Exploring Carbon Tetrachloride Trends in Chemical Manufacturing
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Manufacturing History and Objectives
Carbon tetrachloride (CCl4) has played a significant role in the chemical manufacturing industry since its discovery in the mid-19th century. Initially synthesized by Henri Victor Regnault in 1839, CCl4 quickly gained prominence due to its unique properties and versatile applications. The compound's non-flammability and excellent solvency made it an attractive choice for various industrial processes.
Throughout the early 20th century, CCl4 manufacturing saw substantial growth, driven by its widespread use as a cleaning agent, fire extinguishing medium, and precursor in the production of refrigerants. The chemical's popularity peaked during the 1950s and 1960s, with global production reaching hundreds of thousands of tons annually. This period marked the zenith of CCl4's industrial significance, as it became an integral component in numerous manufacturing processes.
However, the 1970s brought a paradigm shift in the perception and utilization of CCl4. Scientific research revealed its detrimental effects on the ozone layer and potential health hazards. This discovery led to a gradual decline in CCl4 production and usage, as international regulations and environmental concerns began to reshape the chemical manufacturing landscape.
The Montreal Protocol, signed in 1987, marked a turning point in CCl4 manufacturing history. This landmark agreement aimed to phase out the production of ozone-depleting substances, including CCl4. As a result, the chemical industry faced the challenge of finding alternative compounds and developing new manufacturing processes to replace CCl4-dependent operations.
In recent decades, the objectives of CCl4 manufacturing have undergone a significant transformation. While production has drastically reduced, research efforts have focused on developing more environmentally friendly alternatives and improving detection methods for trace amounts of CCl4 in the atmosphere. The industry has also prioritized the development of technologies for the safe disposal and destruction of existing CCl4 stockpiles.
Today, the primary objectives in CCl4 manufacturing revolve around sustainable practices, regulatory compliance, and minimizing environmental impact. Researchers and manufacturers are exploring novel synthesis routes that reduce CCl4 emissions and investigating potential applications in niche areas where its unique properties remain valuable. Additionally, there is a growing emphasis on monitoring and mitigating the long-term effects of historical CCl4 usage on the environment and human health.
As we look towards the future, the trajectory of CCl4 manufacturing continues to evolve. The industry faces the dual challenge of addressing legacy issues while adapting to stringent environmental regulations. Emerging technologies and innovative approaches in green chemistry offer promising avenues for reimagining the role of CCl4 in chemical manufacturing, with a focus on sustainability and responsible production practices.
Throughout the early 20th century, CCl4 manufacturing saw substantial growth, driven by its widespread use as a cleaning agent, fire extinguishing medium, and precursor in the production of refrigerants. The chemical's popularity peaked during the 1950s and 1960s, with global production reaching hundreds of thousands of tons annually. This period marked the zenith of CCl4's industrial significance, as it became an integral component in numerous manufacturing processes.
However, the 1970s brought a paradigm shift in the perception and utilization of CCl4. Scientific research revealed its detrimental effects on the ozone layer and potential health hazards. This discovery led to a gradual decline in CCl4 production and usage, as international regulations and environmental concerns began to reshape the chemical manufacturing landscape.
The Montreal Protocol, signed in 1987, marked a turning point in CCl4 manufacturing history. This landmark agreement aimed to phase out the production of ozone-depleting substances, including CCl4. As a result, the chemical industry faced the challenge of finding alternative compounds and developing new manufacturing processes to replace CCl4-dependent operations.
In recent decades, the objectives of CCl4 manufacturing have undergone a significant transformation. While production has drastically reduced, research efforts have focused on developing more environmentally friendly alternatives and improving detection methods for trace amounts of CCl4 in the atmosphere. The industry has also prioritized the development of technologies for the safe disposal and destruction of existing CCl4 stockpiles.
Today, the primary objectives in CCl4 manufacturing revolve around sustainable practices, regulatory compliance, and minimizing environmental impact. Researchers and manufacturers are exploring novel synthesis routes that reduce CCl4 emissions and investigating potential applications in niche areas where its unique properties remain valuable. Additionally, there is a growing emphasis on monitoring and mitigating the long-term effects of historical CCl4 usage on the environment and human health.
