Fluoroantimonic Acid: A Game Changer in Organic Chemistry
JUN 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid: Background and Objectives
Fluoroantimonic acid, often hailed as the world's strongest superacid, has emerged as a revolutionary compound in the field of organic chemistry. This extraordinarily potent acid, with a Hammett acidity function estimated at -28, surpasses the acidity of pure sulfuric acid by more than a trillion times. Its discovery and subsequent development have opened up new avenues for chemical reactions and transformations previously thought impossible.
The journey of fluoroantimonic acid began in the mid-20th century when researchers were exploring the limits of acidity. It was first synthesized by the reaction of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), resulting in a compound with unprecedented acidic strength. This breakthrough marked a significant milestone in the evolution of superacids and their potential applications in organic synthesis.
As research progressed, scientists began to unravel the unique properties of fluoroantimonic acid. Its extreme acidity is attributed to the formation of very weakly coordinating anions, which allow for the near-complete protonation of organic compounds. This characteristic has proven invaluable in various organic reactions, particularly in the activation of typically unreactive C-H and C-C bonds.
The objectives of exploring fluoroantimonic acid in organic chemistry are multifaceted. Primarily, researchers aim to harness its exceptional protonating ability to facilitate novel synthetic pathways. This includes the development of more efficient methods for producing industrially important compounds, such as high-octane gasoline components and pharmaceutical intermediates.
Furthermore, the study of fluoroantimonic acid seeks to expand our understanding of acid-base chemistry at extreme levels. By pushing the boundaries of acidity, scientists hope to gain insights into the fundamental nature of proton transfer and its effects on molecular structures. This knowledge could potentially lead to the design of new catalysts and the optimization of existing chemical processes.
Another critical objective is to explore the potential of fluoroantimonic acid in activating traditionally inert molecules. Its ability to protonate even weak bases opens up possibilities for transforming unreactive hydrocarbons into more valuable products. This has significant implications for the petrochemical industry and could lead to more sustainable utilization of fossil resources.
As research continues, the scientific community aims to address the challenges associated with handling and applying fluoroantimonic acid. Its extreme corrosiveness and reactivity necessitate the development of specialized containment materials and handling protocols. Overcoming these practical hurdles is crucial for broadening the acid's applicability in both laboratory and industrial settings.
In conclusion, fluoroantimonic acid represents a paradigm shift in organic chemistry, offering unprecedented opportunities for chemical transformations. Its background and the objectives driving its research underscore its potential to revolutionize synthetic methodologies and deepen our understanding of acid-mediated reactions. As scientists continue to explore its capabilities, fluoroantimonic acid stands poised to play a pivotal role in shaping the future of organic synthesis and chemical engineering.
The journey of fluoroantimonic acid began in the mid-20th century when researchers were exploring the limits of acidity. It was first synthesized by the reaction of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), resulting in a compound with unprecedented acidic strength. This breakthrough marked a significant milestone in the evolution of superacids and their potential applications in organic synthesis.
As research progressed, scientists began to unravel the unique properties of fluoroantimonic acid. Its extreme acidity is attributed to the formation of very weakly coordinating anions, which allow for the near-complete protonation of organic compounds. This characteristic has proven invaluable in various organic reactions, particularly in the activation of typically unreactive C-H and C-C bonds.
The objectives of exploring fluoroantimonic acid in organic chemistry are multifaceted. Primarily, researchers aim to harness its exceptional protonating ability to facilitate novel synthetic pathways. This includes the development of more efficient methods for producing industrially important compounds, such as high-octane gasoline components and pharmaceutical intermediates.
Furthermore, the study of fluoroantimonic acid seeks to expand our understanding of acid-base chemistry at extreme levels. By pushing the boundaries of acidity, scientists hope to gain insights into the fundamental nature of proton transfer and its effects on molecular structures. This knowledge could potentially lead to the design of new catalysts and the optimization of existing chemical processes.
Another critical objective is to explore the potential of fluoroantimonic acid in activating traditionally inert molecules. Its ability to protonate even weak bases opens up possibilities for transforming unreactive hydrocarbons into more valuable products. This has significant implications for the petrochemical industry and could lead to more sustainable utilization of fossil resources.
As research continues, the scientific community aims to address the challenges associated with handling and applying fluoroantimonic acid. Its extreme corrosiveness and reactivity necessitate the development of specialized containment materials and handling protocols. Overcoming these practical hurdles is crucial for broadening the acid's applicability in both laboratory and industrial settings.
