Fluoroantimonic Acid and Its Role in Synthesizing Complex Molecules
JUN 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Background and Objectives
Fluoroantimonic acid, often referred to as the world's strongest superacid, has been a subject of intense scientific interest since its discovery in the 1960s. This compound, formed by mixing hydrogen fluoride (HF) and antimony pentafluoride (SbF5), exhibits extraordinary acidity, surpassing the acidity of 100% sulfuric acid by more than a trillion times. Its unique properties have positioned it at the forefront of chemical research, particularly in the realm of complex molecule synthesis.
The development of fluoroantimonic acid marks a significant milestone in the field of superacid chemistry. Its creation was driven by the need for increasingly powerful acidic catalysts capable of facilitating challenging chemical transformations. The ability of fluoroantimonic acid to protonate even extremely weak bases has opened up new avenues for synthetic organic chemistry, enabling reactions that were previously thought impossible.
In the context of synthesizing complex molecules, fluoroantimonic acid plays a crucial role. Its extreme acidity allows it to activate inert substrates, catalyze difficult transformations, and generate highly reactive intermediates. These capabilities are particularly valuable in the synthesis of pharmaceuticals, advanced materials, and other high-value chemical products that require precise control over molecular structure.
The objectives of research into fluoroantimonic acid and its applications in complex molecule synthesis are multifaceted. Primarily, scientists aim to expand the repertoire of reactions that can be catalyzed by this superacid, exploring its potential in creating novel synthetic pathways. There is also a focus on understanding the fundamental mechanisms by which fluoroantimonic acid interacts with various organic compounds, which could lead to more predictable and controllable synthetic processes.
Another key objective is to develop safer and more practical methods for handling and utilizing fluoroantimonic acid in industrial settings. Due to its extreme reactivity, current applications are largely limited to specialized laboratory environments. Overcoming these practical challenges could pave the way for broader industrial adoption, potentially revolutionizing certain aspects of chemical manufacturing.
Furthermore, researchers are investigating the potential of fluoroantimonic acid in the field of asymmetric synthesis. The ability to control stereochemistry in complex molecule synthesis is of paramount importance in many industries, particularly pharmaceuticals. If fluoroantimonic acid can be harnessed to enhance stereoselectivity in certain reactions, it could lead to more efficient production of chiral compounds.
As we look to the future, the continued exploration of fluoroantimonic acid and its role in synthesizing complex molecules promises to push the boundaries of what is possible in organic synthesis. The ongoing research in this field is expected to yield new methodologies, expand our understanding of superacid chemistry, and potentially lead to breakthroughs in the production of advanced materials and pharmaceutical compounds.
The development of fluoroantimonic acid marks a significant milestone in the field of superacid chemistry. Its creation was driven by the need for increasingly powerful acidic catalysts capable of facilitating challenging chemical transformations. The ability of fluoroantimonic acid to protonate even extremely weak bases has opened up new avenues for synthetic organic chemistry, enabling reactions that were previously thought impossible.
In the context of synthesizing complex molecules, fluoroantimonic acid plays a crucial role. Its extreme acidity allows it to activate inert substrates, catalyze difficult transformations, and generate highly reactive intermediates. These capabilities are particularly valuable in the synthesis of pharmaceuticals, advanced materials, and other high-value chemical products that require precise control over molecular structure.
The objectives of research into fluoroantimonic acid and its applications in complex molecule synthesis are multifaceted. Primarily, scientists aim to expand the repertoire of reactions that can be catalyzed by this superacid, exploring its potential in creating novel synthetic pathways. There is also a focus on understanding the fundamental mechanisms by which fluoroantimonic acid interacts with various organic compounds, which could lead to more predictable and controllable synthetic processes.
Another key objective is to develop safer and more practical methods for handling and utilizing fluoroantimonic acid in industrial settings. Due to its extreme reactivity, current applications are largely limited to specialized laboratory environments. Overcoming these practical challenges could pave the way for broader industrial adoption, potentially revolutionizing certain aspects of chemical manufacturing.
Furthermore, researchers are investigating the potential of fluoroantimonic acid in the field of asymmetric synthesis. The ability to control stereochemistry in complex molecule synthesis is of paramount importance in many industries, particularly pharmaceuticals. If fluoroantimonic acid can be harnessed to enhance stereoselectivity in certain reactions, it could lead to more efficient production of chiral compounds.
