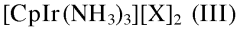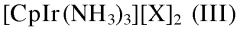Fluoroantimonic Acid: A Frontier in Reaction Science
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Overview and Research Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), represents a pinnacle in the field of reaction science. This extraordinary compound has garnered significant attention due to its unparalleled acidity, surpassing even the strongest mineral acids known to chemistry. The development of fluoroantimonic acid marks a crucial milestone in the ongoing quest for more powerful and efficient catalysts in organic synthesis and industrial processes.
The history of superacids can be traced back to the early 20th century, with significant advancements made in the 1960s and 1970s. However, it was the synthesis and characterization of fluoroantimonic acid that truly pushed the boundaries of acid strength. This superacid system has a Hammett acidity function (H0) estimated to be as low as -28, making it billions of times stronger than 100% sulfuric acid.
The unique properties of fluoroantimonic acid stem from its ability to protonate even extremely weak bases, including normally unreactive hydrocarbons. This characteristic opens up new possibilities in organic synthesis, potentially enabling reactions that were previously thought impossible. The acid's extreme reactivity also presents challenges in handling and storage, requiring specialized equipment and techniques.
Research objectives in the field of fluoroantimonic acid are multifaceted and ambitious. One primary goal is to fully understand the fundamental chemistry behind its extraordinary acidity. This includes investigating the structural dynamics of the acid in solution and its interactions with various substrates at the molecular level. Such knowledge could lead to the design of even more powerful superacid systems or tailored acids for specific applications.
Another critical research direction involves exploring the potential applications of fluoroantimonic acid in industrial processes. Its ability to catalyze reactions under mild conditions could revolutionize certain chemical manufacturing processes, potentially leading to more energy-efficient and environmentally friendly production methods. However, this requires overcoming significant challenges related to the acid's corrosiveness and reactivity.
Researchers are also focusing on developing safer handling methods and more stable formulations of fluoroantimonic acid. This includes investigating novel containment materials that can withstand its extreme corrosiveness and exploring the possibility of immobilized or supported superacid systems that retain the acid's reactivity while mitigating its hazardous nature.
The study of fluoroantimonic acid also extends to theoretical chemistry, where computational models are being developed to predict its behavior and interactions with various compounds. These models could prove invaluable in designing new reactions and understanding the limits of acidity in chemical systems.
The history of superacids can be traced back to the early 20th century, with significant advancements made in the 1960s and 1970s. However, it was the synthesis and characterization of fluoroantimonic acid that truly pushed the boundaries of acid strength. This superacid system has a Hammett acidity function (H0) estimated to be as low as -28, making it billions of times stronger than 100% sulfuric acid.
The unique properties of fluoroantimonic acid stem from its ability to protonate even extremely weak bases, including normally unreactive hydrocarbons. This characteristic opens up new possibilities in organic synthesis, potentially enabling reactions that were previously thought impossible. The acid's extreme reactivity also presents challenges in handling and storage, requiring specialized equipment and techniques.
Research objectives in the field of fluoroantimonic acid are multifaceted and ambitious. One primary goal is to fully understand the fundamental chemistry behind its extraordinary acidity. This includes investigating the structural dynamics of the acid in solution and its interactions with various substrates at the molecular level. Such knowledge could lead to the design of even more powerful superacid systems or tailored acids for specific applications.
Another critical research direction involves exploring the potential applications of fluoroantimonic acid in industrial processes. Its ability to catalyze reactions under mild conditions could revolutionize certain chemical manufacturing processes, potentially leading to more energy-efficient and environmentally friendly production methods. However, this requires overcoming significant challenges related to the acid's corrosiveness and reactivity.
Researchers are also focusing on developing safer handling methods and more stable formulations of fluoroantimonic acid. This includes investigating novel containment materials that can withstand its extreme corrosiveness and exploring the possibility of immobilized or supported superacid systems that retain the acid's reactivity while mitigating its hazardous nature.
The study of fluoroantimonic acid also extends to theoretical chemistry, where computational models are being developed to predict its behavior and interactions with various compounds. These models could prove invaluable in designing new reactions and understanding the limits of acidity in chemical systems.
Industrial Applications and Market Potential
Fluoroantimonic acid, recognized as the strongest known superacid, has garnered significant attention in the field of reaction science due to its exceptional protonating ability. This powerful compound has demonstrated remarkable potential across various industrial applications, driving market interest and research efforts.
In the petrochemical industry, fluoroantimonic acid has shown promise as a catalyst for isomerization and alkylation processes. Its ability to facilitate these reactions under milder conditions than traditional catalysts could lead to more energy-efficient and cost-effective production methods. This application alone represents a substantial market opportunity, given the global scale of petrochemical operations.