In conclusion, fluoroantimonic acid represents a paradigm shift in organic chemistry, offering unprecedented opportunities for chemical transformations. Its background and the objectives driving its research underscore its potential to revolutionize synthetic methodologies and deepen our understanding of acid-mediated reactions. As scientists continue to explore its capabilities, fluoroantimonic acid stands poised to play a pivotal role in shaping the future of organic synthesis and chemical engineering.
Market Analysis for Superacid Applications
The market for superacid applications, particularly those involving fluoroantimonic acid, has shown significant growth potential in recent years. This powerful superacid, known for its extreme acidity and unique chemical properties, has found increasing use in various sectors of the chemical industry. The organic synthesis market, in particular, has seen a surge in demand for fluoroantimonic acid due to its ability to catalyze reactions that were previously difficult or impossible to achieve.
In the petrochemical industry, fluoroantimonic acid has gained traction as a catalyst for isomerization and alkylation processes. These applications have led to improved efficiency in the production of high-octane gasoline components, driving demand from oil refineries and fuel manufacturers. The pharmaceutical sector has also recognized the potential of fluoroantimonic acid in the synthesis of complex organic molecules, opening new avenues for drug discovery and development.
The electronics industry represents another growing market for fluoroantimonic acid applications. Its use in the etching of silicon wafers and the production of advanced semiconductors has become increasingly important as the demand for smaller, more powerful electronic devices continues to rise. This trend is expected to contribute significantly to market growth in the coming years.
Despite the promising market outlook, there are challenges that may impact the widespread adoption of fluoroantimonic acid. Safety concerns and the need for specialized handling equipment due to its highly corrosive nature have limited its use in some applications. Additionally, environmental regulations regarding the disposal of fluorine-containing compounds may pose hurdles for certain industries.
The global market for superacids, including fluoroantimonic acid, is projected to expand at a steady rate. North America and Europe currently dominate the market, with Asia-Pacific regions showing rapid growth potential. Key factors driving this growth include increasing research and development activities in the chemical and pharmaceutical industries, as well as the rising demand for high-performance materials in electronics manufacturing.
As the applications for fluoroantimonic acid continue to diversify, the market is expected to see the entry of new players and increased competition among existing manufacturers. This competition is likely to drive innovation in production methods and application techniques, potentially leading to more cost-effective and safer handling solutions for this powerful superacid.
In the petrochemical industry, fluoroantimonic acid has gained traction as a catalyst for isomerization and alkylation processes. These applications have led to improved efficiency in the production of high-octane gasoline components, driving demand from oil refineries and fuel manufacturers. The pharmaceutical sector has also recognized the potential of fluoroantimonic acid in the synthesis of complex organic molecules, opening new avenues for drug discovery and development.
The electronics industry represents another growing market for fluoroantimonic acid applications. Its use in the etching of silicon wafers and the production of advanced semiconductors has become increasingly important as the demand for smaller, more powerful electronic devices continues to rise. This trend is expected to contribute significantly to market growth in the coming years.
Despite the promising market outlook, there are challenges that may impact the widespread adoption of fluoroantimonic acid. Safety concerns and the need for specialized handling equipment due to its highly corrosive nature have limited its use in some applications. Additionally, environmental regulations regarding the disposal of fluorine-containing compounds may pose hurdles for certain industries.
The global market for superacids, including fluoroantimonic acid, is projected to expand at a steady rate. North America and Europe currently dominate the market, with Asia-Pacific regions showing rapid growth potential. Key factors driving this growth include increasing research and development activities in the chemical and pharmaceutical industries, as well as the rising demand for high-performance materials in electronics manufacturing.
As the applications for fluoroantimonic acid continue to diversify, the market is expected to see the entry of new players and increased competition among existing manufacturers. This competition is likely to drive innovation in production methods and application techniques, potentially leading to more cost-effective and safer handling solutions for this powerful superacid.
Current State and Challenges in Superacid Chemistry
Superacid chemistry has witnessed significant advancements in recent years, with fluoroantimonic acid emerging as a pivotal player in organic synthesis. The current state of superacid research is characterized by intense exploration of novel applications and efforts to overcome existing limitations. Fluoroantimonic acid, a combination of hydrogen fluoride and antimony pentafluoride, stands out as the strongest known superacid, surpassing the acidity of sulfuric acid by orders of magnitude.
