Fluoroantimonic Acid and Superacids: A Comparative Study
JUN 23, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Superacid Evolution
The evolution of superacids represents a significant milestone in the field of chemistry, marking a continuous progression towards increasingly powerful acidic substances. This journey began with the discovery of sulfuric acid in the 8th century, which remained the strongest known acid for centuries. The concept of superacids, however, only emerged in the mid-20th century, revolutionizing our understanding of acidity and catalysis.
In 1927, James Bryant Conant introduced the term "superacid" to describe acids stronger than conventional mineral acids. This laid the foundation for further research into highly acidic substances. The Hammett acidity function, developed by Louis Plack Hammett in the 1930s, provided a crucial tool for measuring and comparing the strength of strong acids, enabling more precise classification and study of superacids.
A significant breakthrough came in 1966 when George A. Olah synthesized magic acid, a mixture of fluorosulfonic acid and antimony pentafluoride. This superacid demonstrated unprecedented acidity, capable of protonating even extremely weak bases. Olah's work paved the way for the development of even stronger superacids, culminating in the creation of fluoroantimonic acid in 1974, currently recognized as the strongest superacid known.
The evolution of superacids has been driven by the quest for stronger proton donors and more stable conjugate bases. This pursuit has led to the exploration of various combinations of strong Lewis acids and Brønsted acids, resulting in the creation of numerous superacid systems with diverse properties and applications.
Parallel to the development of liquid superacids, solid superacids have also emerged as an important area of research. These materials, such as sulfated zirconia and tungstated zirconia, offer advantages in terms of handling and recyclability, expanding the potential applications of superacidic catalysis in industrial processes.
Recent advancements in superacid research have focused on fine-tuning acid strength, improving stability, and enhancing selectivity for specific reactions. The development of ionic liquid-based superacids and the exploration of confined superacidic environments in zeolites and metal-organic frameworks represent cutting-edge directions in this field.
The evolution of superacids continues to push the boundaries of acidity, with ongoing efforts to synthesize even stronger acids and to develop novel superacidic systems with tailored properties for specific applications. This progression not only advances our fundamental understanding of acid-base chemistry but also opens up new possibilities for catalysis, materials science, and chemical synthesis.
In 1927, James Bryant Conant introduced the term "superacid" to describe acids stronger than conventional mineral acids. This laid the foundation for further research into highly acidic substances. The Hammett acidity function, developed by Louis Plack Hammett in the 1930s, provided a crucial tool for measuring and comparing the strength of strong acids, enabling more precise classification and study of superacids.
A significant breakthrough came in 1966 when George A. Olah synthesized magic acid, a mixture of fluorosulfonic acid and antimony pentafluoride. This superacid demonstrated unprecedented acidity, capable of protonating even extremely weak bases. Olah's work paved the way for the development of even stronger superacids, culminating in the creation of fluoroantimonic acid in 1974, currently recognized as the strongest superacid known.
The evolution of superacids has been driven by the quest for stronger proton donors and more stable conjugate bases. This pursuit has led to the exploration of various combinations of strong Lewis acids and Brønsted acids, resulting in the creation of numerous superacid systems with diverse properties and applications.
Parallel to the development of liquid superacids, solid superacids have also emerged as an important area of research. These materials, such as sulfated zirconia and tungstated zirconia, offer advantages in terms of handling and recyclability, expanding the potential applications of superacidic catalysis in industrial processes.
Recent advancements in superacid research have focused on fine-tuning acid strength, improving stability, and enhancing selectivity for specific reactions. The development of ionic liquid-based superacids and the exploration of confined superacidic environments in zeolites and metal-organic frameworks represent cutting-edge directions in this field.
The evolution of superacids continues to push the boundaries of acidity, with ongoing efforts to synthesize even stronger acids and to develop novel superacidic systems with tailored properties for specific applications. This progression not only advances our fundamental understanding of acid-base chemistry but also opens up new possibilities for catalysis, materials science, and chemical synthesis.
Industrial Applications
Fluoroantimonic acid and other superacids have found significant industrial applications across various sectors due to their exceptional acidity and unique chemical properties. In the petrochemical industry, these superacids play a crucial role in catalytic cracking processes, enabling the conversion of heavy hydrocarbons into more valuable lighter fractions. This application has revolutionized the efficiency of oil refining, leading to increased yields of high-quality gasoline and other fuel products.
The electronics industry has also benefited from the use of superacids, particularly in the etching of silicon wafers for semiconductor manufacturing. The extreme acidity of fluoroantimonic acid allows for precise and controlled etching of silicon surfaces, which is essential for creating intricate microchip designs. This has contributed to the ongoing miniaturization of electronic components and the advancement of computing technologies.
In the field of materials science, superacids have proven invaluable in the synthesis of novel polymers and advanced materials. Their ability to catalyze polymerization reactions under mild conditions has led to the development of high-performance plastics with enhanced thermal and chemical resistance. These materials find applications in aerospace, automotive, and industrial sectors, where durability and performance under extreme conditions are paramount.
The pharmaceutical industry utilizes superacids in the synthesis of complex organic compounds, including active pharmaceutical ingredients (APIs). The strong protonating ability of these acids enables challenging chemical transformations that are difficult or impossible to achieve with conventional acids. This has expanded the toolkit of medicinal chemists, potentially leading to the discovery of new drug candidates and more efficient synthesis routes for existing medications.
