Exploring Silicon Micropillars in Biomaterials
JUL 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Silicon Micropillars in Biomaterials: Background and Objectives
Silicon micropillars have emerged as a promising technology in the field of biomaterials, offering unique properties and potential applications in various biomedical domains. The development of silicon micropillars can be traced back to the early 2000s when researchers began exploring the use of micro- and nanostructured surfaces for cell culture and tissue engineering. Since then, the technology has evolved rapidly, driven by advancements in microfabrication techniques and a growing understanding of cell-material interactions.
The primary objective of exploring silicon micropillars in biomaterials is to create biocompatible surfaces with precisely controlled topography and chemistry. These structures aim to mimic the natural extracellular matrix, providing an environment that can influence cell behavior, including adhesion, proliferation, and differentiation. By manipulating the size, shape, and spacing of micropillars, researchers seek to optimize cellular responses for specific applications in regenerative medicine, drug delivery, and biosensing.
One of the key advantages of silicon micropillars lies in their versatility and tunability. Silicon, as a material, offers excellent mechanical properties and can be easily modified through various surface treatments. This allows for the creation of micropillars with different surface chemistries, wettability, and stiffness, enabling researchers to study the impact of these parameters on cell behavior systematically.
The evolution of silicon micropillar technology has been closely linked to advances in microfabrication techniques, particularly photolithography and etching processes. These methods have enabled the production of highly uniform and reproducible micropillar arrays with precise control over their dimensions and spatial distribution. As fabrication techniques continue to improve, there is a growing trend towards creating more complex and hierarchical structures that better mimic the complexity of natural tissues.
Recent years have seen an increased focus on integrating silicon micropillars with other biomaterials and technologies. For instance, researchers are exploring the combination of micropillars with hydrogels, nanoparticles, and bioactive molecules to create multifunctional platforms for tissue engineering and drug delivery. Additionally, there is growing interest in developing dynamic micropillar systems that can respond to external stimuli, offering new possibilities for smart biomaterials and adaptive cell culture substrates.
Looking ahead, the field of silicon micropillars in biomaterials is poised for further growth and innovation. Key areas of development include improving the scalability and cost-effectiveness of fabrication processes, enhancing the long-term stability of micropillar structures in biological environments, and developing new methods for functionalizing micropillar surfaces with bioactive molecules. These advancements are expected to pave the way for novel biomedical applications and contribute to our understanding of cell-material interactions at the microscale.
The primary objective of exploring silicon micropillars in biomaterials is to create biocompatible surfaces with precisely controlled topography and chemistry. These structures aim to mimic the natural extracellular matrix, providing an environment that can influence cell behavior, including adhesion, proliferation, and differentiation. By manipulating the size, shape, and spacing of micropillars, researchers seek to optimize cellular responses for specific applications in regenerative medicine, drug delivery, and biosensing.
One of the key advantages of silicon micropillars lies in their versatility and tunability. Silicon, as a material, offers excellent mechanical properties and can be easily modified through various surface treatments. This allows for the creation of micropillars with different surface chemistries, wettability, and stiffness, enabling researchers to study the impact of these parameters on cell behavior systematically.
The evolution of silicon micropillar technology has been closely linked to advances in microfabrication techniques, particularly photolithography and etching processes. These methods have enabled the production of highly uniform and reproducible micropillar arrays with precise control over their dimensions and spatial distribution. As fabrication techniques continue to improve, there is a growing trend towards creating more complex and hierarchical structures that better mimic the complexity of natural tissues.
Recent years have seen an increased focus on integrating silicon micropillars with other biomaterials and technologies. For instance, researchers are exploring the combination of micropillars with hydrogels, nanoparticles, and bioactive molecules to create multifunctional platforms for tissue engineering and drug delivery. Additionally, there is growing interest in developing dynamic micropillar systems that can respond to external stimuli, offering new possibilities for smart biomaterials and adaptive cell culture substrates.
