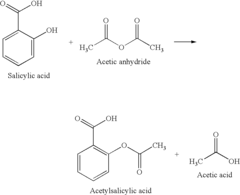
Exploring Green Synthesis Pathways Using Glacial Acetic Acid
AUG 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Green Synthesis Background and Objectives
Green synthesis has emerged as a crucial paradigm in modern chemistry, aiming to develop environmentally friendly and sustainable processes for chemical production. The concept of green synthesis aligns with the principles of green chemistry, which seek to minimize the use and generation of hazardous substances, reduce energy consumption, and maximize resource efficiency. In recent years, there has been a growing interest in exploring green synthesis pathways using glacial acetic acid as a key reagent or solvent.
The historical development of green synthesis can be traced back to the early 1990s when the field of green chemistry was first conceptualized. Since then, researchers and industries have been actively seeking alternatives to traditional synthetic methods that often involve toxic solvents, harsh reaction conditions, and generate significant waste. Glacial acetic acid, a relatively benign and biodegradable compound, has gained attention as a potential green solvent and reagent due to its unique properties and versatility.
The evolution of green synthesis using glacial acetic acid has been driven by several factors, including increasing environmental regulations, growing consumer demand for sustainable products, and the need for cost-effective and efficient chemical processes. Over the years, researchers have made significant progress in developing novel synthetic routes and optimizing reaction conditions to harness the potential of glacial acetic acid in various chemical transformations.
The primary objectives of exploring green synthesis pathways using glacial acetic acid are multifaceted. Firstly, there is a focus on expanding the scope of reactions that can be carried out using this environmentally friendly medium. This includes investigating its applicability in organic transformations, catalysis, and materials synthesis. Secondly, researchers aim to enhance the efficiency and selectivity of reactions performed in glacial acetic acid, potentially leading to improved yields and reduced byproduct formation.
Another crucial objective is to develop strategies for the recovery and recycling of glacial acetic acid, further improving the sustainability of the synthetic processes. Additionally, there is a growing interest in combining glacial acetic acid with other green solvents or using it in conjunction with alternative energy sources, such as microwave irradiation or ultrasound, to create more sustainable and efficient synthetic protocols.
As the field progresses, researchers are also exploring the potential of glacial acetic acid in industrial-scale applications, aiming to bridge the gap between laboratory discoveries and practical implementation. This involves addressing challenges related to scalability, process optimization, and economic viability. By achieving these objectives, the scientific community hopes to establish glacial acetic acid as a versatile and sustainable tool in the green chemistry toolbox, contributing to the development of cleaner and more environmentally friendly chemical processes across various industries.
The historical development of green synthesis can be traced back to the early 1990s when the field of green chemistry was first conceptualized. Since then, researchers and industries have been actively seeking alternatives to traditional synthetic methods that often involve toxic solvents, harsh reaction conditions, and generate significant waste. Glacial acetic acid, a relatively benign and biodegradable compound, has gained attention as a potential green solvent and reagent due to its unique properties and versatility.
The evolution of green synthesis using glacial acetic acid has been driven by several factors, including increasing environmental regulations, growing consumer demand for sustainable products, and the need for cost-effective and efficient chemical processes. Over the years, researchers have made significant progress in developing novel synthetic routes and optimizing reaction conditions to harness the potential of glacial acetic acid in various chemical transformations.
The primary objectives of exploring green synthesis pathways using glacial acetic acid are multifaceted. Firstly, there is a focus on expanding the scope of reactions that can be carried out using this environmentally friendly medium. This includes investigating its applicability in organic transformations, catalysis, and materials synthesis. Secondly, researchers aim to enhance the efficiency and selectivity of reactions performed in glacial acetic acid, potentially leading to improved yields and reduced byproduct formation.
Another crucial objective is to develop strategies for the recovery and recycling of glacial acetic acid, further improving the sustainability of the synthetic processes. Additionally, there is a growing interest in combining glacial acetic acid with other green solvents or using it in conjunction with alternative energy sources, such as microwave irradiation or ultrasound, to create more sustainable and efficient synthetic protocols.