As we look to the future, the continued exploration of fluoroantimonic acid and its role in synthesizing complex molecules promises to push the boundaries of what is possible in organic synthesis. The ongoing research in this field is expected to yield new methodologies, expand our understanding of superacid chemistry, and potentially lead to breakthroughs in the production of advanced materials and pharmaceutical compounds.
Market Analysis for Superacid Applications
The market for superacid applications, particularly those involving fluoroantimonic acid, has shown significant growth in recent years. This growth is primarily driven by the increasing demand for complex molecule synthesis in various industries, including pharmaceuticals, agrochemicals, and materials science. Fluoroantimonic acid, being one of the strongest known superacids, plays a crucial role in facilitating challenging chemical reactions that are otherwise difficult or impossible to achieve with conventional acids.
In the pharmaceutical sector, the use of fluoroantimonic acid has enabled the synthesis of novel drug candidates with complex molecular structures. This has opened up new possibilities for developing treatments for previously intractable diseases. The market for such specialized synthesis services is expected to expand as pharmaceutical companies continue to explore innovative drug designs.
The agrochemical industry has also benefited from the unique properties of fluoroantimonic acid. Its ability to catalyze specific reactions has led to the development of more effective and environmentally friendly pesticides and herbicides. This aligns with the growing trend towards sustainable agriculture and has created a niche market for superacid-enabled agrochemical products.
Materials science represents another significant area of application for fluoroantimonic acid. Its use in the production of advanced polymers and nanomaterials has fueled innovation in sectors such as electronics, aerospace, and automotive industries. The demand for high-performance materials with tailored properties continues to drive the market for superacid applications in this field.
Despite the promising market outlook, there are challenges that impact the widespread adoption of fluoroantimonic acid. Safety concerns and the need for specialized handling equipment have limited its use to highly controlled industrial settings. This has created opportunities for companies offering safer alternatives or improved handling technologies, shaping a competitive landscape within the superacid market.
The global market for superacid applications is geographically diverse, with major centers of activity in North America, Europe, and Asia-Pacific. These regions host the majority of advanced chemical research facilities and industries that require complex molecule synthesis. Emerging economies in Asia, particularly China and India, are showing increased interest in superacid technologies, driven by their growing pharmaceutical and chemical manufacturing sectors.
Looking ahead, the market for superacid applications is poised for continued growth. Advancements in containment and handling technologies are expected to expand the potential user base for fluoroantimonic acid and similar superacids. Additionally, ongoing research into new applications, such as in catalysis for clean energy technologies, suggests that the market may diversify further in the coming years.
In the pharmaceutical sector, the use of fluoroantimonic acid has enabled the synthesis of novel drug candidates with complex molecular structures. This has opened up new possibilities for developing treatments for previously intractable diseases. The market for such specialized synthesis services is expected to expand as pharmaceutical companies continue to explore innovative drug designs.
The agrochemical industry has also benefited from the unique properties of fluoroantimonic acid. Its ability to catalyze specific reactions has led to the development of more effective and environmentally friendly pesticides and herbicides. This aligns with the growing trend towards sustainable agriculture and has created a niche market for superacid-enabled agrochemical products.
Materials science represents another significant area of application for fluoroantimonic acid. Its use in the production of advanced polymers and nanomaterials has fueled innovation in sectors such as electronics, aerospace, and automotive industries. The demand for high-performance materials with tailored properties continues to drive the market for superacid applications in this field.
Despite the promising market outlook, there are challenges that impact the widespread adoption of fluoroantimonic acid. Safety concerns and the need for specialized handling equipment have limited its use to highly controlled industrial settings. This has created opportunities for companies offering safer alternatives or improved handling technologies, shaping a competitive landscape within the superacid market.
The global market for superacid applications is geographically diverse, with major centers of activity in North America, Europe, and Asia-Pacific. These regions host the majority of advanced chemical research facilities and industries that require complex molecule synthesis. Emerging economies in Asia, particularly China and India, are showing increased interest in superacid technologies, driven by their growing pharmaceutical and chemical manufacturing sectors.