Looking ahead, the field of silicon micropillars in biomaterials is poised for further growth and innovation. Key areas of development include improving the scalability and cost-effectiveness of fabrication processes, enhancing the long-term stability of micropillar structures in biological environments, and developing new methods for functionalizing micropillar surfaces with bioactive molecules. These advancements are expected to pave the way for novel biomedical applications and contribute to our understanding of cell-material interactions at the microscale.
Market Analysis for Silicon Micropillar-Based Biomaterials
The market for silicon micropillar-based biomaterials is experiencing significant growth, driven by advancements in nanotechnology and increasing demand for innovative medical solutions. This emerging field combines the unique properties of silicon with the precision engineering of micropillars to create biomaterials with enhanced functionality and biocompatibility.
The healthcare sector represents the primary market for these materials, with applications spanning from drug delivery systems to tissue engineering scaffolds. The global biomaterials market, which encompasses silicon micropillar-based products, is projected to expand substantially in the coming years. This growth is fueled by an aging population, rising incidence of chronic diseases, and the need for more effective medical treatments.
In the pharmaceutical industry, silicon micropillar-based biomaterials are gaining traction for their potential in controlled drug release systems. These materials offer precise control over drug delivery kinetics, potentially improving treatment efficacy and reducing side effects. The drug delivery segment of the biomaterials market is expected to see robust growth, presenting significant opportunities for silicon micropillar technologies.
The orthopedic and dental implant sectors also show promise for silicon micropillar-based biomaterials. These materials can enhance osseointegration and reduce the risk of implant rejection, addressing key challenges in long-term implant success. As the demand for dental and orthopedic implants continues to rise globally, the market for advanced biomaterials in this space is poised for expansion.
Tissue engineering and regenerative medicine represent another crucial market segment for silicon micropillar-based biomaterials. These materials can serve as scaffolds for cell growth and tissue regeneration, offering potential solutions for organ repair and replacement. The regenerative medicine market is anticipated to grow rapidly, driven by increasing research activities and clinical applications.
Geographically, North America and Europe currently lead in the adoption of advanced biomaterials, including silicon micropillar-based products. However, Asia-Pacific is emerging as a significant market, with growing healthcare expenditure and increasing focus on medical technology innovation in countries like China, Japan, and South Korea.
Key market drivers include ongoing research and development efforts, increasing collaborations between academic institutions and industry players, and growing investment in healthcare infrastructure. However, challenges such as high development costs, stringent regulatory requirements, and the need for long-term clinical data may impact market growth rates.
The healthcare sector represents the primary market for these materials, with applications spanning from drug delivery systems to tissue engineering scaffolds. The global biomaterials market, which encompasses silicon micropillar-based products, is projected to expand substantially in the coming years. This growth is fueled by an aging population, rising incidence of chronic diseases, and the need for more effective medical treatments.
In the pharmaceutical industry, silicon micropillar-based biomaterials are gaining traction for their potential in controlled drug release systems. These materials offer precise control over drug delivery kinetics, potentially improving treatment efficacy and reducing side effects. The drug delivery segment of the biomaterials market is expected to see robust growth, presenting significant opportunities for silicon micropillar technologies.
The orthopedic and dental implant sectors also show promise for silicon micropillar-based biomaterials. These materials can enhance osseointegration and reduce the risk of implant rejection, addressing key challenges in long-term implant success. As the demand for dental and orthopedic implants continues to rise globally, the market for advanced biomaterials in this space is poised for expansion.
Tissue engineering and regenerative medicine represent another crucial market segment for silicon micropillar-based biomaterials. These materials can serve as scaffolds for cell growth and tissue regeneration, offering potential solutions for organ repair and replacement. The regenerative medicine market is anticipated to grow rapidly, driven by increasing research activities and clinical applications.
Geographically, North America and Europe currently lead in the adoption of advanced biomaterials, including silicon micropillar-based products. However, Asia-Pacific is emerging as a significant market, with growing healthcare expenditure and increasing focus on medical technology innovation in countries like China, Japan, and South Korea.
Key market drivers include ongoing research and development efforts, increasing collaborations between academic institutions and industry players, and growing investment in healthcare infrastructure. However, challenges such as high development costs, stringent regulatory requirements, and the need for long-term clinical data may impact market growth rates.