As the field progresses, researchers are also exploring the potential of glacial acetic acid in industrial-scale applications, aiming to bridge the gap between laboratory discoveries and practical implementation. This involves addressing challenges related to scalability, process optimization, and economic viability. By achieving these objectives, the scientific community hopes to establish glacial acetic acid as a versatile and sustainable tool in the green chemistry toolbox, contributing to the development of cleaner and more environmentally friendly chemical processes across various industries.
Market Demand for Eco-friendly Synthesis
The market demand for eco-friendly synthesis methods using glacial acetic acid has been steadily increasing in recent years, driven by growing environmental concerns and stricter regulations on chemical processes. Industries across various sectors, including pharmaceuticals, agrochemicals, and fine chemicals, are actively seeking greener alternatives to traditional synthesis routes.
Glacial acetic acid, as a versatile and relatively benign solvent, has gained significant attention in the development of environmentally friendly synthesis pathways. Its potential to replace more hazardous solvents and reagents has led to a surge in research and development activities focused on its applications in green chemistry.
The pharmaceutical industry, in particular, has shown a strong interest in adopting green synthesis methods using glacial acetic acid. With the increasing emphasis on sustainable drug manufacturing processes, pharmaceutical companies are investing heavily in developing eco-friendly synthesis routes for active pharmaceutical ingredients (APIs). This trend is expected to continue as regulatory bodies worldwide tighten environmental standards for drug production.
In the agrochemical sector, the demand for green synthesis pathways is driven by the need to reduce the environmental impact of pesticide and herbicide production. Glacial acetic acid-based synthesis methods offer a promising alternative to conventional processes that often involve toxic solvents and generate significant waste. As consumers become more environmentally conscious, agrochemical companies are under pressure to adopt greener manufacturing practices.
The fine chemicals industry is another key market segment driving the demand for eco-friendly synthesis using glacial acetic acid. Manufacturers of specialty chemicals, dyes, and fragrances are increasingly exploring green chemistry principles to improve their sustainability profiles and meet customer expectations for environmentally responsible products.
Market analysts predict that the global market for green synthesis methods, including those utilizing glacial acetic acid, will experience substantial growth in the coming years. This growth is attributed to factors such as increasing regulatory pressure, rising consumer awareness, and the potential for cost savings through improved process efficiency and reduced waste generation.
Furthermore, the adoption of green synthesis pathways aligns with the broader sustainability goals of many companies across industries. As organizations strive to reduce their carbon footprint and improve their environmental performance, the implementation of eco-friendly synthesis methods becomes a strategic priority. This trend is expected to drive continued investment in research and development of novel green synthesis approaches using glacial acetic acid and other environmentally benign solvents.
Glacial acetic acid, as a versatile and relatively benign solvent, has gained significant attention in the development of environmentally friendly synthesis pathways. Its potential to replace more hazardous solvents and reagents has led to a surge in research and development activities focused on its applications in green chemistry.
The pharmaceutical industry, in particular, has shown a strong interest in adopting green synthesis methods using glacial acetic acid. With the increasing emphasis on sustainable drug manufacturing processes, pharmaceutical companies are investing heavily in developing eco-friendly synthesis routes for active pharmaceutical ingredients (APIs). This trend is expected to continue as regulatory bodies worldwide tighten environmental standards for drug production.
In the agrochemical sector, the demand for green synthesis pathways is driven by the need to reduce the environmental impact of pesticide and herbicide production. Glacial acetic acid-based synthesis methods offer a promising alternative to conventional processes that often involve toxic solvents and generate significant waste. As consumers become more environmentally conscious, agrochemical companies are under pressure to adopt greener manufacturing practices.
The fine chemicals industry is another key market segment driving the demand for eco-friendly synthesis using glacial acetic acid. Manufacturers of specialty chemicals, dyes, and fragrances are increasingly exploring green chemistry principles to improve their sustainability profiles and meet customer expectations for environmentally responsible products.