As we look towards the future, the trajectory of CCl4 manufacturing continues to evolve. The industry faces the dual challenge of addressing legacy issues while adapting to stringent environmental regulations. Emerging technologies and innovative approaches in green chemistry offer promising avenues for reimagining the role of CCl4 in chemical manufacturing, with a focus on sustainability and responsible production practices.
CCl4 Market Demand Analysis
The global market demand for carbon tetrachloride (CCl4) has undergone significant changes in recent decades, primarily due to environmental regulations and shifts in industrial applications. Historically, CCl4 was widely used as a solvent, cleaning agent, and feedstock in various chemical processes. However, its ozone-depleting properties led to strict regulations under the Montreal Protocol, resulting in a sharp decline in its production and consumption for many traditional applications.
Despite these restrictions, CCl4 continues to play a role in specific industrial processes, particularly in the manufacturing of certain chemicals. The current market demand is primarily driven by its use as a feedstock for the production of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs), which are used as refrigerants and blowing agents. This shift has created a more specialized and controlled market for CCl4.
The pharmaceutical industry represents another significant sector contributing to CCl4 demand. It is used in the synthesis of various pharmaceutical intermediates and active pharmaceutical ingredients (APIs). The growth of the global pharmaceutical market, especially in emerging economies, has helped sustain a steady demand for CCl4 in this sector.
In the agrochemical industry, CCl4 finds application in the production of certain pesticides and herbicides. While alternatives are being developed, some processes still rely on CCl4 as a key intermediate. The global push for increased agricultural productivity continues to support this segment of the market.
Geographically, the demand for CCl4 varies significantly. Developed countries have largely phased out its use in many applications, adhering to strict environmental regulations. However, some developing countries still permit its use in certain industrial processes, leading to regional disparities in market demand.
The future market demand for CCl4 is expected to be influenced by several factors. Ongoing research into alternative chemicals and processes may further reduce its use in traditional applications. Conversely, the development of new, highly controlled applications in advanced materials or specialty chemicals could potentially create new demand streams.
Environmental concerns and regulatory pressures continue to shape the CCl4 market. The implementation of the Kigali Amendment to the Montreal Protocol, which aims to phase down HFCs, may indirectly impact CCl4 demand in the refrigerant industry. This could lead to a shift towards more environmentally friendly alternatives, potentially reducing the long-term demand for CCl4 in this sector.
Despite these restrictions, CCl4 continues to play a role in specific industrial processes, particularly in the manufacturing of certain chemicals. The current market demand is primarily driven by its use as a feedstock for the production of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs), which are used as refrigerants and blowing agents. This shift has created a more specialized and controlled market for CCl4.
The pharmaceutical industry represents another significant sector contributing to CCl4 demand. It is used in the synthesis of various pharmaceutical intermediates and active pharmaceutical ingredients (APIs). The growth of the global pharmaceutical market, especially in emerging economies, has helped sustain a steady demand for CCl4 in this sector.
In the agrochemical industry, CCl4 finds application in the production of certain pesticides and herbicides. While alternatives are being developed, some processes still rely on CCl4 as a key intermediate. The global push for increased agricultural productivity continues to support this segment of the market.
Geographically, the demand for CCl4 varies significantly. Developed countries have largely phased out its use in many applications, adhering to strict environmental regulations. However, some developing countries still permit its use in certain industrial processes, leading to regional disparities in market demand.
The future market demand for CCl4 is expected to be influenced by several factors. Ongoing research into alternative chemicals and processes may further reduce its use in traditional applications. Conversely, the development of new, highly controlled applications in advanced materials or specialty chemicals could potentially create new demand streams.
Environmental concerns and regulatory pressures continue to shape the CCl4 market. The implementation of the Kigali Amendment to the Montreal Protocol, which aims to phase down HFCs, may indirectly impact CCl4 demand in the refrigerant industry. This could lead to a shift towards more environmentally friendly alternatives, potentially reducing the long-term demand for CCl4 in this sector.
CCl4 Production Challenges
Carbon tetrachloride (CCl4) production faces numerous challenges in the chemical manufacturing industry. The primary obstacle is the environmental impact associated with its production and use. CCl4 is a known ozone-depleting substance and greenhouse gas, leading to strict regulations and phase-out programs worldwide. This has significantly reduced its demand and production volumes, forcing manufacturers to adapt their processes and explore alternatives.