The exceptional strength of fluoroantimonic acid has opened up new possibilities in organic transformations, particularly in the activation of traditionally unreactive compounds. Its ability to protonate even weak bases has led to breakthroughs in hydrocarbon chemistry, enabling reactions that were previously thought impossible. Researchers have successfully employed fluoroantimonic acid in the isomerization of alkanes, the generation of carbocations, and the facilitation of various electrophilic aromatic substitutions.
Despite its remarkable potential, the widespread adoption of fluoroantimonic acid faces several challenges. The extreme corrosiveness and high reactivity of the acid pose significant safety concerns, necessitating specialized handling techniques and equipment. This has limited its use primarily to controlled laboratory settings, hindering large-scale industrial applications. Additionally, the environmental impact of fluoroantimonic acid and its byproducts remains a subject of concern, prompting research into more sustainable alternatives.
Another challenge lies in the precise control and measurement of superacid strength. The conventional pH scale becomes inadequate for describing the acidity of superacids, leading to the development of alternative acidity functions such as the Hammett acidity function. However, accurately quantifying and comparing the strengths of different superacids remains a complex task, impacting the reproducibility and standardization of superacid-mediated reactions.
The stability and storage of superacids, particularly fluoroantimonic acid, present ongoing challenges. These compounds are highly sensitive to moisture and air, requiring stringent anhydrous conditions for storage and use. Researchers are actively exploring novel containment materials and methods to enhance the longevity and practicality of superacid systems.
As the field progresses, there is a growing focus on developing milder, more selective superacid catalysts that retain high activity while offering improved handling characteristics. This includes the exploration of solid superacids and supported liquid superacids, which promise easier separation and potential for recycling. The quest for new superacid systems with tailored properties continues to drive innovation in this dynamic area of chemistry.
The exceptional strength of fluoroantimonic acid has opened up new possibilities in organic transformations, particularly in the activation of traditionally unreactive compounds. Its ability to protonate even weak bases has led to breakthroughs in hydrocarbon chemistry, enabling reactions that were previously thought impossible. Researchers have successfully employed fluoroantimonic acid in the isomerization of alkanes, the generation of carbocations, and the facilitation of various electrophilic aromatic substitutions.
Despite its remarkable potential, the widespread adoption of fluoroantimonic acid faces several challenges. The extreme corrosiveness and high reactivity of the acid pose significant safety concerns, necessitating specialized handling techniques and equipment. This has limited its use primarily to controlled laboratory settings, hindering large-scale industrial applications. Additionally, the environmental impact of fluoroantimonic acid and its byproducts remains a subject of concern, prompting research into more sustainable alternatives.
Another challenge lies in the precise control and measurement of superacid strength. The conventional pH scale becomes inadequate for describing the acidity of superacids, leading to the development of alternative acidity functions such as the Hammett acidity function. However, accurately quantifying and comparing the strengths of different superacids remains a complex task, impacting the reproducibility and standardization of superacid-mediated reactions.
The stability and storage of superacids, particularly fluoroantimonic acid, present ongoing challenges. These compounds are highly sensitive to moisture and air, requiring stringent anhydrous conditions for storage and use. Researchers are actively exploring novel containment materials and methods to enhance the longevity and practicality of superacid systems.
As the field progresses, there is a growing focus on developing milder, more selective superacid catalysts that retain high activity while offering improved handling characteristics. This includes the exploration of solid superacids and supported liquid superacids, which promise easier separation and potential for recycling. The quest for new superacid systems with tailored properties continues to drive innovation in this dynamic area of chemistry.
Existing Applications of Fluoroantimonic Acid
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Specialized equipment and safety measures are required due to the extreme acidity and reactivity of the compound.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in chemical reactions and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids, making it valuable in organic synthesis and petrochemical industries.
- Material compatibility and storage: Due to its highly corrosive nature, fluoroantimonic acid requires specialized materials for handling and storage. Research has been conducted on developing compatible materials that can withstand its corrosive effects, such as fluoropolymers and certain alloys. Proper storage and containment systems are crucial to prevent degradation of the acid and ensure safety in its handling.
- Safety measures and environmental considerations: Handling fluoroantimonic acid requires strict safety protocols due to its extreme reactivity and corrosiveness. Research has focused on developing improved safety measures, including personal protective equipment, containment systems, and emergency response procedures. Environmental considerations, such as proper disposal methods and containment of potential releases, are also important aspects of working with this superacid.
