Exploring the Chemistry of Carbon Tetrachloride in Various Applications
JUL 2, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Background and Objectives
Carbon tetrachloride (CCl4) has been a subject of scientific interest and industrial application for over a century. This compound, first synthesized in the 1840s, gained prominence due to its unique chemical properties and versatile applications. Initially used as a solvent and cleaning agent, CCl4's role expanded into various industries, including refrigeration, fire extinguishing, and chemical manufacturing.
The evolution of CCl4 usage has been marked by significant milestones. In the early 20th century, it became a popular dry-cleaning solvent and degreasing agent. Its non-flammability made it an attractive option for fire extinguishers. However, the mid-20th century saw a shift in perception as the environmental and health impacts of CCl4 became apparent. This led to a gradual phase-out in many applications, particularly after the Montreal Protocol in 1987 identified it as an ozone-depleting substance.
Despite these restrictions, CCl4 continues to play a role in certain specialized applications, particularly in the chemical industry. Its use as a feedstock for the production of other chlorinated compounds remains significant. This persistence in industrial processes, coupled with its environmental impact, underscores the need for ongoing research and development in this field.
The current technological landscape surrounding CCl4 is characterized by a dual focus: finding safer alternatives for its traditional applications and developing more efficient and environmentally friendly methods for its necessary uses. This includes exploring new synthesis routes that minimize CCl4 as a byproduct and investigating novel remediation techniques for environmental contamination.
The objectives of current research in CCl4 chemistry are multifaceted. Firstly, there is a push to fully understand its atmospheric chemistry and its role in ozone depletion, aiming to mitigate its environmental impact. Secondly, efforts are being made to develop green chemistry alternatives that can replace CCl4 in its remaining applications. Thirdly, there is ongoing work to improve detection and monitoring techniques for CCl4 in the environment, crucial for assessing the effectiveness of global reduction efforts.
Additionally, research is focused on exploring potential new applications of CCl4 that leverage its unique properties while minimizing environmental risks. This includes investigating its role in advanced materials synthesis and as a reagent in specialized chemical reactions. The goal is to balance the compound's utility with stringent safety and environmental considerations.
The evolution of CCl4 usage has been marked by significant milestones. In the early 20th century, it became a popular dry-cleaning solvent and degreasing agent. Its non-flammability made it an attractive option for fire extinguishers. However, the mid-20th century saw a shift in perception as the environmental and health impacts of CCl4 became apparent. This led to a gradual phase-out in many applications, particularly after the Montreal Protocol in 1987 identified it as an ozone-depleting substance.
Despite these restrictions, CCl4 continues to play a role in certain specialized applications, particularly in the chemical industry. Its use as a feedstock for the production of other chlorinated compounds remains significant. This persistence in industrial processes, coupled with its environmental impact, underscores the need for ongoing research and development in this field.
The current technological landscape surrounding CCl4 is characterized by a dual focus: finding safer alternatives for its traditional applications and developing more efficient and environmentally friendly methods for its necessary uses. This includes exploring new synthesis routes that minimize CCl4 as a byproduct and investigating novel remediation techniques for environmental contamination.
The objectives of current research in CCl4 chemistry are multifaceted. Firstly, there is a push to fully understand its atmospheric chemistry and its role in ozone depletion, aiming to mitigate its environmental impact. Secondly, efforts are being made to develop green chemistry alternatives that can replace CCl4 in its remaining applications. Thirdly, there is ongoing work to improve detection and monitoring techniques for CCl4 in the environment, crucial for assessing the effectiveness of global reduction efforts.
Additionally, research is focused on exploring potential new applications of CCl4 that leverage its unique properties while minimizing environmental risks. This includes investigating its role in advanced materials synthesis and as a reagent in specialized chemical reactions. The goal is to balance the compound's utility with stringent safety and environmental considerations.
