equipment and safety protocols to mitigate risks associated with superacid use. Another significant hurdle is the scalability of superacid-mediated reactions, as many processes that show promise in laboratory settings prove difficult to implement on an industrial scale.
Efforts to expand the applicability of superacids in organic synthesis have led to breakthroughs in carbon-carbon bond formation and the activation of typically unreactive compounds. However, the extreme reactivity of superacids often results in unwanted side reactions, necessitating the development of more selective catalytic systems. This challenge has spurred research into tailored superacid catalysts with improved specificity and reduced environmental impact.
The environmental concerns surrounding superacid chemistry present another significant challenge. The production and use of fluoroantimonic acid and other superacids generate hazardous waste, prompting investigations into greener alternatives and more efficient recycling methods. Some researchers are exploring ionic liquid-based superacids as potentially more environmentally friendly options.
In the realm of materials science, superacids are finding novel applications in the synthesis of advanced materials, including nanomaterials and functional polymers. However, controlling the reactivity of superacids in these processes remains a formidable challenge, requiring precise reaction conditions and innovative quenching techniques.
The analytical aspects of superacid chemistry also present unique challenges. Conventional pH measurement techniques are inadequate for quantifying the acidity of these extreme systems, necessitating the development of new analytical methods. Researchers are exploring advanced spectroscopic techniques and computational models to better understand and characterize superacid behavior.
As the field progresses, there is a growing focus on harnessing the power of superacids for sustainable chemistry applications. This includes their potential use in biomass conversion, CO2 activation, and the development of next-generation energy storage materials. However, realizing these applications requires overcoming significant technical hurdles related to selectivity, stability, and process integration.
Existing Protocols for Fluoroantimonic Acid Reactions
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized by combining hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of these highly reactive compounds under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the resulting superacid.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in chemical reactions and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. The acid's extreme acidity allows it to catalyze reactions that are difficult or impossible with conventional acids, making it valuable in organic synthesis and petrochemical industries.
- Material compatibility and storage: Due to its highly corrosive nature, fluoroantimonic acid requires specialized materials for handling and storage. Research has been conducted on developing resistant materials, such as certain fluoropolymers and specially treated metals, that can withstand the acid's corrosive properties. Proper storage and containment systems are crucial for safe handling and prevention of environmental contamination.
- Safety measures and environmental considerations: Handling fluoroantimonic acid requires strict safety protocols due to its extreme reactivity and corrosiveness. Research has focused on developing improved safety measures, including specialized personal protective equipment, containment systems, and neutralization techniques. Environmental impact assessments and waste management strategies have also been studied to minimize potential risks associated with its use and disposal.
- Analytical applications and detection methods: Fluoroantimonic acid has found use in analytical chemistry for specialized applications. Research has been conducted on developing methods for detecting and quantifying the acid, as well as its potential use in analytical techniques. These applications leverage the acid's unique properties to enable certain types of chemical analysis or sample preparation.
02 Applications in chemical reactions and catalysis
Fluoroantimonic acid is utilized as a powerful catalyst in various chemical reactions due to its extremely high acidity. It is particularly effective in promoting alkylation, isomerization, and polymerization reactions. The superacid's catalytic properties are exploited in the petrochemical industry and in the synthesis of specialty chemicals.Expand Specific Solutions03 Material compatibility and storage
Due to its highly corrosive nature, fluoroantimonic acid requires specialized materials for handling and storage. Research has been conducted on developing compatible materials, such as fluoropolymers and certain alloys, that can withstand the acid's aggressive properties. Proper containment and storage techniques are crucial for safe handling and preventing environmental contamination.Expand Specific Solutions04 Safety measures and environmental considerations
Handling fluoroantimonic acid requires strict safety protocols due to its extreme reactivity and corrosiveness. Research has focused on developing improved safety measures, including personal protective equipment, containment systems, and emergency response procedures. Environmental impact assessments and waste management strategies have also been studied to minimize potential hazards.Expand Specific Solutions05 Analytical applications and characterization
Fluoroantimonic acid has found use in analytical chemistry and materials characterization. Its unique properties make it valuable for studying the behavior of compounds under extreme acidic conditions. Researchers have developed methods to utilize the superacid in spectroscopic techniques, surface analysis, and the investigation of novel materials and chemical structures.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Research and Production
The development of Fluoroantimonic Acid for next-generation reaction protocols is in its early stages, with significant potential for growth. The market size is expanding as researchers explore its applications in organic synthesis and catalysis. Technologically, it's still evolving, with varying levels of maturity across different companies. Key players like Honeywell International Technologies Ltd., Air Products & Chemicals, Inc., and Solvay Specialty Polymers Italy SpA are at the forefront, leveraging their expertise in chemical manufacturing. Academic institutions such as Peking University and Tsinghua University are contributing to fundamental research. Pharmaceutical companies like Novartis AG and Daiichi Sankyo Co., Ltd. are exploring its potential in drug discovery. The competitive landscape is diverse, with both established chemical companies and emerging research institutions vying for breakthroughs in this promising field.