- Analytical applications and detection methods: Fluoroantimonic acid has found use in analytical chemistry and materials characterization. Research has been conducted on developing methods for detecting and quantifying the acid, as well as utilizing its properties for analyzing other compounds. These applications include spectroscopic techniques, electrochemical methods, and specialized sensors for monitoring the presence or concentration of fluoroantimonic acid.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid serves as a powerful superacid catalyst in various organic synthesis reactions. It is particularly useful in alkylation, isomerization, and polymerization processes. The extreme acidity of fluoroantimonic acid enables it to catalyze reactions that are difficult or impossible with conventional acids.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science for surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials. The extreme reactivity of the acid allows for unique surface modifications that are not achievable with milder acids.Expand Specific Solutions04 Safety considerations and handling procedures
Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized safety measures and handling procedures. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage, transport, and disposal. Proper training and safety systems are essential for working with this superacid.Expand Specific Solutions05 Analytical and characterization techniques
Specialized analytical and characterization techniques are employed for studying fluoroantimonic acid and its reactions. These may include spectroscopic methods, electrochemical analyses, and advanced computational modeling. The extreme nature of the acid necessitates adapted analytical approaches to understand its properties and behavior in various chemical systems.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Research
The field of Fluoroantimonic Acid in Organic Chemistry is in a nascent stage of development, with significant potential for growth. The market size is relatively small but expanding rapidly due to increasing applications in various industries. Technologically, it's still in the early stages of maturity, with ongoing research and development efforts. Key players like DuPont de Nemours, Inc., California Institute of Technology, and Zhejiang University of Technology are at the forefront of innovation. Other notable contributors include Central Glass Co., Ltd. and Mitsui Chemicals Crop & Life Solutions, Inc. The competitive landscape is characterized by a mix of academic institutions and industrial players, each bringing unique expertise to advance the field. As the technology matures, we can expect increased collaboration and potential market consolidation.
Central Glass Co., Ltd.
Technical Solution: Central Glass has pioneered a novel approach to fluoroantimonic acid production and application, focusing on its use as a superacid catalyst in petrochemical processes. Their method involves a continuous flow reactor system that allows for the controlled generation and immediate use of fluoroantimonic acid, minimizing handling risks. The company has also developed specialized glass-lined reactors resistant to the corrosive nature of the acid, enabling longer-term storage and transport. Central Glass's innovation extends to the development of supported fluoroantimonic acid catalysts, which offer enhanced stability and recyclability in organic synthesis reactions.
Strengths: Innovative reactor design, expertise in handling corrosive materials, and development of supported catalysts. Weaknesses: Limited to specific industrial applications, potentially high initial investment costs for implementation.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for the synthesis and handling of fluoroantimonic acid, utilizing specialized containment systems made of fluoropolymers. Their method involves the controlled reaction of hydrogen fluoride with antimony pentafluoride under precise temperature and pressure conditions. DuPont's approach also includes innovative purification techniques to achieve ultra-high purity levels, crucial for advanced organic synthesis applications. The company has integrated this technology into their broader portfolio of fluorine chemistry, enabling the development of novel fluorinated compounds and catalysts.
Strengths: Extensive experience in fluorine chemistry, advanced containment technology, and integration with existing product lines. Weaknesses: High production costs and stringent safety requirements may limit widespread adoption.
Core Innovations in Fluoroantimonic Acid Synthesis
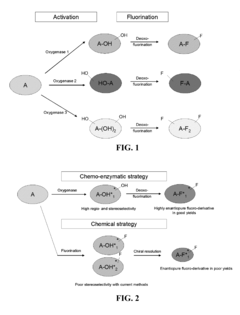
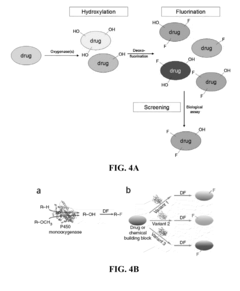
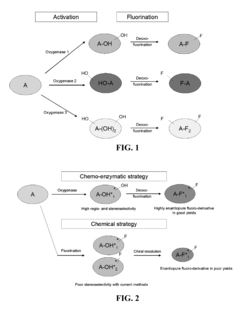
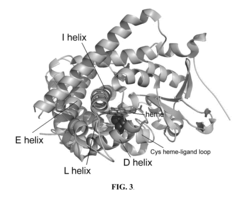
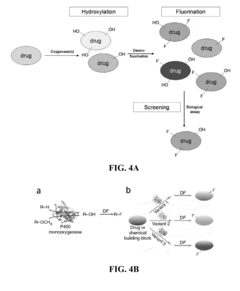
Methods and systems for selective fluorination of organic molecules
PatentActiveUS8252559B2
Innovation
- A method involving cytochrome P450-catalyzed oxygenation followed by deoxofluorination to selectively fluorinate oxidizable carbon atoms in organic molecules, using an oxidizing agent to introduce an oxygen-containing functional group and a fluorinating agent to replace it with fluorine, allowing for regioselective mono- or poly-fluorination and control of chirality.