Another major challenge is the toxicity of CCl4 and its precursors. Exposure to CCl4 can cause severe health issues, including liver and kidney damage. This necessitates stringent safety measures in production facilities, increasing operational costs and complexity. The handling, storage, and transportation of CCl4 also require specialized equipment and procedures to minimize risks, further adding to the production challenges.
The traditional production methods for CCl4, such as the chlorination of methane or carbon disulfide, are energy-intensive and generate significant amounts of waste. This not only raises environmental concerns but also impacts the economic viability of CCl4 production. Manufacturers are under pressure to develop more efficient and environmentally friendly production processes, which often require substantial investments in research and development.
Regulatory compliance presents another significant hurdle for CCl4 producers. The Montreal Protocol and subsequent amendments have mandated the phase-out of CCl4 production for dispersive uses. While some exemptions exist for specific applications, such as feedstock for other chemicals, producers must navigate a complex regulatory landscape. This includes obtaining necessary permits, adhering to strict emission controls, and maintaining detailed records of production and sales.
The declining market for CCl4 due to environmental regulations has led to overcapacity in the industry. Many manufacturers have been forced to shut down or repurpose their production facilities, leading to economic challenges for companies heavily invested in CCl4 production. This situation has created a need for strategic planning and diversification to maintain profitability in the face of shrinking demand.
Lastly, the search for suitable alternatives to CCl4 in various applications poses a significant challenge. While progress has been made in finding substitutes for many uses, some niche applications still rely on CCl4 due to its unique properties. Developing effective and economically viable alternatives requires ongoing research and development efforts, adding to the overall challenges faced by the industry in transitioning away from CCl4 production.
Another major challenge is the toxicity of CCl4 and its precursors. Exposure to CCl4 can cause severe health issues, including liver and kidney damage. This necessitates stringent safety measures in production facilities, increasing operational costs and complexity. The handling, storage, and transportation of CCl4 also require specialized equipment and procedures to minimize risks, further adding to the production challenges.
The traditional production methods for CCl4, such as the chlorination of methane or carbon disulfide, are energy-intensive and generate significant amounts of waste. This not only raises environmental concerns but also impacts the economic viability of CCl4 production. Manufacturers are under pressure to develop more efficient and environmentally friendly production processes, which often require substantial investments in research and development.
Regulatory compliance presents another significant hurdle for CCl4 producers. The Montreal Protocol and subsequent amendments have mandated the phase-out of CCl4 production for dispersive uses. While some exemptions exist for specific applications, such as feedstock for other chemicals, producers must navigate a complex regulatory landscape. This includes obtaining necessary permits, adhering to strict emission controls, and maintaining detailed records of production and sales.
The declining market for CCl4 due to environmental regulations has led to overcapacity in the industry. Many manufacturers have been forced to shut down or repurpose their production facilities, leading to economic challenges for companies heavily invested in CCl4 production. This situation has created a need for strategic planning and diversification to maintain profitability in the face of shrinking demand.
Lastly, the search for suitable alternatives to CCl4 in various applications poses a significant challenge. While progress has been made in finding substitutes for many uses, some niche applications still rely on CCl4 due to its unique properties. Developing effective and economically viable alternatives requires ongoing research and development efforts, adding to the overall challenges faced by the industry in transitioning away from CCl4 production.
Current CCl4 Production Methods
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, showcasing its versatility in chemical manufacturing.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes studies on its effects on the ozone layer and strategies for reducing its use in industrial processes.
- Detection and analysis methods: Various techniques and apparatus have been developed for detecting and analyzing carbon tetrachloride in different environments. These include spectroscopic methods, chromatography, and specialized sensors designed to measure carbon tetrachloride concentrations in air, water, or other media.