Looking ahead, the market for superacid applications is poised for continued growth. Advancements in containment and handling technologies are expected to expand the potential user base for fluoroantimonic acid and similar superacids. Additionally, ongoing research into new applications, such as in catalysis for clean energy technologies, suggests that the market may diversify further in the coming years.
Current Challenges in Fluoroantimonic Acid Synthesis
The synthesis of fluoroantimonic acid presents several significant challenges that researchers and industry professionals continue to grapple with. One of the primary obstacles is the extreme reactivity and corrosiveness of this superacid, which makes handling and storage exceptionally difficult. Conventional materials used in laboratory equipment and industrial processes are often inadequate to withstand the highly aggressive nature of fluoroantimonic acid, necessitating the development of specialized containment systems and handling protocols.
Another major challenge lies in the precise control of the reaction conditions required for the synthesis of fluoroantimonic acid. The process involves the combination of hydrogen fluoride (HF) and antimony pentafluoride (SbF5) in specific ratios, typically 2:1. Maintaining the exact stoichiometry and preventing side reactions or decomposition products is crucial for achieving the desired superacid properties. This level of control becomes increasingly difficult when scaling up production for industrial applications.
The environmental and safety concerns associated with fluoroantimonic acid synthesis also pose significant challenges. The precursor materials, particularly hydrogen fluoride, are highly toxic and pose severe health risks. Ensuring proper containment, ventilation, and safety measures throughout the synthesis process is paramount but often complex and costly to implement effectively.
Furthermore, the purification and characterization of fluoroantimonic acid present their own set of challenges. Traditional analytical techniques are often inadequate due to the extreme reactivity of the superacid. Developing reliable methods for assessing the purity and composition of synthesized fluoroantimonic acid remains an ongoing area of research.
The cost-effectiveness of fluoroantimonic acid synthesis is another hurdle, especially for large-scale production. The high-purity precursors required and the specialized equipment needed for safe handling and storage contribute to significant production costs. This economic factor limits the widespread adoption of fluoroantimonic acid in industrial processes, despite its potential applications in organic synthesis and catalysis.
Lastly, the long-term stability of fluoroantimonic acid under various storage conditions remains a challenge. The superacid's tendency to react with moisture and many common materials necessitates the development of innovative storage solutions that can maintain its potency over extended periods without degradation or contamination.
Another major challenge lies in the precise control of the reaction conditions required for the synthesis of fluoroantimonic acid. The process involves the combination of hydrogen fluoride (HF) and antimony pentafluoride (SbF5) in specific ratios, typically 2:1. Maintaining the exact stoichiometry and preventing side reactions or decomposition products is crucial for achieving the desired superacid properties. This level of control becomes increasingly difficult when scaling up production for industrial applications.
The environmental and safety concerns associated with fluoroantimonic acid synthesis also pose significant challenges. The precursor materials, particularly hydrogen fluoride, are highly toxic and pose severe health risks. Ensuring proper containment, ventilation, and safety measures throughout the synthesis process is paramount but often complex and costly to implement effectively.
Furthermore, the purification and characterization of fluoroantimonic acid present their own set of challenges. Traditional analytical techniques are often inadequate due to the extreme reactivity of the superacid. Developing reliable methods for assessing the purity and composition of synthesized fluoroantimonic acid remains an ongoing area of research.
The cost-effectiveness of fluoroantimonic acid synthesis is another hurdle, especially for large-scale production. The high-purity precursors required and the specialized equipment needed for safe handling and storage contribute to significant production costs. This economic factor limits the widespread adoption of fluoroantimonic acid in industrial processes, despite its potential applications in organic synthesis and catalysis.
Lastly, the long-term stability of fluoroantimonic acid under various storage conditions remains a challenge. The superacid's tendency to react with moisture and many common materials necessitates the development of innovative storage solutions that can maintain its potency over extended periods without degradation or contamination.
Existing Fluoroantimonic Acid Synthesis Methods
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Specialized equipment and safety measures are required due to the extreme acidity and reactivity of the compound.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly corrosive and reactive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in catalysis and organic synthesis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acid catalysts, making it valuable in the production of specialty chemicals and advanced materials.
- Use in materials science and surface treatment: Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, enhance adhesion properties, and create specialized coatings. The acid's unique properties make it suitable for treating metals, semiconductors, and other materials in advanced manufacturing processes.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and storage procedures. Safety measures include the use of specialized containment materials, personal protective equipment, and controlled environments. Proper neutralization and disposal methods are essential to prevent environmental contamination and ensure worker safety.