Current Challenges in Silicon Micropillar Fabrication
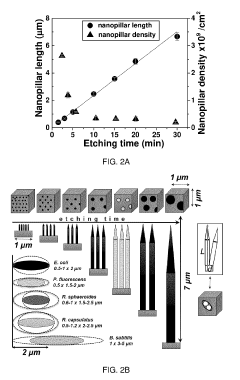
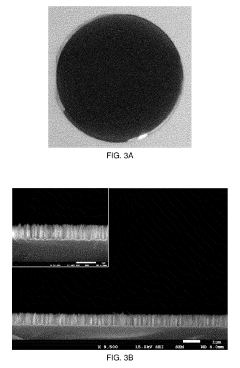
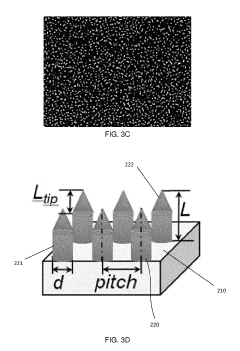
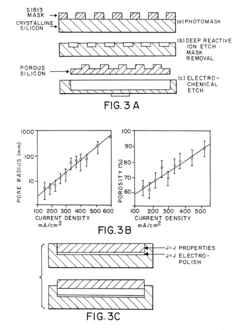
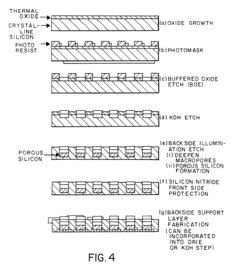
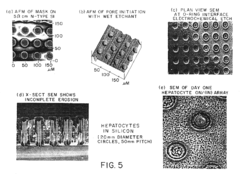
The fabrication of silicon micropillars for biomaterial applications faces several significant challenges that hinder their widespread adoption and optimal performance. One of the primary obstacles is achieving precise control over the dimensions and geometry of the micropillars. The ability to create uniform, high-aspect-ratio structures with consistent spacing and alignment is crucial for their effectiveness in biological interfaces. Current fabrication techniques often struggle to maintain this level of precision, especially when scaling up production.
Another major challenge lies in the surface modification of silicon micropillars. While silicon offers excellent mechanical properties and biocompatibility, its surface characteristics may not always be ideal for specific biological applications. Researchers face difficulties in developing reliable and scalable methods for functionalizing the micropillar surfaces without compromising their structural integrity or altering their mechanical properties.
The integration of silicon micropillars with other biomaterials and sensing elements presents additional complexities. Creating seamless interfaces between the micropillars and surrounding matrices or substrates is essential for maintaining the overall functionality of the biomaterial system. This integration often requires innovative approaches to ensure proper adhesion, signal transduction, and long-term stability under physiological conditions.
Durability and long-term performance of silicon micropillars in biological environments remain significant concerns. The potential degradation or fouling of these structures over time can impact their effectiveness and reliability in biomedical applications. Developing strategies to enhance the resistance of silicon micropillars to biological and chemical degradation without compromising their biocompatibility is an ongoing challenge.
The cost-effective and scalable production of silicon micropillars for biomaterials is another hurdle that researchers and manufacturers face. Current fabrication methods often involve complex, multi-step processes that can be time-consuming and expensive. Streamlining these processes while maintaining the required precision and quality is crucial for the commercial viability of silicon micropillar-based biomaterials.
Lastly, the characterization and quality control of silicon micropillars pose significant challenges. Developing reliable, non-destructive methods for assessing the structural integrity, surface properties, and functionality of micropillars in complex biological environments is essential for ensuring consistent performance and regulatory compliance. This requires advanced imaging and analytical techniques that can provide detailed information about the micropillars without disrupting their delicate structures or interfering with their biological interactions.
Another major challenge lies in the surface modification of silicon micropillars. While silicon offers excellent mechanical properties and biocompatibility, its surface characteristics may not always be ideal for specific biological applications. Researchers face difficulties in developing reliable and scalable methods for functionalizing the micropillar surfaces without compromising their structural integrity or altering their mechanical properties.