Market Analysis for CCl4
The global market for carbon tetrachloride (CCl4) has undergone significant changes in recent decades due to environmental regulations and shifting industrial applications. Historically, CCl4 was widely used as a solvent, cleaning agent, and feedstock for various chemical processes. However, its market has contracted substantially since the implementation of the Montreal Protocol in 1987, which phased out the production of ozone-depleting substances.
Despite these restrictions, CCl4 continues to find niche applications in certain industries. The pharmaceutical sector remains a key consumer, utilizing CCl4 in the production of some medicines and as a reagent in analytical chemistry. Additionally, the agrochemical industry employs CCl4 in the synthesis of certain pesticides and herbicides.
The electronics industry also maintains a demand for high-purity CCl4, particularly in the manufacturing of semiconductors and optical fibers. This sector values CCl4 for its unique properties in plasma etching processes and as a precursor in chemical vapor deposition.
Market trends indicate a gradual decline in overall CCl4 consumption, driven by stringent environmental regulations and the development of alternative substances. However, this decline is partially offset by the growth in specialized applications where suitable replacements are yet to be found.
Geographically, the Asia-Pacific region, particularly China and India, represents the largest market for CCl4, primarily due to their robust pharmaceutical and agrochemical industries. North America and Europe have seen more significant reductions in CCl4 usage, reflecting stricter environmental policies and a shift towards greener alternatives.
The market dynamics of CCl4 are heavily influenced by regulatory frameworks. The continued implementation of the Montreal Protocol and other environmental agreements has led to increased monitoring and control of CCl4 production and trade. This has resulted in a more consolidated market, with fewer producers and tighter supply chains.
Looking ahead, the CCl4 market is expected to face ongoing challenges. The push for sustainable chemistry and environmentally friendly processes will likely further constrain its use. However, opportunities may arise in recycling and recovery technologies, as well as in the development of more efficient and targeted applications that minimize environmental impact while maximizing the unique properties of CCl4.
Despite these restrictions, CCl4 continues to find niche applications in certain industries. The pharmaceutical sector remains a key consumer, utilizing CCl4 in the production of some medicines and as a reagent in analytical chemistry. Additionally, the agrochemical industry employs CCl4 in the synthesis of certain pesticides and herbicides.
The electronics industry also maintains a demand for high-purity CCl4, particularly in the manufacturing of semiconductors and optical fibers. This sector values CCl4 for its unique properties in plasma etching processes and as a precursor in chemical vapor deposition.
Market trends indicate a gradual decline in overall CCl4 consumption, driven by stringent environmental regulations and the development of alternative substances. However, this decline is partially offset by the growth in specialized applications where suitable replacements are yet to be found.
Geographically, the Asia-Pacific region, particularly China and India, represents the largest market for CCl4, primarily due to their robust pharmaceutical and agrochemical industries. North America and Europe have seen more significant reductions in CCl4 usage, reflecting stricter environmental policies and a shift towards greener alternatives.
The market dynamics of CCl4 are heavily influenced by regulatory frameworks. The continued implementation of the Montreal Protocol and other environmental agreements has led to increased monitoring and control of CCl4 production and trade. This has resulted in a more consolidated market, with fewer producers and tighter supply chains.
Looking ahead, the CCl4 market is expected to face ongoing challenges. The push for sustainable chemistry and environmentally friendly processes will likely further constrain its use. However, opportunities may arise in recycling and recovery technologies, as well as in the development of more efficient and targeted applications that minimize environmental impact while maximizing the unique properties of CCl4.
CCl4 Technical Challenges
Carbon tetrachloride (CCl4) presents several technical challenges in its various applications, primarily due to its unique chemical properties and environmental concerns. One of the main difficulties lies in its high toxicity and potential for ozone depletion, which has led to strict regulations on its use and production. This has necessitated the development of alternative compounds and processes in many industries where CCl4 was once widely utilized.