Sinochem Lantian Co., Ltd.
Technical Solution: Sinochem Lantian has developed a proprietary process for the synthesis and purification of fluoroantimonic acid, utilizing advanced fluorination technology and high-purity antimony pentafluoride. Their method involves a controlled reaction between hydrogen fluoride and antimony pentafluoride under precise temperature and pressure conditions. The company has also implemented specialized handling and storage protocols to maintain the stability and purity of the superacid.
Strengths: High-purity product, scalable production process. Weaknesses: High production costs, stringent safety requirements.
Air Products & Chemicals, Inc.
Technical Solution: Air Products has developed a novel approach to fluoroantimonic acid production using a continuous flow reactor system. This method allows for precise control of reaction conditions and enables the production of high-quality fluoroantimonic acid with consistent properties. The company has also implemented advanced purification techniques to remove trace impurities, resulting in a product with exceptional purity levels suitable for next-generation reaction protocols.
Strengths: Consistent product quality, efficient production process. Weaknesses: High initial investment costs, complex equipment maintenance.
Core Innovations in Fluoroantimonic Acid Applications
Fluorinated compounds as PH-sensitive analgesics
PatentWO2020006563A1
Innovation
- Development of fluorinated fentanyl analogs with a fluorine attached to carbon 2 of the side chain, which are pH-sensitive, selectively targeting inflamed tissues and having superior selectivity for peripheral receptors, thereby reducing CNS-related side effects and abuse potential.
Use of trifluoroacetamide for n-terminal protection
PatentInactiveUS20150011778A1
Innovation
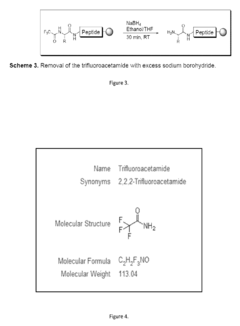
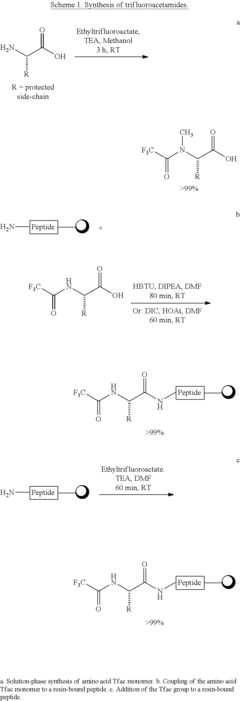
- The use of trifluoroacetamide (Tfac) as a novel amine protecting group in solid phase peptide synthesis, which is stable under reaction conditions, easily removable with sodium borohydride in a mixed ethanol/tetrahydrofuran solvent system, and facilitates site-specific N-methylation under Mitsunobu conditions, offering improved orthogonality and atom economy.
Safety and Handling Protocols for Fluoroantimonic Acid
Fluoroantimonic acid, recognized as one of the strongest superacids, demands rigorous safety and handling protocols due to its extreme corrosiveness and reactivity. The implementation of comprehensive safety measures is paramount to mitigate risks associated with its use in next-generation reaction protocols.