The integration of silicon micropillars with other biomaterials and sensing elements presents additional complexities. Creating seamless interfaces between the micropillars and surrounding matrices or substrates is essential for maintaining the overall functionality of the biomaterial system. This integration often requires innovative approaches to ensure proper adhesion, signal transduction, and long-term stability under physiological conditions.
Durability and long-term performance of silicon micropillars in biological environments remain significant concerns. The potential degradation or fouling of these structures over time can impact their effectiveness and reliability in biomedical applications. Developing strategies to enhance the resistance of silicon micropillars to biological and chemical degradation without compromising their biocompatibility is an ongoing challenge.
The cost-effective and scalable production of silicon micropillars for biomaterials is another hurdle that researchers and manufacturers face. Current fabrication methods often involve complex, multi-step processes that can be time-consuming and expensive. Streamlining these processes while maintaining the required precision and quality is crucial for the commercial viability of silicon micropillar-based biomaterials.
Lastly, the characterization and quality control of silicon micropillars pose significant challenges. Developing reliable, non-destructive methods for assessing the structural integrity, surface properties, and functionality of micropillars in complex biological environments is essential for ensuring consistent performance and regulatory compliance. This requires advanced imaging and analytical techniques that can provide detailed information about the micropillars without disrupting their delicate structures or interfering with their biological interactions.
Existing Silicon Micropillar Fabrication Techniques
01 Fabrication methods for silicon micropillars
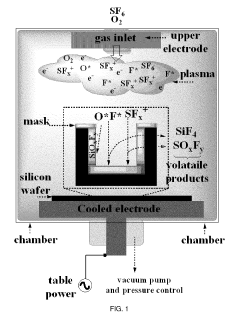
Various techniques are employed to create silicon micropillars, including etching processes, lithography, and deposition methods. These fabrication methods allow for precise control over the size, shape, and spacing of the micropillars, which is crucial for their performance in different applications.- Fabrication methods for silicon micropillars: Various techniques are employed to create silicon micropillars, including etching processes, lithography, and deposition methods. These fabrication methods allow for precise control over the size, shape, and arrangement of the micropillars, which is crucial for their performance in different applications.
- Applications in solar cells and photovoltaics: Silicon micropillars are utilized in solar cell and photovoltaic technologies to enhance light absorption and improve overall efficiency. The unique structure of micropillars allows for better light trapping and increased surface area, leading to improved energy conversion rates.
- Use in microfluidic and lab-on-a-chip devices: Silicon micropillars play a significant role in microfluidic systems and lab-on-a-chip devices. They can be used for fluid manipulation, particle separation, and as structural elements in miniaturized analytical systems, enabling more efficient and precise microfluidic operations.
- Integration with sensors and MEMS devices: Silicon micropillars are integrated into various sensors and MEMS (Micro-Electro-Mechanical Systems) devices. Their unique properties allow for enhanced sensitivity in chemical and biological sensors, as well as improved performance in mechanical sensing applications.
- Surface modification and functionalization: The surface of silicon micropillars can be modified and functionalized to tailor their properties for specific applications. This includes coating with various materials, chemical treatments, and the addition of functional groups to enhance their performance in areas such as catalysis, sensing, and biomedical applications.