- Historical uses and patents: Early patents and historical documents reveal the diverse applications of carbon tetrachloride in the past, including its use in fire extinguishers, dry cleaning, and as a degreasing agent. These historical patents provide insight into the evolution of carbon tetrachloride's industrial applications over time.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its unique properties make it valuable in certain industrial applications, despite environmental concerns.Expand Specific Solutions03 Environmental and safety considerations
Due to its ozone-depleting properties and potential health hazards, the use of carbon tetrachloride has been restricted. Research focuses on developing safer alternatives and methods for its proper handling, disposal, and environmental remediation.Expand Specific Solutions04 Detection and analysis methods
Various analytical techniques and instruments have been developed for detecting and quantifying carbon tetrachloride in different matrices, including air, water, and soil. These methods are crucial for environmental monitoring and quality control in industrial processes.Expand Specific Solutions05 Historical uses and patents
Early patents and historical documents reveal the diverse applications of carbon tetrachloride, including its use as a fire extinguishing agent, cleaning solvent, and in the production of refrigerants. Many of these applications have since been phased out due to safety and environmental concerns.Expand Specific Solutions
Key CCl4 Manufacturers
The carbon tetrachloride market in chemical manufacturing is in a mature phase, with a global market size estimated at around $30-40 million annually. The industry is characterized by stringent regulations due to environmental concerns, leading to a decline in production and use. Major players like DuPont de Nemours, Occidental Chemical Corp., and The Chemours Co. have significant market presence, leveraging their advanced technologies and extensive distribution networks. The technology for carbon tetrachloride production is well-established, with companies focusing on developing alternative, more environmentally friendly substitutes. Emerging players such as Wacker Chemie AG and Bayer AG are investing in research and development to explore new applications and safer production methods, aiming to capture market share in this evolving landscape.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has developed advanced chlorination processes for carbon tetrachloride production, focusing on improving efficiency and reducing environmental impact. Their technology utilizes a proprietary catalyst system that enhances selectivity and yield in the chlorination of methane or other hydrocarbons[1]. The process operates at optimized temperatures and pressures to minimize energy consumption while maximizing product purity. Additionally, they have implemented closed-loop recycling systems to capture and reuse unreacted chlorine, significantly reducing waste and emissions[3]. The company has also invested in state-of-the-art scrubbing and treatment technologies to further mitigate environmental concerns associated with carbon tetrachloride production[5].
Strengths: High efficiency, reduced environmental impact, and improved product purity. Weaknesses: Potential high capital costs for implementation and ongoing catalyst management requirements.
DuPont de Nemours, Inc.
Technical Solution: DuPont has pioneered innovative approaches to carbon tetrachloride production and handling, with a strong focus on sustainability and safety. Their technology incorporates advanced process control systems that optimize reaction conditions in real-time, ensuring consistent product quality while minimizing energy consumption[2]. DuPont has also developed specialized containment and handling equipment designed to prevent leaks and emissions during production and storage. Furthermore, they have implemented a comprehensive life cycle assessment approach to track and reduce the environmental footprint of carbon tetrachloride throughout its production and use[4]. The company's research efforts have also led to the development of alternative compounds that can replace carbon tetrachloride in some applications, contributing to overall reduction in its production and use[6].
Strengths: Advanced process control, strong safety measures, and focus on sustainable alternatives. Weaknesses: Potential higher production costs due to advanced technologies and safety measures.
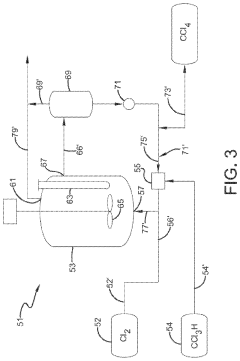
CCl4 Production Innovations
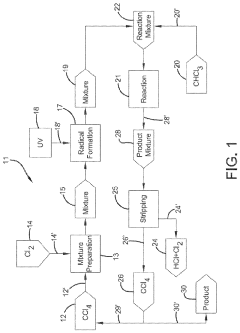
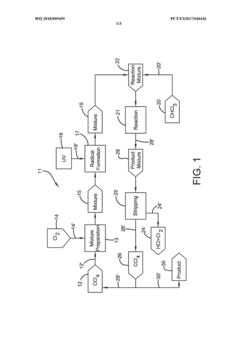
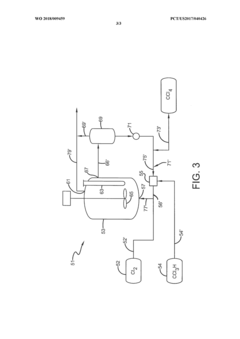
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
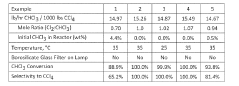
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
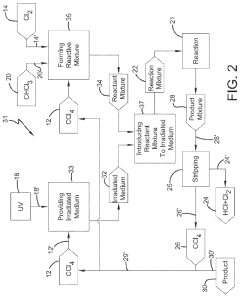
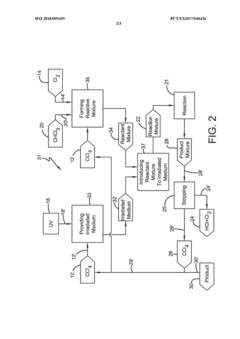
Photochlorination of chloroform to carbon tetrachloride
PatentWO2018009459A1
Innovation
- A photochlorination process involving the reaction of chlorine with chloroform in the presence of electromagnetic radiation within a carbon tetrachloride medium, maintaining low chloroform concentrations and stoichiometric levels of chlorine, to selectively convert chloroform to carbon tetrachloride, with the reaction mixture being well-mixed and subjected to UV light of specific wavelengths.