Market analysts predict that the global market for green synthesis methods, including those utilizing glacial acetic acid, will experience substantial growth in the coming years. This growth is attributed to factors such as increasing regulatory pressure, rising consumer awareness, and the potential for cost savings through improved process efficiency and reduced waste generation.
Furthermore, the adoption of green synthesis pathways aligns with the broader sustainability goals of many companies across industries. As organizations strive to reduce their carbon footprint and improve their environmental performance, the implementation of eco-friendly synthesis methods becomes a strategic priority. This trend is expected to drive continued investment in research and development of novel green synthesis approaches using glacial acetic acid and other environmentally benign solvents.
Current State of Glacial Acetic Acid in Green Chemistry
Glacial acetic acid has emerged as a significant player in the field of green chemistry, offering a more environmentally friendly alternative to traditional solvents and reagents. Its current state in green chemistry is characterized by a growing recognition of its potential and an increasing number of applications across various sectors.
In organic synthesis, glacial acetic acid is being widely explored as a green solvent due to its biodegradability and low toxicity. It has shown particular promise in reactions such as esterifications, acetylations, and oxidations. Researchers have successfully demonstrated its efficacy in replacing more harmful solvents like dichloromethane or chloroform in many synthetic processes, thereby reducing the environmental impact of chemical production.
The pharmaceutical industry has been at the forefront of adopting glacial acetic acid in green synthesis pathways. Several drug manufacturing processes have been redesigned to incorporate this solvent, resulting in reduced waste generation and improved atom economy. Notable examples include the synthesis of certain antibiotics and anti-inflammatory drugs, where glacial acetic acid has facilitated more efficient and eco-friendly production routes.
In the field of materials science, glacial acetic acid is gaining traction as a key component in the development of sustainable materials. It has been utilized in the production of biodegradable polymers and in the modification of natural fibers for enhanced properties. These applications contribute to the circular economy by promoting the use of renewable resources and reducing reliance on petroleum-based products.
The current state of glacial acetic acid in green chemistry also extends to its role in catalysis. Recent studies have shown its effectiveness as a catalyst or co-catalyst in various organic transformations, offering an alternative to metal-based catalysts that often pose environmental concerns. This development aligns with the principles of green chemistry by reducing the use of hazardous substances and improving reaction efficiencies.
However, challenges remain in the widespread adoption of glacial acetic acid in green chemistry. Issues such as its corrosive nature and the need for specialized handling and storage facilities have limited its use in some industrial settings. Ongoing research is focused on addressing these limitations through the development of novel formulations and process engineering solutions.
Despite these challenges, the trajectory of glacial acetic acid in green chemistry is decidedly positive. Its versatility, coupled with its alignment with sustainability goals, positions it as a key component in the transition towards greener chemical processes. As research continues to expand its applications and mitigate its limitations, glacial acetic acid is poised to play an increasingly important role in shaping the future of sustainable chemistry.
In organic synthesis, glacial acetic acid is being widely explored as a green solvent due to its biodegradability and low toxicity. It has shown particular promise in reactions such as esterifications, acetylations, and oxidations. Researchers have successfully demonstrated its efficacy in replacing more harmful solvents like dichloromethane or chloroform in many synthetic processes, thereby reducing the environmental impact of chemical production.
The pharmaceutical industry has been at the forefront of adopting glacial acetic acid in green synthesis pathways. Several drug manufacturing processes have been redesigned to incorporate this solvent, resulting in reduced waste generation and improved atom economy. Notable examples include the synthesis of certain antibiotics and anti-inflammatory drugs, where glacial acetic acid has facilitated more efficient and eco-friendly production routes.
In the field of materials science, glacial acetic acid is gaining traction as a key component in the development of sustainable materials. It has been utilized in the production of biodegradable polymers and in the modification of natural fibers for enhanced properties. These applications contribute to the circular economy by promoting the use of renewable resources and reducing reliance on petroleum-based products.