02 Applications in solar cells and photovoltaics
Silicon micropillars are utilized in solar cell and photovoltaic technologies to enhance light absorption and improve overall efficiency. The unique structure of micropillars allows for better light trapping and increased surface area, leading to improved energy conversion in solar devices.Expand Specific Solutions03 Use in microfluidic and lab-on-a-chip devices
Silicon micropillars play a significant role in microfluidic systems and lab-on-a-chip devices. They can be used for fluid manipulation, particle separation, and as structural elements in miniaturized analytical systems, enabling more efficient and precise microfluidic operations.Expand Specific Solutions04 Integration with sensors and MEMS devices
Silicon micropillars are integrated into various sensors and MEMS (Micro-Electro-Mechanical Systems) devices. Their unique properties allow for enhanced sensitivity in chemical and biological sensors, as well as improved performance in mechanical sensing applications.Expand Specific Solutions05 Surface modification and functionalization
The surface of silicon micropillars can be modified and functionalized to tailor their properties for specific applications. This includes coating with various materials, chemical treatments, and the addition of functional groups to enhance their performance in areas such as catalysis, sensing, and biomedical applications.Expand Specific Solutions
Key Players in Silicon Micropillar Research and Development
The exploration of silicon micropillars in biomaterials represents an emerging field at the intersection of nanotechnology and bioengineering. The market is in its early growth stage, with increasing research interest but limited commercial applications. The global market size for biomaterials is projected to reach $47 billion by 2025, with silicon-based materials playing a growing role. Technologically, silicon micropillar fabrication is advancing rapidly, with key players like Massachusetts Institute of Technology, The Regents of the University of California, and Shanghai Huali Microelectronics Corp. leading research efforts. However, the technology is still in the development phase, with challenges in scalability and biocompatibility to be addressed before widespread adoption in medical devices and tissue engineering applications.
The Regents of the University of California
Technical Solution: The University of California has made significant strides in silicon micropillar research for biomaterials. Their approach focuses on creating bioactive silicon micropillars through a combination of lithography and etching techniques[4]. These micropillars are designed to mimic the extracellular matrix, providing a 3D environment for cell growth and differentiation. The UC team has demonstrated the ability to control pillar height, diameter, and spacing with nanometer precision, allowing for tailored cell-material interactions[5]. Additionally, they have explored the incorporation of bioactive molecules into the micropillar structures, enhancing their functionality for tissue engineering and drug delivery applications[6].
Strengths: Advanced control over micropillar properties, integration of bioactive molecules. Weaknesses: Complex fabrication process, potential challenges in scaling up production.
The Charles Stark Draper Laboratory, Inc.
Technical Solution: Draper Laboratory has developed innovative approaches to silicon micropillar fabrication for biomedical applications. Their research focuses on creating multifunctional micropillar arrays that can serve as both structural supports and sensing elements in biomaterials[7]. Using a combination of photolithography and deep silicon etching, Draper has produced micropillars with high aspect ratios and tailored surface chemistries. These structures have been applied to create 3D cell culture platforms that more accurately mimic in vivo environments[8]. Additionally, Draper has explored the integration of these micropillars with microfluidic systems, enabling real-time monitoring of cellular responses and drug screening applications[9].
Strengths: Integration of sensing capabilities, compatibility with microfluidic systems. Weaknesses: Potentially high production costs, limited to specific biomedical applications.
Innovative Applications of Silicon Micropillars in Biomaterials
Nanotextured materials
PatentInactiveUS20190093150A1
Innovation
- Development of nanotextured materials with tunable nanopillar structures, such as black silicon, that mimic natural surfaces, providing selective bactericidal activity through mechanical interaction with bacterial cells, including sharp and blunt nanopillars that can stretch, tear, or pierce cell membranes, depending on the bacterial species.
Nanoporous silicon support containing macropores for use as a bioreactor
PatentInactiveUS6734000B2
Innovation
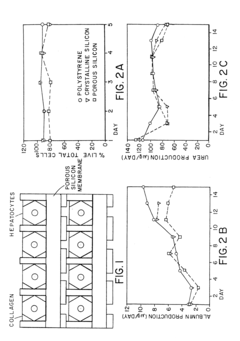
- A nanoporous silicon bioreactor is developed, featuring macropores of defined sizes to accommodate cells and nanopores for media passage, allowing for the integration of biosensors and enhanced surface area, which supports the growth and differentiation of primary hepatocytes and other cell types, enabling pseudo-3D interactions and bidirectional mass transfer.
Biocompatibility and Safety Considerations
The integration of silicon micropillars in biomaterials necessitates a thorough examination of biocompatibility and safety considerations. These factors are paramount in ensuring the successful application of such materials in biological systems, particularly in medical and bioengineering contexts.