- Analytical and characterization techniques: Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analysis, and specialized titration procedures. Advanced instrumentation and methodologies are employed to determine the acid's properties, purity, and behavior in different chemical environments.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid serves as a powerful superacid catalyst in various organic synthesis reactions. It is particularly useful in alkylation, isomerization, and polymerization processes. The extreme acidity of fluoroantimonic acid enables it to catalyze reactions that are difficult or impossible with conventional acids.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science for surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials. The extreme reactivity of the acid allows for unique surface modifications that are not achievable with milder acids.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and storage procedures. Safety measures include the use of specialized containment materials, personal protective equipment, and strict protocols for handling and disposal. Proper training and safety precautions are essential for working with this superacid.Expand Specific Solutions05 Analytical and research applications
Fluoroantimonic acid is used in various analytical and research applications due to its unique properties. It can be employed in spectroscopic studies, as a reagent in chemical analysis, and in the development of new materials and compounds. The superacidity of fluoroantimonic acid makes it valuable for studying extreme acid-base interactions and reaction mechanisms.Expand Specific Solutions
Key Players in Superacid Research and Industry
The field of Fluoroantimonic Acid and its application in synthesizing complex molecules is in a nascent stage of development, with significant potential for growth. The market size is relatively small but expanding as researchers explore its unique properties. Technologically, it's still in the early phases of maturity, with ongoing research to fully understand and harness its capabilities. Key players like Hunan University, Centre National de la Recherche Scientifique, and Université Toulouse III - Paul Sabatier are leading academic research, while companies such as DSM IP Assets BV, Pfizer Inc., and AGC, Inc. are exploring industrial applications. The competitive landscape is characterized by a mix of academic institutions and pharmaceutical companies, indicating a strong focus on both fundamental research and practical applications in drug synthesis and materials science.
Pfizer Inc.
Technical Solution: Pfizer has developed a novel approach to synthesizing complex molecules using fluoroantimonic acid as a key catalyst. Their method involves a controlled, step-wise addition of fluoroantimonic acid to carefully selected precursor molecules under precisely regulated temperature and pressure conditions. This process allows for the formation of highly reactive carbocation intermediates, which can then undergo a series of rearrangements and additions to form complex molecular structures. Pfizer's technique also incorporates in-situ spectroscopic monitoring to optimize reaction conditions in real-time, ensuring high yields and purity of the final products.
Strengths: High efficiency in synthesizing complex pharmaceutical compounds; precise control over reaction conditions. Weaknesses: Requires specialized equipment for handling extremely corrosive fluoroantimonic acid; potential safety concerns in large-scale production.
AGC, Inc. (Japan)
Technical Solution: AGC has pioneered a unique application of fluoroantimonic acid in the synthesis of advanced materials, particularly focusing on the creation of novel fluoropolymers. Their proprietary process utilizes a specially designed reactor system that allows for the controlled exposure of monomers to fluoroantimonic acid under varying pressures and temperatures. This method enables the formation of highly fluorinated polymers with exceptional chemical and thermal resistance. AGC's innovation also includes a patented neutralization and purification process to remove residual acid and byproducts, ensuring the final product meets stringent quality standards for industrial and technological applications.
Strengths: Production of high-performance materials with unique properties; scalable for industrial production. Weaknesses: High cost of raw materials and specialized equipment; environmental concerns related to fluorinated compounds.
Innovations in Fluoroantimonic Acid Applications
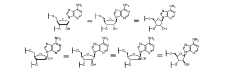
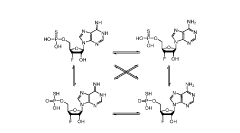
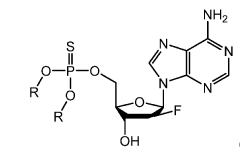
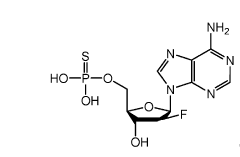
Synthesis of fluorinated nucleotides
PatentWO2022035917A1
Innovation
- A synthetic process using stereo-controlled electrophilic fluorination and glycosylation with new organocatalysts to produce 2'-fluorinated nucleosides, avoiding dimeric and polymeric impurities, and employing inexpensive raw materials, stable intermediates, and crystalline forms, while replacing hazardous reagents with safer alternatives.