In the field of organic synthesis, replacing CCl4 as a solvent and reagent has proven challenging. Its excellent solvating properties for non-polar compounds and its ability to act as a chlorine source in certain reactions make it difficult to find suitable substitutes that offer the same versatility and efficiency. Researchers are exploring greener alternatives, but many lack the specific reactivity profile of CCl4, leading to reduced yields or altered reaction pathways.
The use of CCl4 in fire extinguishers has been phased out due to its ozone-depleting potential, but finding equally effective and environmentally friendly replacements has been problematic. New fire suppression agents must balance efficacy, safety, and environmental impact, a combination that has proven elusive in many cases.
In the realm of analytical chemistry, CCl4 has been valuable for its use in infrared spectroscopy and as a solvent in various analytical techniques. Developing new methodologies that do not rely on CCl4 while maintaining the same level of accuracy and sensitivity has been a significant challenge for analytical chemists.
The remediation of CCl4 contamination in soil and groundwater presents another set of technical hurdles. Its high density and low water solubility make it a dense non-aqueous phase liquid (DNAPL), which can penetrate deep into aquifers and be difficult to locate and treat. Conventional pump-and-treat methods are often ineffective, necessitating the development of innovative in-situ remediation technologies.
In industrial cleaning and degreasing applications, where CCl4 was once commonly used, finding alternatives that match its cleaning power without the associated health and environmental risks has been challenging. Many substitutes require changes in cleaning processes or equipment, leading to increased costs and potential reductions in efficiency.
The production of chlorofluorocarbons (CFCs) and their alternatives has also faced challenges with the phaseout of CCl4 as a feedstock. Developing new synthesis routes that do not rely on CCl4 while maintaining product quality and production efficiency has required significant research and development efforts in the chemical industry.
In the field of organic synthesis, replacing CCl4 as a solvent and reagent has proven challenging. Its excellent solvating properties for non-polar compounds and its ability to act as a chlorine source in certain reactions make it difficult to find suitable substitutes that offer the same versatility and efficiency. Researchers are exploring greener alternatives, but many lack the specific reactivity profile of CCl4, leading to reduced yields or altered reaction pathways.
The use of CCl4 in fire extinguishers has been phased out due to its ozone-depleting potential, but finding equally effective and environmentally friendly replacements has been problematic. New fire suppression agents must balance efficacy, safety, and environmental impact, a combination that has proven elusive in many cases.
In the realm of analytical chemistry, CCl4 has been valuable for its use in infrared spectroscopy and as a solvent in various analytical techniques. Developing new methodologies that do not rely on CCl4 while maintaining the same level of accuracy and sensitivity has been a significant challenge for analytical chemists.
The remediation of CCl4 contamination in soil and groundwater presents another set of technical hurdles. Its high density and low water solubility make it a dense non-aqueous phase liquid (DNAPL), which can penetrate deep into aquifers and be difficult to locate and treat. Conventional pump-and-treat methods are often ineffective, necessitating the development of innovative in-situ remediation technologies.
In industrial cleaning and degreasing applications, where CCl4 was once commonly used, finding alternatives that match its cleaning power without the associated health and environmental risks has been challenging. Many substitutes require changes in cleaning processes or equipment, leading to increased costs and potential reductions in efficiency.
The production of chlorofluorocarbons (CFCs) and their alternatives has also faced challenges with the phaseout of CCl4 as a feedstock. Developing new synthesis routes that do not rely on CCl4 while maintaining product quality and production efficiency has required significant research and development efforts in the chemical industry.
Current CCl4 Applications
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, such as pharmaceuticals, plastics, and agrochemicals.
- Environmental and safety considerations: Due to its environmental impact and health hazards, methods for detecting, monitoring, and safely handling carbon tetrachloride are developed. This includes techniques for environmental remediation and workplace safety measures to minimize exposure risks.