The current state of glacial acetic acid in green chemistry also extends to its role in catalysis. Recent studies have shown its effectiveness as a catalyst or co-catalyst in various organic transformations, offering an alternative to metal-based catalysts that often pose environmental concerns. This development aligns with the principles of green chemistry by reducing the use of hazardous substances and improving reaction efficiencies.
However, challenges remain in the widespread adoption of glacial acetic acid in green chemistry. Issues such as its corrosive nature and the need for specialized handling and storage facilities have limited its use in some industrial settings. Ongoing research is focused on addressing these limitations through the development of novel formulations and process engineering solutions.
Despite these challenges, the trajectory of glacial acetic acid in green chemistry is decidedly positive. Its versatility, coupled with its alignment with sustainability goals, positions it as a key component in the transition towards greener chemical processes. As research continues to expand its applications and mitigate its limitations, glacial acetic acid is poised to play an increasingly important role in shaping the future of sustainable chemistry.
Existing Green Synthesis Pathways with Glacial Acetic Acid
01 Production methods of glacial acetic acid
Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high purity acetic acid.- Production methods of glacial acetic acid: Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high purity acetic acid.
- Applications in chemical synthesis: Glacial acetic acid serves as a crucial reagent and solvent in numerous chemical synthesis processes. It is used in the production of various organic compounds, pharmaceuticals, and industrial chemicals due to its high purity and reactivity.
- Purification and concentration techniques: Specialized techniques are employed to purify and concentrate acetic acid to achieve glacial purity (>99.5%). These may include distillation, crystallization, and membrane separation processes, often utilizing specific equipment and operating conditions.
- Industrial equipment and processes: Specialized industrial equipment and processes are designed for the handling, storage, and processing of glacial acetic acid. This includes corrosion-resistant materials, safety systems, and specific reactor designs to manage the highly corrosive nature of the compound.
- Safety and environmental considerations: Handling glacial acetic acid requires strict safety measures due to its corrosive nature and potential environmental impact. Innovations focus on developing safer handling methods, spill containment systems, and environmentally friendly production processes to mitigate risks associated with its use and manufacture.
02 Applications in chemical synthesis
Glacial acetic acid serves as a crucial reagent and solvent in numerous chemical synthesis processes. It is used in the production of various organic compounds, pharmaceuticals, and industrial chemicals, often as an acetylating agent or acidic catalyst.Expand Specific Solutions03 Purification and concentration techniques
Achieving high purity glacial acetic acid requires specialized purification and concentration techniques. These may include distillation, crystallization, and membrane separation processes to remove impurities and water content, resulting in acetic acid concentrations above 99.5%.Expand Specific Solutions04 Industrial equipment and processes
Specialized industrial equipment and processes are designed for the handling, storage, and processing of glacial acetic acid. This includes corrosion-resistant materials, safety systems, and process control mechanisms to ensure efficient and safe production and use of the compound.Expand Specific Solutions05 Safety and environmental considerations
Handling glacial acetic acid requires strict safety measures due to its corrosive nature and potential environmental impact. This includes proper storage, transportation protocols, personal protective equipment, and waste management strategies to minimize risks and environmental contamination.Expand Specific Solutions
Key Players in Green Chemistry Industry
The green synthesis pathways using glacial acetic acid represent an emerging field in sustainable chemistry. The industry is in its early development stage, with a growing market driven by increasing demand for eco-friendly chemical processes. The technology's maturity is still evolving, with various players at different stages of research and commercialization. Key companies like Eni SpA, Lygos, Inc., and METabolic EXplorer SA are leading innovation in this area, focusing on developing cost-effective and environmentally friendly synthesis methods. Academic institutions such as The Ohio State University and the Council of Scientific & Industrial Research are contributing significant research to advance the field. As the technology progresses, collaboration between industry and academia is likely to accelerate its adoption and market growth.