Fluorophosphonocinnamic compounds, synthesis and uses for treating disorders caused by oxidative stress
PatentWO2006037869A2
Innovation
- A process involving a complex formed between a tertiary amine and hydrogen fluoride is used to fluorinate phosphonocinnamic compounds, allowing for the production of fluorophosphonocinnamic compounds with improved antioxidant activity, using easily accessible and safer reagents at room temperature or lower temperatures.
Safety and Handling Protocols for Fluoroantimonic Acid
Fluoroantimonic acid, being one of the strongest known superacids, requires exceptionally stringent safety measures and handling protocols. The extreme corrosiveness and reactivity of this compound necessitate specialized equipment and highly trained personnel for its safe manipulation.
Personal protective equipment (PPE) is of paramount importance when working with fluoroantimonic acid. This includes chemical-resistant suits made of materials such as Teflon or Viton, which can withstand the acid's corrosive nature. Full-face respirators with appropriate acid gas cartridges are essential to prevent inhalation of toxic fumes. Double-layered gloves, with an inner layer of fluoroelastomer and an outer layer of neoprene, provide the necessary hand protection.
The storage and transportation of fluoroantimonic acid demand specialized containers made of materials resistant to its corrosive effects. Polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkane (PFA) containers are commonly used due to their inertness to the acid. These containers must be sealed tightly and stored in a cool, dry place away from any potential reactants or moisture sources.
Handling procedures for fluoroantimonic acid must be conducted in a designated area with proper ventilation systems. Fume hoods equipped with acid-resistant liners and scrubbers are crucial for containing and neutralizing any vapors or spills. All work surfaces should be covered with PTFE-coated materials to prevent contamination and potential reactions.
Emergency response protocols are critical when working with fluoroantimonic acid. Specialized spill kits containing neutralizing agents such as sodium bicarbonate or calcium carbonate should be readily available. Eyewash stations and safety showers must be easily accessible and regularly maintained. A detailed emergency response plan, including evacuation procedures and contact information for hazardous material response teams, should be in place and regularly reviewed.
Training and education form an integral part of the safety protocols. All personnel working with or around fluoroantimonic acid must undergo comprehensive training on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to ensure that all staff members are up-to-date with the latest safety practices and emergency procedures.
Waste disposal of fluoroantimonic acid and related materials requires careful consideration. Neutralization processes must be carried out in controlled environments, typically involving the slow addition of the acid to large volumes of ice-cold alkaline solutions. The resulting waste must be treated as hazardous and disposed of according to local and national regulations, often requiring specialized waste management services.
Personal protective equipment (PPE) is of paramount importance when working with fluoroantimonic acid. This includes chemical-resistant suits made of materials such as Teflon or Viton, which can withstand the acid's corrosive nature. Full-face respirators with appropriate acid gas cartridges are essential to prevent inhalation of toxic fumes. Double-layered gloves, with an inner layer of fluoroelastomer and an outer layer of neoprene, provide the necessary hand protection.
The storage and transportation of fluoroantimonic acid demand specialized containers made of materials resistant to its corrosive effects. Polytetrafluoroethylene (PTFE) or perfluoroalkoxy alkane (PFA) containers are commonly used due to their inertness to the acid. These containers must be sealed tightly and stored in a cool, dry place away from any potential reactants or moisture sources.
Handling procedures for fluoroantimonic acid must be conducted in a designated area with proper ventilation systems. Fume hoods equipped with acid-resistant liners and scrubbers are crucial for containing and neutralizing any vapors or spills. All work surfaces should be covered with PTFE-coated materials to prevent contamination and potential reactions.
Emergency response protocols are critical when working with fluoroantimonic acid. Specialized spill kits containing neutralizing agents such as sodium bicarbonate or calcium carbonate should be readily available. Eyewash stations and safety showers must be easily accessible and regularly maintained. A detailed emergency response plan, including evacuation procedures and contact information for hazardous material response teams, should be in place and regularly reviewed.
Training and education form an integral part of the safety protocols. All personnel working with or around fluoroantimonic acid must undergo comprehensive training on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to ensure that all staff members are up-to-date with the latest safety practices and emergency procedures.