Silicon, as a base material, has demonstrated favorable biocompatibility in various forms. However, the unique structure of micropillars introduces new variables that require careful evaluation. The increased surface area and potential for mechanical interaction with cells and tissues demand a comprehensive assessment of both short-term and long-term biological responses.
One primary concern is the potential for silicon micropillars to induce inflammatory responses. Studies have shown that the size, shape, and surface properties of these structures can significantly influence cellular interactions. Optimizing these parameters is crucial to minimize adverse reactions and promote healthy tissue integration. Additionally, the degradation behavior of silicon micropillars in physiological environments must be thoroughly investigated to ensure that any breakdown products do not pose toxicity risks.
The mechanical properties of silicon micropillars also play a critical role in their biocompatibility. While silicon is generally considered a rigid material, the micropillar structure can introduce flexibility and compressibility. This altered mechanical behavior may affect cellular adhesion, proliferation, and differentiation. Careful design and testing are necessary to achieve a balance between structural integrity and biomechanical compatibility with surrounding tissues.
Surface functionalization of silicon micropillars offers opportunities to enhance biocompatibility and introduce specific biological functions. Techniques such as plasma treatment, chemical modification, or coating with bioactive molecules can modulate cell-material interactions and improve overall integration. However, these modifications must be rigorously evaluated for stability and potential long-term effects on the host system.
Safety considerations extend beyond immediate biological responses to include potential long-term implications. The durability of silicon micropillars under physiological conditions, their resistance to fatigue and wear, and the possibility of particle shedding must be thoroughly assessed. Furthermore, the potential for these structures to interfere with diagnostic imaging techniques or therapeutic interventions should be carefully evaluated.
In the context of medical applications, sterilization compatibility is a crucial aspect of safety. The delicate nature of micropillar structures may necessitate the development of specialized sterilization protocols that maintain structural integrity while ensuring complete microbial elimination. This challenge underscores the need for interdisciplinary collaboration between materials scientists, microbiologists, and medical device engineers.
Silicon, as a base material, has demonstrated favorable biocompatibility in various forms. However, the unique structure of micropillars introduces new variables that require careful evaluation. The increased surface area and potential for mechanical interaction with cells and tissues demand a comprehensive assessment of both short-term and long-term biological responses.
One primary concern is the potential for silicon micropillars to induce inflammatory responses. Studies have shown that the size, shape, and surface properties of these structures can significantly influence cellular interactions. Optimizing these parameters is crucial to minimize adverse reactions and promote healthy tissue integration. Additionally, the degradation behavior of silicon micropillars in physiological environments must be thoroughly investigated to ensure that any breakdown products do not pose toxicity risks.
The mechanical properties of silicon micropillars also play a critical role in their biocompatibility. While silicon is generally considered a rigid material, the micropillar structure can introduce flexibility and compressibility. This altered mechanical behavior may affect cellular adhesion, proliferation, and differentiation. Careful design and testing are necessary to achieve a balance between structural integrity and biomechanical compatibility with surrounding tissues.
Surface functionalization of silicon micropillars offers opportunities to enhance biocompatibility and introduce specific biological functions. Techniques such as plasma treatment, chemical modification, or coating with bioactive molecules can modulate cell-material interactions and improve overall integration. However, these modifications must be rigorously evaluated for stability and potential long-term effects on the host system.
Safety considerations extend beyond immediate biological responses to include potential long-term implications. The durability of silicon micropillars under physiological conditions, their resistance to fatigue and wear, and the possibility of particle shedding must be thoroughly assessed. Furthermore, the potential for these structures to interfere with diagnostic imaging techniques or therapeutic interventions should be carefully evaluated.
In the context of medical applications, sterilization compatibility is a crucial aspect of safety. The delicate nature of micropillar structures may necessitate the development of specialized sterilization protocols that maintain structural integrity while ensuring complete microbial elimination. This challenge underscores the need for interdisciplinary collaboration between materials scientists, microbiologists, and medical device engineers.