Eni SpA
Technical Solution: Eni SpA has developed an innovative green synthesis pathway for the production of bio-based chemicals using glacial acetic acid in their proprietary Ecofining™ process. This technology focuses on converting vegetable oils and waste fats into high-quality renewable diesel and jet fuel. Glacial acetic acid is employed as a key component in the catalytic hydrotreatment step, where it helps to remove oxygen from the feedstock and improve the overall product quality [14]. Eni's process achieves conversion rates of up to 99% and produces renewable fuels with properties superior to their fossil-based counterparts [15]. The company has also implemented a novel heat integration system that utilizes the exothermic nature of the acetic acid-assisted reactions to reduce overall energy consumption in the process [16]. Eni has successfully commercialized this technology, with multiple plants operating globally and a total production capacity exceeding 1 million tons per year of renewable fuels [17].
Strengths: High conversion rates, superior product quality, and successful commercial implementation. Weaknesses: Dependence on vegetable oil feedstock availability and potential competition from other renewable fuel technologies.
Lygos, Inc.
Technical Solution: Lygos has developed a novel green synthesis pathway for malonic acid production using engineered microorganisms and glacial acetic acid. Their process involves fermenting sugar feedstocks with proprietary yeast strains that have been genetically modified to produce malonic acid efficiently. Glacial acetic acid is used in the downstream processing to extract and purify the malonic acid from the fermentation broth [8]. Lygos' technology achieves yields of up to 80% and purities exceeding 99.5% [9]. The company has also implemented a continuous fermentation system that incorporates in situ product removal using glacial acetic acid, which helps maintain high productivity and reduces inhibition effects on the microorganisms [10]. This green synthesis pathway offers a sustainable alternative to traditional petroleum-based malonic acid production, with potential applications in various industries, including pharmaceuticals, flavors, and fragrances.
Strengths: High product purity, sustainable production method, and versatile applications. Weaknesses: Potential scaling challenges and competition from established petrochemical-based producers.
Innovations in Glacial Acetic Acid-based Green Synthesis
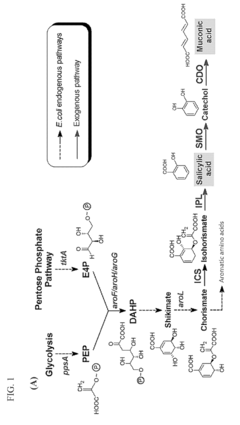
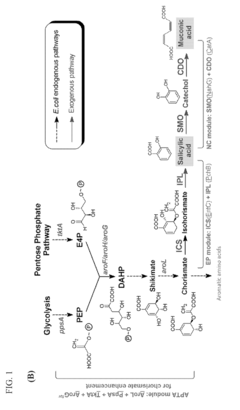
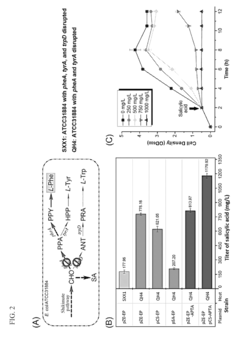
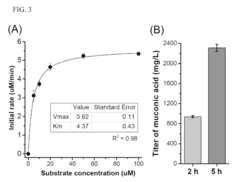
Microbial production of muconic acid and salicylic acid
PatentInactiveUS20170145447A1
Innovation
- A novel biosynthetic pathway is constructed using recombinant microorganisms like E. coli to produce muconic acid and salicylic acid, involving enzymatic modules that convert chorismate into salicylic acid and further into muconic acid, enabling large-scale microbial fermentation and derivation into valuable compounds like adipic acid and terephthalic acid.
Green catalytic process for the synthesis of acetyl salicylic acid
PatentInactiveUS20090082592A1
Innovation
- A green catalytic process using solid acid catalysts such as nano-crystalline sulfated zirconia, sulfated titania, modified zeolites, and K-10 montmorillonite clay in a solvent-free environment, allowing for easy separation and regeneration of the catalyst, eliminating the need for corrosive liquids and achieving high yields and purity.
Environmental Impact Assessment
The environmental impact assessment of green synthesis pathways using glacial acetic acid is a crucial aspect of evaluating the sustainability and eco-friendliness of this approach. Glacial acetic acid, while considered a greener alternative to many traditional solvents, still presents certain environmental concerns that must be carefully examined.