Environmental Impact of CCl4
Carbon tetrachloride (CCl4) has been a significant concern in chemical manufacturing due to its severe environmental impacts. The release of CCl4 into the atmosphere has been linked to ozone depletion, contributing to the expansion of the ozone hole and increased ultraviolet radiation reaching the Earth's surface. This has led to various environmental and health issues, including increased risks of skin cancer and cataracts in humans, as well as damage to terrestrial and aquatic ecosystems.
In aquatic environments, CCl4 contamination poses a threat to marine life and water quality. Its persistence in water bodies can lead to bioaccumulation in aquatic organisms, potentially disrupting food chains and ecosystems. Groundwater contamination by CCl4 is particularly problematic, as it can persist for extended periods and spread over large areas, potentially affecting drinking water sources for both humans and wildlife.
Soil contamination is another significant environmental impact of CCl4. When released into soil, it can volatilize and contaminate the air or leach into groundwater. This contamination can have long-lasting effects on soil ecosystems, affecting microbial communities, plant growth, and soil fertility. The remediation of CCl4-contaminated soil and groundwater is often a complex and costly process.
The global warming potential of CCl4 is also a concern. Although not as potent as some other greenhouse gases, its long atmospheric lifetime contributes to its overall impact on climate change. The phaseout of CCl4 under the Montreal Protocol has helped reduce its environmental impact, but its legacy effects continue to be a concern for environmental scientists and policymakers.
Industrial accidents and improper disposal of CCl4 have led to localized environmental disasters. These incidents have resulted in acute environmental damage, including immediate toxicity to local flora and fauna, as well as long-term contamination of affected areas. Such events have highlighted the need for stringent safety measures and proper handling protocols in chemical manufacturing facilities.
The environmental impacts of CCl4 extend beyond its direct effects on ecosystems. Its production and use in chemical manufacturing processes often involve energy-intensive operations, contributing to indirect environmental impacts through increased carbon emissions. Additionally, the development and implementation of alternative substances and processes to replace CCl4 have their own environmental considerations, which must be carefully evaluated to ensure a net positive impact on the environment.
In aquatic environments, CCl4 contamination poses a threat to marine life and water quality. Its persistence in water bodies can lead to bioaccumulation in aquatic organisms, potentially disrupting food chains and ecosystems. Groundwater contamination by CCl4 is particularly problematic, as it can persist for extended periods and spread over large areas, potentially affecting drinking water sources for both humans and wildlife.
Soil contamination is another significant environmental impact of CCl4. When released into soil, it can volatilize and contaminate the air or leach into groundwater. This contamination can have long-lasting effects on soil ecosystems, affecting microbial communities, plant growth, and soil fertility. The remediation of CCl4-contaminated soil and groundwater is often a complex and costly process.
The global warming potential of CCl4 is also a concern. Although not as potent as some other greenhouse gases, its long atmospheric lifetime contributes to its overall impact on climate change. The phaseout of CCl4 under the Montreal Protocol has helped reduce its environmental impact, but its legacy effects continue to be a concern for environmental scientists and policymakers.
Industrial accidents and improper disposal of CCl4 have led to localized environmental disasters. These incidents have resulted in acute environmental damage, including immediate toxicity to local flora and fauna, as well as long-term contamination of affected areas. Such events have highlighted the need for stringent safety measures and proper handling protocols in chemical manufacturing facilities.