- Alternatives and substitutes for carbon tetrachloride: Research into alternatives and substitutes for carbon tetrachloride is ongoing, aiming to replace its use in various applications with less harmful substances. This includes developing new compounds or processes that can perform similar functions without the associated environmental and health risks.
- Historical uses and developments: The historical uses of carbon tetrachloride, including its early applications in fire extinguishers, dry cleaning, and as a refrigerant, are documented. This point also covers the evolution of its production methods and the gradual phase-out of its use due to environmental concerns.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in organic synthesis. It plays a role in the production of other chlorinated compounds and in certain industrial applications.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research focuses on alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes developing environmentally friendly substitutes and improving containment strategies.Expand Specific Solutions04 Detection and analysis methods
Techniques for detecting and analyzing carbon tetrachloride in various matrices are developed. These include spectroscopic methods, chromatographic techniques, and sensor-based approaches for monitoring carbon tetrachloride levels in air, water, and soil.Expand Specific Solutions05 Historical uses and phaseout
Carbon tetrachloride was historically used in fire extinguishers, cleaning agents, and as a refrigerant. However, due to its ozone-depleting properties and toxicity, its use has been phased out in many applications. Research now focuses on understanding its long-term environmental impact and developing remediation strategies.Expand Specific Solutions
Key CCl4 Industry Players
The exploration of carbon tetrachloride chemistry in various applications is currently in a mature stage, with a well-established market and extensive research history. The global market size for carbon tetrachloride is relatively stable, primarily driven by its use in specific industrial processes and as a feedstock for other chemicals. Technologically, the field is well-developed, with companies like Occidental Chemical Corp., BASF Corp., and Sumitomo Chemical Co., Ltd. leading in production and application research. However, due to environmental concerns and regulatory restrictions, there's a shift towards finding safer alternatives, spurring innovation in green chemistry. Academic institutions such as the University of Warwick and Central South University are contributing to this evolving landscape through advanced research and development efforts.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has developed proprietary technologies for the production and handling of carbon tetrachloride, focusing on safety and environmental protection. Their approach includes advanced process control systems that optimize CCl4 synthesis, reducing energy consumption and minimizing byproduct formation[8]. They have also implemented state-of-the-art containment and scrubbing technologies to prevent CCl4 emissions during production and storage. In applications, Occidental has developed specialized grades of CCl4 for use in the pharmaceutical industry, meeting stringent purity requirements[9].
Strengths: Advanced process control, emission reduction technologies, and specialized product grades. Weaknesses: Continued reliance on a chemical with significant environmental concerns and potential regulatory limitations.
BASF Corp.
Technical Solution: BASF has developed a comprehensive approach to CCl4 chemistry, focusing on both production and application technologies. They have implemented a novel synthesis route that utilizes chloromethanes as feedstock, resulting in higher yields and reduced energy consumption[4]. BASF's technology also includes advanced handling and storage solutions that minimize the risk of environmental release. In applications, they have developed CCl4-based formulations for use as solvents in specific industrial processes, optimizing performance while adhering to strict environmental regulations[5].
Strengths: Efficient synthesis route, advanced handling solutions, and optimized industrial applications. Weaknesses: Dependence on chloromethane feedstock availability and ongoing regulatory scrutiny of CCl4 use.
CCl4 Core Chemical Properties
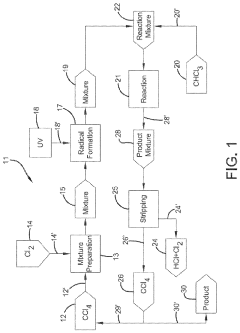
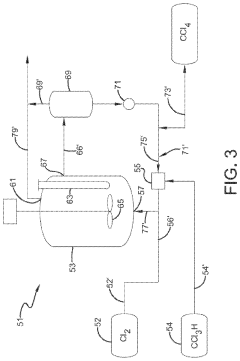
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
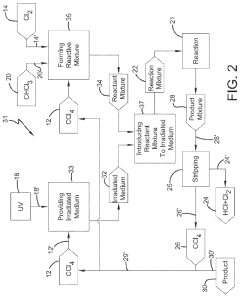
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Environmental Impact of CCl4
Carbon tetrachloride (CCl4) has been widely used in various industrial applications due to its unique chemical properties. However, its environmental impact has become a significant concern in recent decades. The release of CCl4 into the atmosphere has been identified as a major contributor to ozone depletion, particularly in the stratosphere.