The semiconductor industry has also identified potential uses for fluoroantimonic acid in etching and cleaning processes. Its extreme acidity allows for precise and rapid etching of silicon wafers, potentially improving manufacturing efficiency and product quality. As the demand for advanced semiconductors continues to grow, the market for specialized chemical processes is expected to expand correspondingly.
In materials science, fluoroantimonic acid's unique properties make it valuable for synthesizing novel compounds and materials. Its ability to protonate even weak bases opens up new possibilities for creating advanced polymers, nanostructures, and other high-performance materials. This application could drive innovation across multiple sectors, including aerospace, automotive, and electronics.
The pharmaceutical industry is exploring the use of fluoroantimonic acid in the synthesis of complex organic molecules. Its strong protonating ability can enable reactions that are difficult or impossible with conventional acids, potentially leading to more efficient drug manufacturing processes and the development of new therapeutic compounds.
While the industrial potential of fluoroantimonic acid is significant, it's important to note that its extreme reactivity also presents challenges. Handling and containment issues, as well as environmental and safety concerns, must be carefully addressed before widespread adoption can occur. These factors may influence market growth and require substantial investment in safety measures and specialized equipment.
The global market for superacids, including fluoroantimonic acid, is expected to grow as research continues to uncover new applications. However, precise market size estimates are challenging due to the specialized nature of the compound and its current limited use. The potential for fluoroantimonic acid to enable breakthrough technologies in various industries suggests that its market value could increase significantly in the coming years, particularly if safer handling methods and more diverse applications are developed.
In the petrochemical industry, fluoroantimonic acid has shown promise as a catalyst for isomerization and alkylation processes. Its ability to facilitate these reactions under milder conditions than traditional catalysts could lead to more energy-efficient and cost-effective production methods. This application alone represents a substantial market opportunity, given the global scale of petrochemical operations.
The semiconductor industry has also identified potential uses for fluoroantimonic acid in etching and cleaning processes. Its extreme acidity allows for precise and rapid etching of silicon wafers, potentially improving manufacturing efficiency and product quality. As the demand for advanced semiconductors continues to grow, the market for specialized chemical processes is expected to expand correspondingly.
In materials science, fluoroantimonic acid's unique properties make it valuable for synthesizing novel compounds and materials. Its ability to protonate even weak bases opens up new possibilities for creating advanced polymers, nanostructures, and other high-performance materials. This application could drive innovation across multiple sectors, including aerospace, automotive, and electronics.
The pharmaceutical industry is exploring the use of fluoroantimonic acid in the synthesis of complex organic molecules. Its strong protonating ability can enable reactions that are difficult or impossible with conventional acids, potentially leading to more efficient drug manufacturing processes and the development of new therapeutic compounds.
While the industrial potential of fluoroantimonic acid is significant, it's important to note that its extreme reactivity also presents challenges. Handling and containment issues, as well as environmental and safety concerns, must be carefully addressed before widespread adoption can occur. These factors may influence market growth and require substantial investment in safety measures and specialized equipment.
The global market for superacids, including fluoroantimonic acid, is expected to grow as research continues to uncover new applications. However, precise market size estimates are challenging due to the specialized nature of the compound and its current limited use. The potential for fluoroantimonic acid to enable breakthrough technologies in various industries suggests that its market value could increase significantly in the coming years, particularly if safer handling methods and more diverse applications are developed.
Current Challenges in Synthesis and Handling
Fluoroantimonic acid, known as the world's strongest superacid, presents significant challenges in its synthesis and handling due to its extreme reactivity and corrosive nature. The primary obstacle in synthesizing this compound lies in the precise combination of hydrogen fluoride (HF) and antimony pentafluoride (SbF5) under strictly controlled conditions. The highly exothermic reaction requires specialized equipment capable of withstanding extreme temperatures and pressures.
The handling of fluoroantimonic acid poses even greater difficulties. Its ability to protonate virtually any substance it contacts makes containment a critical issue. Traditional laboratory glassware and most metals are rapidly corroded by this superacid, necessitating the use of specialized materials such as Teflon or fluorinated polymers for storage and transfer. Even these materials may degrade over time, requiring frequent replacement and careful monitoring.
Safety concerns are paramount when working with fluoroantimonic acid. Its extreme corrosivity can cause severe burns and tissue damage upon contact with skin or eyes. Inhalation of its vapors can lead to serious respiratory issues. Consequently, stringent safety protocols, including the use of specialized personal protective equipment and well-ventilated fume hoods, are essential for any laboratory handling this substance.