Scalability and Cost-Effectiveness Analysis
The scalability and cost-effectiveness of silicon micropillars in biomaterials are critical factors determining their potential for widespread adoption and commercial viability. As the technology advances, it is essential to evaluate these aspects to ensure successful implementation in various biomedical applications.
Scalability of silicon micropillar production is a key consideration. Current fabrication methods, such as photolithography and etching techniques, have demonstrated the ability to create uniform micropillar arrays on silicon substrates. However, scaling up these processes for large-scale production presents challenges. The precision required for creating consistent micropillar dimensions and spacing across larger surface areas may lead to increased production times and potential quality control issues.
Advancements in nanoimprint lithography and roll-to-roll manufacturing techniques show promise for improving scalability. These methods could potentially allow for continuous production of silicon micropillar arrays on flexible substrates, significantly increasing throughput. However, further research and development are needed to optimize these processes for biomaterial applications while maintaining the desired micropillar properties.
Cost-effectiveness is another crucial factor in the adoption of silicon micropillars in biomaterials. The initial investment in equipment and facilities for silicon micropillar fabrication can be substantial. However, as production scales up and processes become more efficient, the per-unit cost is expected to decrease. The use of silicon as the base material is advantageous due to its abundance and established supply chains in the semiconductor industry.
To improve cost-effectiveness, researchers are exploring alternative materials and hybrid approaches. For instance, combining silicon micropillars with polymer-based biomaterials could potentially reduce overall costs while maintaining the desired functionality. Additionally, the development of reusable silicon micropillar templates for creating polymer-based replicas could offer a more cost-effective solution for certain applications.
The long-term durability and biocompatibility of silicon micropillars in biomaterials also contribute to their cost-effectiveness. If these structures can maintain their functionality over extended periods without degradation or adverse biological responses, it could reduce the need for frequent replacements or interventions, ultimately lowering overall costs for end-users.
As the technology matures, economies of scale are likely to play a significant role in improving both scalability and cost-effectiveness. Increased demand for silicon micropillar-based biomaterials could drive investments in more efficient production methods and foster innovation in manufacturing processes. This, in turn, could lead to reduced costs and improved accessibility of these advanced biomaterials across various biomedical applications.
Scalability of silicon micropillar production is a key consideration. Current fabrication methods, such as photolithography and etching techniques, have demonstrated the ability to create uniform micropillar arrays on silicon substrates. However, scaling up these processes for large-scale production presents challenges. The precision required for creating consistent micropillar dimensions and spacing across larger surface areas may lead to increased production times and potential quality control issues.
Advancements in nanoimprint lithography and roll-to-roll manufacturing techniques show promise for improving scalability. These methods could potentially allow for continuous production of silicon micropillar arrays on flexible substrates, significantly increasing throughput. However, further research and development are needed to optimize these processes for biomaterial applications while maintaining the desired micropillar properties.
Cost-effectiveness is another crucial factor in the adoption of silicon micropillars in biomaterials. The initial investment in equipment and facilities for silicon micropillar fabrication can be substantial. However, as production scales up and processes become more efficient, the per-unit cost is expected to decrease. The use of silicon as the base material is advantageous due to its abundance and established supply chains in the semiconductor industry.
To improve cost-effectiveness, researchers are exploring alternative materials and hybrid approaches. For instance, combining silicon micropillars with polymer-based biomaterials could potentially reduce overall costs while maintaining the desired functionality. Additionally, the development of reusable silicon micropillar templates for creating polymer-based replicas could offer a more cost-effective solution for certain applications.
The long-term durability and biocompatibility of silicon micropillars in biomaterials also contribute to their cost-effectiveness. If these structures can maintain their functionality over extended periods without degradation or adverse biological responses, it could reduce the need for frequent replacements or interventions, ultimately lowering overall costs for end-users.
As the technology matures, economies of scale are likely to play a significant role in improving both scalability and cost-effectiveness. Increased demand for silicon micropillar-based biomaterials could drive investments in more efficient production methods and foster innovation in manufacturing processes. This, in turn, could lead to reduced costs and improved accessibility of these advanced biomaterials across various biomedical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!