When CCl4 reaches the stratosphere, it undergoes photolysis by ultraviolet radiation, releasing chlorine atoms. These chlorine atoms catalyze the destruction of ozone molecules, leading to the thinning of the ozone layer. This process has far-reaching consequences for both human health and ecosystems, as the ozone layer plays a crucial role in protecting the Earth from harmful UV radiation.
In addition to its effects on the ozone layer, CCl4 also contributes to global warming. It is classified as a greenhouse gas with a global warming potential approximately 1,400 times that of carbon dioxide over a 100-year period. This means that even small emissions of CCl4 can have a significant impact on climate change.
The persistence of CCl4 in the environment is another cause for concern. With an atmospheric lifetime of about 26 years, it can remain in the atmosphere for extended periods, continuing to cause damage long after its release. This persistence also allows CCl4 to be transported over long distances, affecting areas far from its original source of emission.
In aquatic environments, CCl4 can accumulate in sediments and bioaccumulate in marine organisms. This poses risks to aquatic ecosystems and potentially to human health through the food chain. Studies have shown that CCl4 can cause liver and kidney damage in various aquatic species, disrupting ecosystem balance.
Soil contamination by CCl4 is also a significant issue, particularly in areas with a history of industrial activity. Once in the soil, CCl4 can leach into groundwater, potentially contaminating drinking water sources. This contamination can persist for years, posing long-term risks to both human health and the environment.
Recognizing these environmental impacts, international efforts have been made to phase out the production and use of CCl4. The Montreal Protocol, an international treaty designed to protect the ozone layer, has been instrumental in reducing CCl4 emissions. However, despite these efforts, unexpected levels of CCl4 continue to be detected in the atmosphere, suggesting ongoing emissions from unknown sources or legacy contamination.
When CCl4 reaches the stratosphere, it undergoes photolysis by ultraviolet radiation, releasing chlorine atoms. These chlorine atoms catalyze the destruction of ozone molecules, leading to the thinning of the ozone layer. This process has far-reaching consequences for both human health and ecosystems, as the ozone layer plays a crucial role in protecting the Earth from harmful UV radiation.
In addition to its effects on the ozone layer, CCl4 also contributes to global warming. It is classified as a greenhouse gas with a global warming potential approximately 1,400 times that of carbon dioxide over a 100-year period. This means that even small emissions of CCl4 can have a significant impact on climate change.
The persistence of CCl4 in the environment is another cause for concern. With an atmospheric lifetime of about 26 years, it can remain in the atmosphere for extended periods, continuing to cause damage long after its release. This persistence also allows CCl4 to be transported over long distances, affecting areas far from its original source of emission.
In aquatic environments, CCl4 can accumulate in sediments and bioaccumulate in marine organisms. This poses risks to aquatic ecosystems and potentially to human health through the food chain. Studies have shown that CCl4 can cause liver and kidney damage in various aquatic species, disrupting ecosystem balance.
Soil contamination by CCl4 is also a significant issue, particularly in areas with a history of industrial activity. Once in the soil, CCl4 can leach into groundwater, potentially contaminating drinking water sources. This contamination can persist for years, posing long-term risks to both human health and the environment.
Recognizing these environmental impacts, international efforts have been made to phase out the production and use of CCl4. The Montreal Protocol, an international treaty designed to protect the ozone layer, has been instrumental in reducing CCl4 emissions. However, despite these efforts, unexpected levels of CCl4 continue to be detected in the atmosphere, suggesting ongoing emissions from unknown sources or legacy contamination.