Methods and systems for selective fluorination of organic molecules
PatentActiveUS20090209010A1
Innovation
- A method involving cytochrome P450-catalyzed oxygenation followed by deoxofluorination to selectively fluorinate organic molecules at specific sites, using an oxidizing agent to introduce an oxygen-containing functional group and a fluorinating agent to replace it with fluorine, allowing for regioselective mono- or poly-fluorination and control of chirality.
Safety and Handling Protocols
Fluoroantimonic acid, being one of the strongest known superacids, requires exceptionally stringent safety and handling protocols. The extreme corrosiveness and reactivity of this compound necessitate specialized equipment and procedures to ensure the safety of personnel and the integrity of research facilities.
Personal protective equipment (PPE) is of paramount importance when working with fluoroantimonic acid. Researchers must wear fully encapsulating chemical-resistant suits, preferably made of fluorinated materials such as Teflon or Viton. Multiple layers of chemical-resistant gloves are essential, with the outer layer being changed frequently. Full-face respirators with acid gas cartridges or self-contained breathing apparatus (SCBA) are mandatory to protect against toxic fumes.
The storage and containment of fluoroantimonic acid demand specialized materials. Containers must be constructed from highly resistant fluoropolymers like polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA). Glass, metal, and most conventional plastics are unsuitable due to the acid's extreme corrosiveness. Storage areas must be well-ventilated, cool, and dry, with strict access controls and comprehensive spill containment systems.
Handling procedures for fluoroantimonic acid require meticulous planning and execution. All operations must be conducted in a fume hood with a face velocity of at least 100 feet per minute. Transfer of the acid should be done using specialized pumps and tubing made of compatible materials. Dilution of the acid must never be attempted by adding water, as this can lead to violent reactions and potential explosions.
Emergency response protocols are critical when working with fluoroantimonic acid. Facilities must have readily accessible safety showers and eyewash stations. Spill kits specifically designed for superacids, containing neutralizing agents like sodium bicarbonate or calcium carbonate, should be strategically placed. Personnel must be trained in emergency procedures, including evacuation routes and the use of specialized fire suppression systems.
Waste disposal of fluoroantimonic acid and related materials requires careful consideration. Neutralization should be performed gradually and under controlled conditions, typically using large quantities of ice-cold sodium or potassium hydroxide solutions. The resulting waste must be treated as hazardous and disposed of according to local regulations, often requiring specialized waste management services.
Regular safety audits and equipment inspections are essential to maintain the integrity of safety protocols. This includes checking the condition of PPE, verifying the functionality of ventilation systems, and ensuring the proper labeling and storage of all chemicals. Comprehensive training programs must be implemented for all personnel working with or around fluoroantimonic acid, covering not only handling procedures but also the chemical properties and potential hazards associated with this powerful superacid.
Personal protective equipment (PPE) is of paramount importance when working with fluoroantimonic acid. Researchers must wear fully encapsulating chemical-resistant suits, preferably made of fluorinated materials such as Teflon or Viton. Multiple layers of chemical-resistant gloves are essential, with the outer layer being changed frequently. Full-face respirators with acid gas cartridges or self-contained breathing apparatus (SCBA) are mandatory to protect against toxic fumes.
The storage and containment of fluoroantimonic acid demand specialized materials. Containers must be constructed from highly resistant fluoropolymers like polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkanes (PFA). Glass, metal, and most conventional plastics are unsuitable due to the acid's extreme corrosiveness. Storage areas must be well-ventilated, cool, and dry, with strict access controls and comprehensive spill containment systems.
Handling procedures for fluoroantimonic acid require meticulous planning and execution. All operations must be conducted in a fume hood with a face velocity of at least 100 feet per minute. Transfer of the acid should be done using specialized pumps and tubing made of compatible materials. Dilution of the acid must never be attempted by adding water, as this can lead to violent reactions and potential explosions.
Emergency response protocols are critical when working with fluoroantimonic acid. Facilities must have readily accessible safety showers and eyewash stations. Spill kits specifically designed for superacids, containing neutralizing agents like sodium bicarbonate or calcium carbonate, should be strategically placed. Personnel must be trained in emergency procedures, including evacuation routes and the use of specialized fire suppression systems.