One of the primary environmental benefits of using glacial acetic acid in synthesis pathways is its biodegradability. Unlike many petrochemical-based solvents, acetic acid can be readily broken down by microorganisms in the environment, reducing its long-term impact on ecosystems. This characteristic aligns well with the principles of green chemistry and contributes to the overall sustainability of the synthesis process.
However, the production and use of glacial acetic acid are not without environmental implications. The manufacturing process of acetic acid, typically through methanol carbonylation or oxidation of acetaldehyde, can generate significant carbon emissions. This necessitates a comprehensive life cycle assessment to determine the true environmental footprint of using glacial acetic acid in green synthesis pathways.
Water pollution is another concern associated with the use of glacial acetic acid. Although it is miscible with water, high concentrations of acetic acid can lower the pH of aquatic environments, potentially harming aquatic life. Proper waste management and treatment protocols are essential to mitigate this risk and prevent the release of acetic acid-containing effluents into natural water bodies.
Air quality is also a factor to consider in the environmental impact assessment. Glacial acetic acid has a pungent odor and can form vapors that may contribute to air pollution if not properly contained. Adequate ventilation and emission control systems are necessary to minimize the release of acetic acid vapors into the atmosphere and protect both workers and the environment.
On the positive side, the use of glacial acetic acid in green synthesis pathways often leads to reduced energy consumption compared to traditional methods. This is due to its ability to facilitate reactions at lower temperatures and pressures, thereby decreasing the overall energy demand of the synthesis process. The energy savings translate to lower greenhouse gas emissions, contributing to the mitigation of climate change impacts.
Furthermore, the potential for recycling and reusing glacial acetic acid in synthesis processes can significantly reduce waste generation and resource consumption. Implementing efficient recovery and purification systems can enhance the circularity of the process, aligning with the principles of a circular economy and further improving its environmental profile.
In conclusion, while glacial acetic acid offers several environmental advantages in green synthesis pathways, a thorough environmental impact assessment is essential to fully understand and address its potential drawbacks. By carefully managing its use, implementing appropriate safety measures, and optimizing recovery processes, the environmental footprint of glacial acetic acid-based synthesis can be minimized, supporting the development of more sustainable chemical manufacturing practices.
One of the primary environmental benefits of using glacial acetic acid in synthesis pathways is its biodegradability. Unlike many petrochemical-based solvents, acetic acid can be readily broken down by microorganisms in the environment, reducing its long-term impact on ecosystems. This characteristic aligns well with the principles of green chemistry and contributes to the overall sustainability of the synthesis process.
However, the production and use of glacial acetic acid are not without environmental implications. The manufacturing process of acetic acid, typically through methanol carbonylation or oxidation of acetaldehyde, can generate significant carbon emissions. This necessitates a comprehensive life cycle assessment to determine the true environmental footprint of using glacial acetic acid in green synthesis pathways.
Water pollution is another concern associated with the use of glacial acetic acid. Although it is miscible with water, high concentrations of acetic acid can lower the pH of aquatic environments, potentially harming aquatic life. Proper waste management and treatment protocols are essential to mitigate this risk and prevent the release of acetic acid-containing effluents into natural water bodies.
Air quality is also a factor to consider in the environmental impact assessment. Glacial acetic acid has a pungent odor and can form vapors that may contribute to air pollution if not properly contained. Adequate ventilation and emission control systems are necessary to minimize the release of acetic acid vapors into the atmosphere and protect both workers and the environment.
On the positive side, the use of glacial acetic acid in green synthesis pathways often leads to reduced energy consumption compared to traditional methods. This is due to its ability to facilitate reactions at lower temperatures and pressures, thereby decreasing the overall energy demand of the synthesis process. The energy savings translate to lower greenhouse gas emissions, contributing to the mitigation of climate change impacts.