Personal protective equipment (PPE) forms the first line of defense when working with fluoroantimonic acid. Chemists and laboratory personnel must don impermeable, acid-resistant suits, gloves, and footwear. Face shields and respirators with appropriate filters are essential to protect against potential splashes or fumes.
Storage and containment of fluoroantimonic acid require specialized materials resistant to its corrosive nature. Teflon (PTFE) containers are commonly used due to their chemical inertness. These containers must be sealed tightly and stored in a cool, dry area away from incompatible substances, particularly those containing water or organic compounds.
Handling procedures for fluoroantimonic acid necessitate the use of a fume hood with proper ventilation to prevent exposure to harmful vapors. All transfers and reactions involving this superacid should be conducted in inert atmospheres, typically using dry nitrogen or argon, to prevent unwanted reactions with atmospheric moisture.
Emergency response protocols are crucial when working with fluoroantimonic acid. Laboratories must be equipped with easily accessible emergency showers, eyewash stations, and spill containment kits. Staff should be trained in proper spill cleanup procedures, which typically involve neutralization with appropriate bases followed by absorption with inert materials.
Waste disposal of fluoroantimonic acid and related materials requires careful consideration. Neutralization is generally the first step, followed by proper packaging and labeling of waste containers. Disposal must comply with local, state, and federal regulations governing hazardous waste management.
Training and education play a vital role in ensuring the safe handling of fluoroantimonic acid. Regular safety briefings, hands-on training sessions, and up-to-date standard operating procedures (SOPs) should be provided to all personnel working with or around this superacid.
Monitoring and maintenance of safety equipment and infrastructure are essential for long-term safety. Regular inspections of PPE, storage facilities, and emergency response equipment should be conducted and documented. Any deficiencies must be addressed promptly to maintain a safe working environment.
Personal protective equipment (PPE) forms the first line of defense when working with fluoroantimonic acid. Chemists and laboratory personnel must don impermeable, acid-resistant suits, gloves, and footwear. Face shields and respirators with appropriate filters are essential to protect against potential splashes or fumes.
Storage and containment of fluoroantimonic acid require specialized materials resistant to its corrosive nature. Teflon (PTFE) containers are commonly used due to their chemical inertness. These containers must be sealed tightly and stored in a cool, dry area away from incompatible substances, particularly those containing water or organic compounds.
Handling procedures for fluoroantimonic acid necessitate the use of a fume hood with proper ventilation to prevent exposure to harmful vapors. All transfers and reactions involving this superacid should be conducted in inert atmospheres, typically using dry nitrogen or argon, to prevent unwanted reactions with atmospheric moisture.
Emergency response protocols are crucial when working with fluoroantimonic acid. Laboratories must be equipped with easily accessible emergency showers, eyewash stations, and spill containment kits. Staff should be trained in proper spill cleanup procedures, which typically involve neutralization with appropriate bases followed by absorption with inert materials.
Waste disposal of fluoroantimonic acid and related materials requires careful consideration. Neutralization is generally the first step, followed by proper packaging and labeling of waste containers. Disposal must comply with local, state, and federal regulations governing hazardous waste management.
Training and education play a vital role in ensuring the safe handling of fluoroantimonic acid. Regular safety briefings, hands-on training sessions, and up-to-date standard operating procedures (SOPs) should be provided to all personnel working with or around this superacid.
Monitoring and maintenance of safety equipment and infrastructure are essential for long-term safety. Regular inspections of PPE, storage facilities, and emergency response equipment should be conducted and documented. Any deficiencies must be addressed promptly to maintain a safe working environment.
Environmental Impact and Sustainability Considerations
Fluoroantimonic acid, while a powerful tool for next-generation reaction protocols, raises significant environmental and sustainability concerns. The production, use, and disposal of this superacid require careful consideration to minimize its ecological impact. One of the primary environmental risks associated with fluoroantimonic acid is its extreme corrosiveness and reactivity. Any accidental release could lead to severe damage to ecosystems, potentially causing long-lasting harm to soil, water bodies, and local flora and fauna.