Waste disposal of fluoroantimonic acid and related materials requires careful consideration. Neutralization should be performed gradually and under controlled conditions, typically using large quantities of ice-cold sodium or potassium hydroxide solutions. The resulting waste must be treated as hazardous and disposed of according to local regulations, often requiring specialized waste management services.
Regular safety audits and equipment inspections are essential to maintain the integrity of safety protocols. This includes checking the condition of PPE, verifying the functionality of ventilation systems, and ensuring the proper labeling and storage of all chemicals. Comprehensive training programs must be implemented for all personnel working with or around fluoroantimonic acid, covering not only handling procedures but also the chemical properties and potential hazards associated with this powerful superacid.
Environmental Impact Assessment
Fluoroantimonic acid, while a powerful tool in organic chemistry, poses significant environmental concerns that require careful assessment and management. The production, use, and disposal of this superacid can have far-reaching impacts on ecosystems and human health if not properly controlled.
The primary environmental risk associated with fluoroantimonic acid stems from its extreme corrosiveness and reactivity. Any accidental release or improper handling could lead to severe soil and water contamination. The acid's ability to react violently with water and many other substances means it can rapidly alter the pH of aquatic environments, potentially causing widespread damage to aquatic life and disrupting entire ecosystems.
Air pollution is another critical concern. The volatile nature of fluoroantimonic acid components, particularly hydrogen fluoride, can result in the release of toxic fumes. These emissions can contribute to air quality degradation, potentially affecting both human and animal respiratory health in surrounding areas. Long-term exposure to such pollutants may lead to chronic health issues in affected populations.
The production process of fluoroantimonic acid also raises environmental concerns. The synthesis typically involves the use of other hazardous materials, such as antimony pentafluoride and hydrogen fluoride. The manufacturing facilities must implement stringent safety measures and emission control systems to prevent environmental contamination and protect worker health.
Waste management presents another significant challenge. The disposal of fluoroantimonic acid and related byproducts requires specialized treatment facilities and protocols. Improper disposal can lead to long-term environmental contamination, affecting soil quality, groundwater resources, and potentially entering the food chain.
From a broader perspective, the use of fluoroantimonic acid in industrial processes may indirectly contribute to environmental issues. While it can enhance the efficiency of certain chemical reactions, potentially reducing overall energy consumption and waste production, the environmental costs of its production and handling must be carefully weighed against these benefits.
To mitigate these environmental risks, comprehensive safety protocols, advanced containment systems, and rigorous monitoring programs are essential in facilities using fluoroantimonic acid. Additionally, ongoing research into safer alternatives or more environmentally friendly synthesis methods is crucial for reducing the long-term environmental impact of this powerful chemical tool in organic chemistry.
The primary environmental risk associated with fluoroantimonic acid stems from its extreme corrosiveness and reactivity. Any accidental release or improper handling could lead to severe soil and water contamination. The acid's ability to react violently with water and many other substances means it can rapidly alter the pH of aquatic environments, potentially causing widespread damage to aquatic life and disrupting entire ecosystems.
Air pollution is another critical concern. The volatile nature of fluoroantimonic acid components, particularly hydrogen fluoride, can result in the release of toxic fumes. These emissions can contribute to air quality degradation, potentially affecting both human and animal respiratory health in surrounding areas. Long-term exposure to such pollutants may lead to chronic health issues in affected populations.
The production process of fluoroantimonic acid also raises environmental concerns. The synthesis typically involves the use of other hazardous materials, such as antimony pentafluoride and hydrogen fluoride. The manufacturing facilities must implement stringent safety measures and emission control systems to prevent environmental contamination and protect worker health.
Waste management presents another significant challenge. The disposal of fluoroantimonic acid and related byproducts requires specialized treatment facilities and protocols. Improper disposal can lead to long-term environmental contamination, affecting soil quality, groundwater resources, and potentially entering the food chain.
From a broader perspective, the use of fluoroantimonic acid in industrial processes may indirectly contribute to environmental issues. While it can enhance the efficiency of certain chemical reactions, potentially reducing overall energy consumption and waste production, the environmental costs of its production and handling must be carefully weighed against these benefits.
To mitigate these environmental risks, comprehensive safety protocols, advanced containment systems, and rigorous monitoring programs are essential in facilities using fluoroantimonic acid. Additionally, ongoing research into safer alternatives or more environmentally friendly synthesis methods is crucial for reducing the long-term environmental impact of this powerful chemical tool in organic chemistry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!