The disposal of fluoroantimonic acid and its byproducts presents another significant challenge. Due to its reactivity, it cannot be simply neutralized like other acids. Specialized waste treatment procedures are necessary to safely decompose the compound without generating hazardous byproducts or causing environmental damage.
Furthermore, the instability of fluoroantimonic acid in the presence of moisture complicates its storage and use. Trace amounts of water can lead to rapid decomposition, potentially resulting in explosive reactions. This necessitates storage and handling under strictly anhydrous conditions, often requiring the use of inert gas atmospheres and moisture-free environments.
The extreme reactivity of fluoroantimonic acid also limits its practical applications in many chemical processes. While its superacidic properties make it valuable for certain specialized reactions, such as the isomerization of alkanes or the protonation of exceptionally weak bases, its use is often impractical or too dangerous for large-scale industrial processes.
The handling of fluoroantimonic acid poses even greater difficulties. Its ability to protonate virtually any substance it contacts makes containment a critical issue. Traditional laboratory glassware and most metals are rapidly corroded by this superacid, necessitating the use of specialized materials such as Teflon or fluorinated polymers for storage and transfer. Even these materials may degrade over time, requiring frequent replacement and careful monitoring.
Safety concerns are paramount when working with fluoroantimonic acid. Its extreme corrosivity can cause severe burns and tissue damage upon contact with skin or eyes. Inhalation of its vapors can lead to serious respiratory issues. Consequently, stringent safety protocols, including the use of specialized personal protective equipment and well-ventilated fume hoods, are essential for any laboratory handling this substance.
The disposal of fluoroantimonic acid and its byproducts presents another significant challenge. Due to its reactivity, it cannot be simply neutralized like other acids. Specialized waste treatment procedures are necessary to safely decompose the compound without generating hazardous byproducts or causing environmental damage.
Furthermore, the instability of fluoroantimonic acid in the presence of moisture complicates its storage and use. Trace amounts of water can lead to rapid decomposition, potentially resulting in explosive reactions. This necessitates storage and handling under strictly anhydrous conditions, often requiring the use of inert gas atmospheres and moisture-free environments.
The extreme reactivity of fluoroantimonic acid also limits its practical applications in many chemical processes. While its superacidic properties make it valuable for certain specialized reactions, such as the isomerization of alkanes or the protonation of exceptionally weak bases, its use is often impractical or too dangerous for large-scale industrial processes.
Existing Synthesis and Containment Methods
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in organic synthesis and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acid catalysts, making it valuable in the production of certain chemicals and materials.
- Use in materials science and surface treatment: Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials. The acid's unique properties make it suitable for generating highly reactive surfaces or creating specific chemical functionalities.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and safety protocols. This includes the use of specific containment materials, personal protective equipment, and controlled environments. Proper storage, transportation, and disposal methods are crucial to prevent accidents and environmental contamination.
- Analytical and characterization techniques: Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These methods include spectroscopic techniques, electrochemical analysis, and specialized apparatus for handling and measuring superacidic systems. Such techniques are essential for understanding the acid's properties, reaction mechanisms, and ensuring quality control in its production and use.
02 Applications in chemical reactions and catalysis
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acid catalysts.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, improve adhesion properties, and create specialized coatings. The acid's unique properties allow for the development of advanced materials with enhanced characteristics.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, handling fluoroantimonic acid requires stringent safety measures. Specialized equipment, containment systems, and personal protective gear are essential when working with this superacid. Proper storage, transportation, and disposal protocols must be followed to prevent accidents and environmental contamination.Expand Specific Solutions05 Analytical and research applications
Fluoroantimonic acid is used in various analytical and research applications. Its superacidic properties make it valuable for studying reaction mechanisms, probing molecular structures, and developing new analytical techniques. It can be employed in spectroscopic studies and as a reagent in specialized chemical analyses.Expand Specific Solutions
Key Players in Superacid Research and Production
The field of Fluoroantimonic Acid research is in its early developmental stage, characterized by a niche market with significant potential for growth. The global market size for this superacid is relatively small but expanding, driven by its applications in organic synthesis and catalysis. The technology maturity varies among key players, with companies like Central Glass Co., Ltd., DAIKIN INDUSTRIES Ltd., and DuPont de Nemours, Inc. leading in research and development. Academic institutions such as Oxford University and the University of Tokyo are also contributing to advancements in this field. The competitive landscape is marked by a mix of established chemical companies and specialized research organizations, each striving to unlock the full potential of Fluoroantimonic Acid in reaction science.