The environmental impacts of CCl4 extend beyond its direct effects on ecosystems. Its production and use in chemical manufacturing processes often involve energy-intensive operations, contributing to indirect environmental impacts through increased carbon emissions. Additionally, the development and implementation of alternative substances and processes to replace CCl4 have their own environmental considerations, which must be carefully evaluated to ensure a net positive impact on the environment.
CCl4 Regulations and Compliance
Carbon tetrachloride (CCl4) has been subject to increasingly stringent regulations due to its ozone-depleting properties and potential health hazards. The Montreal Protocol, implemented in 1989, marked a significant turning point in CCl4 regulation, mandating the phase-out of its production and consumption for non-feedstock uses. This international agreement has been instrumental in reducing global CCl4 emissions and protecting the ozone layer.
In the United States, the Environmental Protection Agency (EPA) has implemented strict controls on CCl4 under the Clean Air Act. The chemical is classified as a hazardous air pollutant and is subject to National Emission Standards for Hazardous Air Pollutants (NESHAP). These standards require chemical manufacturers to implement maximum achievable control technology (MACT) to minimize CCl4 emissions.
The European Union has also taken decisive action through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its PBT (persistent, bioaccumulative, and toxic) properties. This classification imposes strict requirements on manufacturers, importers, and downstream users of CCl4, including the need for authorization for specific uses.
Compliance with these regulations has necessitated significant changes in chemical manufacturing processes. Many companies have invested in alternative technologies and substitutes to reduce or eliminate CCl4 use. For instance, the electronics industry has largely phased out CCl4 as a cleaning solvent, replacing it with less harmful alternatives.
Monitoring and reporting requirements play a crucial role in ensuring compliance. Chemical manufacturers must maintain detailed records of CCl4 production, use, and disposal. Regular emissions monitoring and reporting to regulatory authorities are mandatory in many jurisdictions. Advanced leak detection and repair (LDAR) programs have become standard practice in facilities handling CCl4.
Despite these stringent measures, challenges remain in achieving full compliance. Legacy uses of CCl4, particularly in developing countries, continue to pose environmental risks. Additionally, the chemical's role as a feedstock in the production of other substances complicates complete phase-out efforts. Regulatory bodies are continuously refining their approaches to address these challenges, with a focus on improving enforcement mechanisms and promoting international cooperation.
As regulations evolve, chemical manufacturers must stay vigilant and adaptive. Ongoing research into safer alternatives and more efficient production processes is essential for long-term compliance and sustainability in the industry. The trend towards stricter CCl4 regulations is expected to continue, driving further innovations in chemical manufacturing and environmental protection technologies.
In the United States, the Environmental Protection Agency (EPA) has implemented strict controls on CCl4 under the Clean Air Act. The chemical is classified as a hazardous air pollutant and is subject to National Emission Standards for Hazardous Air Pollutants (NESHAP). These standards require chemical manufacturers to implement maximum achievable control technology (MACT) to minimize CCl4 emissions.
The European Union has also taken decisive action through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its PBT (persistent, bioaccumulative, and toxic) properties. This classification imposes strict requirements on manufacturers, importers, and downstream users of CCl4, including the need for authorization for specific uses.
Compliance with these regulations has necessitated significant changes in chemical manufacturing processes. Many companies have invested in alternative technologies and substitutes to reduce or eliminate CCl4 use. For instance, the electronics industry has largely phased out CCl4 as a cleaning solvent, replacing it with less harmful alternatives.
Monitoring and reporting requirements play a crucial role in ensuring compliance. Chemical manufacturers must maintain detailed records of CCl4 production, use, and disposal. Regular emissions monitoring and reporting to regulatory authorities are mandatory in many jurisdictions. Advanced leak detection and repair (LDAR) programs have become standard practice in facilities handling CCl4.
Despite these stringent measures, challenges remain in achieving full compliance. Legacy uses of CCl4, particularly in developing countries, continue to pose environmental risks. Additionally, the chemical's role as a feedstock in the production of other substances complicates complete phase-out efforts. Regulatory bodies are continuously refining their approaches to address these challenges, with a focus on improving enforcement mechanisms and promoting international cooperation.
As regulations evolve, chemical manufacturers must stay vigilant and adaptive. Ongoing research into safer alternatives and more efficient production processes is essential for long-term compliance and sustainability in the industry. The trend towards stricter CCl4 regulations is expected to continue, driving further innovations in chemical manufacturing and environmental protection technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!