CCl4 Safety Regulations
Carbon tetrachloride (CCl4) has been subject to stringent safety regulations due to its potential health and environmental hazards. The Montreal Protocol, an international treaty designed to protect the ozone layer, has played a crucial role in phasing out the production and consumption of CCl4 along with other ozone-depleting substances. As a result, its use has been severely restricted in many countries.
In the United States, the Environmental Protection Agency (EPA) has implemented strict regulations on CCl4 under the Toxic Substances Control Act (TSCA). The chemical is listed as a hazardous air pollutant under the Clean Air Act and is subject to reporting requirements under the Emergency Planning and Community Right-to-Know Act (EPCRA). The Occupational Safety and Health Administration (OSHA) has set permissible exposure limits for workers, with a time-weighted average of 10 ppm over an 8-hour workday.
The European Union has also imposed rigorous controls on CCl4 through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Its use is heavily restricted, and any application requires specific authorization. The Classification, Labeling, and Packaging (CLP) Regulation classifies CCl4 as a substance of very high concern due to its carcinogenic properties and environmental impact.
In the realm of transportation, CCl4 is classified as a dangerous good under international regulations. The United Nations Model Regulations on the Transport of Dangerous Goods categorize it as a Class 6.1 toxic substance. This classification necessitates specific packaging, labeling, and handling requirements during transportation to minimize risks.
Safety data sheets (SDS) for CCl4 must provide detailed information on its hazards, safe handling procedures, and emergency measures. These documents are crucial for ensuring proper risk management in laboratories and industrial settings where CCl4 may still be used in limited quantities for specific applications.
Waste management regulations also apply strictly to CCl4. In many jurisdictions, it is classified as hazardous waste and must be disposed of through specialized facilities. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal governs its international transport and disposal.
As research continues to explore alternatives and safer substitutes for CCl4 in various applications, regulatory bodies worldwide maintain vigilant oversight. Regular reviews and updates to safety regulations ensure that the use of CCl4 remains tightly controlled, balancing its limited necessary applications with the imperative to protect human health and the environment.
In the United States, the Environmental Protection Agency (EPA) has implemented strict regulations on CCl4 under the Toxic Substances Control Act (TSCA). The chemical is listed as a hazardous air pollutant under the Clean Air Act and is subject to reporting requirements under the Emergency Planning and Community Right-to-Know Act (EPCRA). The Occupational Safety and Health Administration (OSHA) has set permissible exposure limits for workers, with a time-weighted average of 10 ppm over an 8-hour workday.
The European Union has also imposed rigorous controls on CCl4 through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Its use is heavily restricted, and any application requires specific authorization. The Classification, Labeling, and Packaging (CLP) Regulation classifies CCl4 as a substance of very high concern due to its carcinogenic properties and environmental impact.
In the realm of transportation, CCl4 is classified as a dangerous good under international regulations. The United Nations Model Regulations on the Transport of Dangerous Goods categorize it as a Class 6.1 toxic substance. This classification necessitates specific packaging, labeling, and handling requirements during transportation to minimize risks.
Safety data sheets (SDS) for CCl4 must provide detailed information on its hazards, safe handling procedures, and emergency measures. These documents are crucial for ensuring proper risk management in laboratories and industrial settings where CCl4 may still be used in limited quantities for specific applications.
Waste management regulations also apply strictly to CCl4. In many jurisdictions, it is classified as hazardous waste and must be disposed of through specialized facilities. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal governs its international transport and disposal.
As research continues to explore alternatives and safer substitutes for CCl4 in various applications, regulatory bodies worldwide maintain vigilant oversight. Regular reviews and updates to safety regulations ensure that the use of CCl4 remains tightly controlled, balancing its limited necessary applications with the imperative to protect human health and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!