Central Glass Co., Ltd.
Technical Solution: Central Glass Co., Ltd. has developed a proprietary process for the production and handling of fluoroantimonic acid. Their method involves a controlled reaction between hydrogen fluoride and antimony pentafluoride in specialized corrosion-resistant reactors. The company has implemented advanced safety protocols and containment systems to manage the highly reactive nature of fluoroantimonic acid. They have also developed novel applications for this superacid in organic synthesis and catalysis, particularly in the production of high-performance fluoropolymers and specialty chemicals.
Strengths: Expertise in fluorine chemistry, advanced safety protocols, and specialized equipment for handling superacids. Weaknesses: High production costs and limited commercial applications due to the extreme reactivity of fluoroantimonic acid.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN has developed a novel approach to utilizing fluoroantimonic acid in the synthesis of advanced fluoropolymers. Their technique involves a controlled, low-temperature reaction environment where fluoroantimonic acid acts as a catalyst for the polymerization of fluorinated monomers. This process allows for the creation of ultra-high molecular weight fluoropolymers with exceptional chemical resistance and thermal stability. DAIKIN has also engineered specialized reactor systems with multiple layers of containment and neutralization capabilities to ensure safe handling of the superacid.
Strengths: Innovative application in fluoropolymer synthesis, advanced containment systems. Weaknesses: High operational costs, limited scalability due to safety concerns.
Breakthrough Innovations in Fluoroantimonic Acid Research
4,5-difluorophthaloyl fluoride and its preparation
PatentInactiveEP0148366A3
Innovation
- A process involving the reaction of chlorophthalic anhydrides with potassium fluoride or cesium fluoride, using polyether catalysts, to produce 4,5-difluorophthaloyl fluoride, which serves as an intermediate for synthesizing fluoroanthranilic acids, fluoroanilines, and other fluorinated compounds, optimizing conditions such as temperature and solvent use to enhance yield and purity.
Process for the production of furanic compounds comprising at least one amine function
PatentWO2014198057A1
Innovation
- A process involving the reaction of a furanic compound with a specific amine in the presence of an iridium catalyst, such as [CpIrX2]2, and optionally a reductant agent, to achieve N-alkylation through dehydrogenation and transfer hydrogenation, allowing for the formation of furanic compounds with amine functions with improved yields and selectivity.
Safety and Environmental Considerations
Fluoroantimonic acid, as one of the strongest known superacids, presents significant safety and environmental challenges that must be carefully addressed in its handling, storage, and application. The extreme corrosiveness and reactivity of this compound necessitate stringent safety protocols and specialized containment measures to protect both personnel and the environment.
From a safety perspective, exposure to fluoroantimonic acid can cause severe and immediate tissue damage, with potential for life-threatening injuries. The acid's ability to react violently with water and many common materials further compounds the risks associated with its use. Consequently, specialized personal protective equipment (PPE) is essential, including chemical-resistant suits, gloves, and respiratory protection. Facilities working with this superacid must implement robust emergency response plans and decontamination procedures.
Environmental considerations are equally critical when dealing with fluoroantimonic acid. Its extreme reactivity means that any release into the environment could have catastrophic consequences for ecosystems and water sources. The acid's potential to generate toxic fumes, particularly hydrogen fluoride, poses additional risks to air quality and human health in surrounding areas. As such, stringent containment and disposal protocols are paramount to prevent environmental contamination.
The storage and transport of fluoroantimonic acid require specialized containers made of materials resistant to its corrosive properties, such as polytetrafluoroethylene (PTFE) or certain fluoropolymers. These containers must be regularly inspected and maintained to ensure their integrity. Furthermore, the acid must be kept away from moisture and incompatible substances to prevent uncontrolled reactions.
In research and industrial settings, the use of fluoroantimonic acid demands purpose-built facilities with advanced ventilation systems, spill containment measures, and dedicated waste treatment processes. The implementation of closed-system handling techniques can significantly reduce the risks of exposure and environmental release.
Given the extreme hazards associated with fluoroantimonic acid, ongoing research into safer alternatives or modified formulations that retain its unique chemical properties while mitigating its risks is crucial. This includes exploring the potential for immobilized or supported forms of the acid that could offer improved safety profiles without compromising reactivity.
From a safety perspective, exposure to fluoroantimonic acid can cause severe and immediate tissue damage, with potential for life-threatening injuries. The acid's ability to react violently with water and many common materials further compounds the risks associated with its use. Consequently, specialized personal protective equipment (PPE) is essential, including chemical-resistant suits, gloves, and respiratory protection. Facilities working with this superacid must implement robust emergency response plans and decontamination procedures.