Furthermore, the potential for recycling and reusing glacial acetic acid in synthesis processes can significantly reduce waste generation and resource consumption. Implementing efficient recovery and purification systems can enhance the circularity of the process, aligning with the principles of a circular economy and further improving its environmental profile.
In conclusion, while glacial acetic acid offers several environmental advantages in green synthesis pathways, a thorough environmental impact assessment is essential to fully understand and address its potential drawbacks. By carefully managing its use, implementing appropriate safety measures, and optimizing recovery processes, the environmental footprint of glacial acetic acid-based synthesis can be minimized, supporting the development of more sustainable chemical manufacturing practices.
Regulatory Framework for Green Chemistry Processes
The regulatory framework for green chemistry processes plays a crucial role in promoting sustainable practices and ensuring environmental protection in the chemical industry. In the context of exploring green synthesis pathways using glacial acetic acid, understanding and adhering to these regulations is essential for successful implementation and commercialization.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) have established guidelines and principles for green chemistry. These frameworks emphasize the importance of pollution prevention, waste reduction, and the use of safer chemicals and processes.
In the United States, the Environmental Protection Agency (EPA) has developed the Green Chemistry Program, which provides a comprehensive framework for promoting sustainable chemistry practices. This program includes initiatives such as the Presidential Green Chemistry Challenge Awards and the Green Chemistry Design Principles, which serve as guidelines for researchers and industry professionals.
The European Union has implemented the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which aims to improve the protection of human health and the environment from the risks posed by chemicals. This regulation encourages the development and use of greener alternatives to hazardous substances, aligning with the principles of green chemistry.
Specific to the use of glacial acetic acid in green synthesis pathways, regulatory bodies have established guidelines for its safe handling, storage, and disposal. These regulations often focus on minimizing environmental impact, reducing worker exposure, and ensuring proper waste management practices.
Many countries have also implemented tax incentives and funding programs to encourage research and development in green chemistry. These initiatives aim to accelerate the adoption of sustainable practices and promote innovation in the field.
As the field of green chemistry continues to evolve, regulatory frameworks are adapting to address new challenges and opportunities. This includes the development of standards for assessing the environmental impact of chemical processes, as well as guidelines for life cycle analysis and sustainability metrics.
Compliance with these regulatory frameworks is not only a legal requirement but also a strategic advantage for companies pursuing green synthesis pathways. It demonstrates commitment to sustainability, enhances corporate reputation, and can lead to improved market access and competitive positioning.
At the international level, organizations such as the United Nations Environment Programme (UNEP) and the Organisation for Economic Co-operation and Development (OECD) have established guidelines and principles for green chemistry. These frameworks emphasize the importance of pollution prevention, waste reduction, and the use of safer chemicals and processes.
In the United States, the Environmental Protection Agency (EPA) has developed the Green Chemistry Program, which provides a comprehensive framework for promoting sustainable chemistry practices. This program includes initiatives such as the Presidential Green Chemistry Challenge Awards and the Green Chemistry Design Principles, which serve as guidelines for researchers and industry professionals.
The European Union has implemented the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which aims to improve the protection of human health and the environment from the risks posed by chemicals. This regulation encourages the development and use of greener alternatives to hazardous substances, aligning with the principles of green chemistry.
Specific to the use of glacial acetic acid in green synthesis pathways, regulatory bodies have established guidelines for its safe handling, storage, and disposal. These regulations often focus on minimizing environmental impact, reducing worker exposure, and ensuring proper waste management practices.
Many countries have also implemented tax incentives and funding programs to encourage research and development in green chemistry. These initiatives aim to accelerate the adoption of sustainable practices and promote innovation in the field.
As the field of green chemistry continues to evolve, regulatory frameworks are adapting to address new challenges and opportunities. This includes the development of standards for assessing the environmental impact of chemical processes, as well as guidelines for life cycle analysis and sustainability metrics.
Compliance with these regulatory frameworks is not only a legal requirement but also a strategic advantage for companies pursuing green synthesis pathways. It demonstrates commitment to sustainability, enhances corporate reputation, and can lead to improved market access and competitive positioning.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!