Waste disposal of fluoroantimonic acid and related materials requires careful consideration. Neutralization processes must be carried out in controlled environments, typically involving the slow addition of the acid to large volumes of ice-cold alkaline solutions. The resulting waste must be treated as hazardous and disposed of according to local and national regulations, often requiring specialized waste management services.
Environmental Impact of Fluoroantimonic Acid Production
The production of fluoroantimonic acid, one of the strongest known superacids, poses significant environmental concerns due to its highly corrosive and reactive nature. The manufacturing process involves the combination of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), both of which are hazardous substances with potential environmental impacts.
The primary environmental risk associated with fluoroantimonic acid production is the potential release of fluorine-containing compounds into the atmosphere. These emissions can contribute to the formation of acid rain, which has detrimental effects on ecosystems, including soil acidification and damage to vegetation. Additionally, fluorine compounds can persist in the environment and accumulate in living organisms, potentially disrupting food chains and biodiversity.
Water pollution is another critical concern. Accidental spills or improper disposal of fluoroantimonic acid or its precursors can contaminate water sources, leading to severe ecological damage. The extreme acidity of the compound can drastically alter the pH of water bodies, making them uninhabitable for aquatic life and rendering the water unsafe for human consumption.
The production process also generates hazardous waste, including spent acid and contaminated materials. Proper handling, treatment, and disposal of these wastes are essential to prevent soil contamination and groundwater pollution. Specialized containment and neutralization procedures are required to manage these wastes safely.
Energy consumption during the production of fluoroantimonic acid is another environmental consideration. The synthesis process typically requires high temperatures and pressures, contributing to greenhouse gas emissions if non-renewable energy sources are used. Implementing energy-efficient technologies and exploring renewable energy options can help mitigate this impact.
To address these environmental concerns, stringent safety measures and regulatory compliance are crucial in fluoroantimonic acid production facilities. This includes implementing robust containment systems, advanced air and water treatment technologies, and comprehensive emergency response plans. Regular environmental monitoring and audits are essential to ensure compliance with environmental standards and to detect and address potential issues promptly.
Research into alternative synthesis methods or less environmentally harmful substitutes for fluoroantimonic acid in complex molecule synthesis is ongoing. These efforts aim to reduce the environmental footprint of superacid production while maintaining its effectiveness in chemical processes. Developing greener alternatives could significantly mitigate the environmental risks associated with fluoroantimonic acid production and use.
The primary environmental risk associated with fluoroantimonic acid production is the potential release of fluorine-containing compounds into the atmosphere. These emissions can contribute to the formation of acid rain, which has detrimental effects on ecosystems, including soil acidification and damage to vegetation. Additionally, fluorine compounds can persist in the environment and accumulate in living organisms, potentially disrupting food chains and biodiversity.
Water pollution is another critical concern. Accidental spills or improper disposal of fluoroantimonic acid or its precursors can contaminate water sources, leading to severe ecological damage. The extreme acidity of the compound can drastically alter the pH of water bodies, making them uninhabitable for aquatic life and rendering the water unsafe for human consumption.
The production process also generates hazardous waste, including spent acid and contaminated materials. Proper handling, treatment, and disposal of these wastes are essential to prevent soil contamination and groundwater pollution. Specialized containment and neutralization procedures are required to manage these wastes safely.
Energy consumption during the production of fluoroantimonic acid is another environmental consideration. The synthesis process typically requires high temperatures and pressures, contributing to greenhouse gas emissions if non-renewable energy sources are used. Implementing energy-efficient technologies and exploring renewable energy options can help mitigate this impact.
To address these environmental concerns, stringent safety measures and regulatory compliance are crucial in fluoroantimonic acid production facilities. This includes implementing robust containment systems, advanced air and water treatment technologies, and comprehensive emergency response plans. Regular environmental monitoring and audits are essential to ensure compliance with environmental standards and to detect and address potential issues promptly.
Research into alternative synthesis methods or less environmentally harmful substitutes for fluoroantimonic acid in complex molecule synthesis is ongoing. These efforts aim to reduce the environmental footprint of superacid production while maintaining its effectiveness in chemical processes. Developing greener alternatives could significantly mitigate the environmental risks associated with fluoroantimonic acid production and use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!