Environmental considerations are equally critical when dealing with fluoroantimonic acid. Its extreme reactivity means that any release into the environment could have catastrophic consequences for ecosystems and water sources. The acid's potential to generate toxic fumes, particularly hydrogen fluoride, poses additional risks to air quality and human health in surrounding areas. As such, stringent containment and disposal protocols are paramount to prevent environmental contamination.
The storage and transport of fluoroantimonic acid require specialized containers made of materials resistant to its corrosive properties, such as polytetrafluoroethylene (PTFE) or certain fluoropolymers. These containers must be regularly inspected and maintained to ensure their integrity. Furthermore, the acid must be kept away from moisture and incompatible substances to prevent uncontrolled reactions.
In research and industrial settings, the use of fluoroantimonic acid demands purpose-built facilities with advanced ventilation systems, spill containment measures, and dedicated waste treatment processes. The implementation of closed-system handling techniques can significantly reduce the risks of exposure and environmental release.
Given the extreme hazards associated with fluoroantimonic acid, ongoing research into safer alternatives or modified formulations that retain its unique chemical properties while mitigating its risks is crucial. This includes exploring the potential for immobilized or supported forms of the acid that could offer improved safety profiles without compromising reactivity.
Regulatory Framework for Superacid Research
The regulatory framework for superacid research, particularly concerning fluoroantimonic acid, is a complex and evolving landscape. Given the extreme reactivity and potential hazards associated with superacids, stringent regulations have been implemented across various jurisdictions to ensure safe handling, storage, and use in research and industrial settings.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a foundation for the classification of superacids. Fluoroantimonic acid, being one of the strongest known superacids, falls under the highest hazard categories for corrosivity and reactivity. This classification mandates specific labeling requirements and safety data sheet information to be provided by manufacturers and suppliers.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific guidelines for the handling of highly corrosive substances, including superacids. These regulations outline requirements for personal protective equipment, emergency response procedures, and workplace safety measures. The Environmental Protection Agency (EPA) also regulates the production, use, and disposal of superacids under the Toxic Substances Control Act (TSCA).
European Union regulations, governed by the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework, impose strict controls on the manufacture, import, and use of superacids. Researchers and industries working with fluoroantimonic acid must comply with extensive documentation and risk assessment requirements.
In academic and industrial research settings, institutional review boards and safety committees play a crucial role in overseeing experiments involving superacids. These bodies are responsible for ensuring compliance with local and national regulations, as well as implementing additional safety protocols specific to the research environment.
Transportation of superacids is subject to rigorous international regulations, such as those set by the International Air Transport Association (IATA) for air shipments and the International Maritime Dangerous Goods (IMDG) Code for sea transport. These regulations dictate specific packaging, labeling, and documentation requirements to minimize risks during transit.
As research into superacids continues to advance, regulatory frameworks are expected to evolve. Emerging areas of focus include the development of more sophisticated containment technologies, enhanced waste management protocols, and the establishment of specialized training programs for researchers and industrial workers handling these extremely reactive substances.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a foundation for the classification of superacids. Fluoroantimonic acid, being one of the strongest known superacids, falls under the highest hazard categories for corrosivity and reactivity. This classification mandates specific labeling requirements and safety data sheet information to be provided by manufacturers and suppliers.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific guidelines for the handling of highly corrosive substances, including superacids. These regulations outline requirements for personal protective equipment, emergency response procedures, and workplace safety measures. The Environmental Protection Agency (EPA) also regulates the production, use, and disposal of superacids under the Toxic Substances Control Act (TSCA).
European Union regulations, governed by the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework, impose strict controls on the manufacture, import, and use of superacids. Researchers and industries working with fluoroantimonic acid must comply with extensive documentation and risk assessment requirements.
In academic and industrial research settings, institutional review boards and safety committees play a crucial role in overseeing experiments involving superacids. These bodies are responsible for ensuring compliance with local and national regulations, as well as implementing additional safety protocols specific to the research environment.
Transportation of superacids is subject to rigorous international regulations, such as those set by the International Air Transport Association (IATA) for air shipments and the International Maritime Dangerous Goods (IMDG) Code for sea transport. These regulations dictate specific packaging, labeling, and documentation requirements to minimize risks during transit.
As research into superacids continues to advance, regulatory frameworks are expected to evolve. Emerging areas of focus include the development of more sophisticated containment technologies, enhanced waste management protocols, and the establishment of specialized training programs for researchers and industrial workers handling these extremely reactive substances.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!