The synthesis of fluoroantimonic acid involves the use of hydrofluoric acid and antimony pentafluoride, both of which are hazardous substances with their own environmental implications. The production process may contribute to greenhouse gas emissions and energy consumption, further impacting climate change. Additionally, the storage and transportation of fluoroantimonic acid pose risks of spills or leaks, necessitating robust safety measures and containment protocols.
In terms of sustainability, the use of fluoroantimonic acid in industrial processes must be evaluated against alternative, more environmentally friendly catalysts or reaction methods. While its exceptional catalytic properties may lead to more efficient reactions and potentially reduce overall resource consumption, this benefit must be weighed against the environmental risks and the challenges of safe handling and disposal.
The disposal of fluoroantimonic acid and its byproducts presents another significant environmental challenge. Neutralization and treatment of waste streams containing this superacid require specialized facilities and processes to prevent contamination of water sources and soil. The potential for long-term environmental persistence of fluorine and antimony compounds derived from fluoroantimonic acid must also be considered in lifecycle assessments.
To address these environmental and sustainability concerns, research efforts should focus on developing greener alternatives or improving the efficiency and safety of fluoroantimonic acid-based processes. This may include exploring ionic liquid alternatives, designing closed-loop systems to minimize waste, or developing more effective neutralization and disposal methods. Additionally, implementing strict regulatory frameworks and industry best practices for the handling, use, and disposal of fluoroantimonic acid is crucial to mitigate its environmental impact.
As the chemical industry moves towards more sustainable practices, the role of fluoroantimonic acid in next-generation reaction protocols must be carefully balanced with its environmental implications. Continuous monitoring, risk assessment, and improvement of safety measures are essential to ensure that the benefits of this powerful tool do not come at an unacceptable environmental cost. Future research should prioritize the development of equally effective but more environmentally benign alternatives to fluoroantimonic acid, aligning with global sustainability goals and the principles of green chemistry.
The synthesis of fluoroantimonic acid involves the use of hydrofluoric acid and antimony pentafluoride, both of which are hazardous substances with their own environmental implications. The production process may contribute to greenhouse gas emissions and energy consumption, further impacting climate change. Additionally, the storage and transportation of fluoroantimonic acid pose risks of spills or leaks, necessitating robust safety measures and containment protocols.
In terms of sustainability, the use of fluoroantimonic acid in industrial processes must be evaluated against alternative, more environmentally friendly catalysts or reaction methods. While its exceptional catalytic properties may lead to more efficient reactions and potentially reduce overall resource consumption, this benefit must be weighed against the environmental risks and the challenges of safe handling and disposal.
The disposal of fluoroantimonic acid and its byproducts presents another significant environmental challenge. Neutralization and treatment of waste streams containing this superacid require specialized facilities and processes to prevent contamination of water sources and soil. The potential for long-term environmental persistence of fluorine and antimony compounds derived from fluoroantimonic acid must also be considered in lifecycle assessments.
To address these environmental and sustainability concerns, research efforts should focus on developing greener alternatives or improving the efficiency and safety of fluoroantimonic acid-based processes. This may include exploring ionic liquid alternatives, designing closed-loop systems to minimize waste, or developing more effective neutralization and disposal methods. Additionally, implementing strict regulatory frameworks and industry best practices for the handling, use, and disposal of fluoroantimonic acid is crucial to mitigate its environmental impact.
As the chemical industry moves towards more sustainable practices, the role of fluoroantimonic acid in next-generation reaction protocols must be carefully balanced with its environmental implications. Continuous monitoring, risk assessment, and improvement of safety measures are essential to ensure that the benefits of this powerful tool do not come at an unacceptable environmental cost. Future research should prioritize the development of equally effective but more environmentally benign alternatives to fluoroantimonic acid, aligning with global sustainability goals and the principles of green chemistry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!