In the field of energy storage, superacids have shown promise in the development of advanced battery technologies. Research into superacid-based electrolytes for lithium-ion batteries has demonstrated potential improvements in energy density and cycle life. This could have far-reaching implications for electric vehicles and renewable energy storage systems, addressing key challenges in the transition to sustainable energy sources.
The nuclear industry has also found applications for superacids, particularly in the processing and recycling of nuclear fuel. The extreme acidity of these compounds allows for the efficient dissolution of uranium and plutonium oxides, facilitating the separation and purification of fissile materials. This application contributes to the overall efficiency and sustainability of nuclear power generation.
The electronics industry has also benefited from the use of superacids, particularly in the etching of silicon wafers for semiconductor manufacturing. The extreme acidity of fluoroantimonic acid allows for precise and controlled etching of silicon surfaces, which is essential for creating intricate microchip designs. This has contributed to the ongoing miniaturization of electronic components and the advancement of computing technologies.
In the field of materials science, superacids have proven invaluable in the synthesis of novel polymers and advanced materials. Their ability to catalyze polymerization reactions under mild conditions has led to the development of high-performance plastics with enhanced thermal and chemical resistance. These materials find applications in aerospace, automotive, and industrial sectors, where durability and performance under extreme conditions are paramount.
The pharmaceutical industry utilizes superacids in the synthesis of complex organic compounds, including active pharmaceutical ingredients (APIs). The strong protonating ability of these acids enables challenging chemical transformations that are difficult or impossible to achieve with conventional acids. This has expanded the toolkit of medicinal chemists, potentially leading to the discovery of new drug candidates and more efficient synthesis routes for existing medications.
In the field of energy storage, superacids have shown promise in the development of advanced battery technologies. Research into superacid-based electrolytes for lithium-ion batteries has demonstrated potential improvements in energy density and cycle life. This could have far-reaching implications for electric vehicles and renewable energy storage systems, addressing key challenges in the transition to sustainable energy sources.
The nuclear industry has also found applications for superacids, particularly in the processing and recycling of nuclear fuel. The extreme acidity of these compounds allows for the efficient dissolution of uranium and plutonium oxides, facilitating the separation and purification of fissile materials. This application contributes to the overall efficiency and sustainability of nuclear power generation.
Synthesis Challenges
The synthesis of fluoroantimonic acid and other superacids presents significant challenges due to their extreme reactivity and corrosive nature. One of the primary difficulties lies in the handling and containment of these substances. Traditional laboratory glassware and most common materials are unsuitable, as they are rapidly attacked and degraded by superacids. Specialized equipment, such as fluoropolymer-lined vessels and perfluorinated plastics, must be employed to withstand the harsh conditions.
The preparation of fluoroantimonic acid, in particular, involves the combination of hydrogen fluoride (HF) and antimony pentafluoride (SbF5). This process requires stringent safety measures and precise control over reaction conditions. The highly exothermic nature of the mixing process necessitates careful temperature management to prevent runaway reactions. Moreover, the extreme hygroscopicity of both reactants and the product demands rigorous exclusion of moisture at all stages of synthesis.
Another significant challenge in superacid synthesis is the purification and characterization of the final products. Traditional analytical techniques are often inadequate due to the extreme acidity and reactivity of these compounds. Spectroscopic methods must be adapted to accommodate the unique properties of superacids, often requiring specialized equipment and expertise. The quantification of acidity for superacids also poses difficulties, as conventional pH scales are insufficient to measure their strength accurately.
The scalability of superacid synthesis presents additional hurdles. While laboratory-scale production may be manageable with appropriate precautions, scaling up to industrial quantities introduces new complexities. Large-scale handling of precursors like hydrogen fluoride and antimony pentafluoride requires sophisticated engineering controls and safety systems. The heat management and reaction control become increasingly critical as batch sizes increase, necessitating advanced reactor designs and cooling systems.
Environmental and safety considerations further complicate the synthesis process. The potential for release of highly toxic and corrosive substances demands robust containment strategies and emergency response protocols. Waste management and disposal of byproducts or unreacted materials pose significant environmental challenges, requiring specialized treatment and neutralization procedures.
In the context of comparative studies between fluoroantimonic acid and other superacids, researchers must navigate the varying synthesis requirements for each compound. Different superacids may demand unique precursors, reaction conditions, and handling protocols. This diversity complicates standardization efforts and makes direct comparisons of synthetic routes challenging. Researchers must develop innovative approaches to ensure consistent quality and purity across different superacid samples for meaningful comparative analyses.
The preparation of fluoroantimonic acid, in particular, involves the combination of hydrogen fluoride (HF) and antimony pentafluoride (SbF5). This process requires stringent safety measures and precise control over reaction conditions. The highly exothermic nature of the mixing process necessitates careful temperature management to prevent runaway reactions. Moreover, the extreme hygroscopicity of both reactants and the product demands rigorous exclusion of moisture at all stages of synthesis.
Another significant challenge in superacid synthesis is the purification and characterization of the final products. Traditional analytical techniques are often inadequate due to the extreme acidity and reactivity of these compounds. Spectroscopic methods must be adapted to accommodate the unique properties of superacids, often requiring specialized equipment and expertise. The quantification of acidity for superacids also poses difficulties, as conventional pH scales are insufficient to measure their strength accurately.
The scalability of superacid synthesis presents additional hurdles. While laboratory-scale production may be manageable with appropriate precautions, scaling up to industrial quantities introduces new complexities. Large-scale handling of precursors like hydrogen fluoride and antimony pentafluoride requires sophisticated engineering controls and safety systems. The heat management and reaction control become increasingly critical as batch sizes increase, necessitating advanced reactor designs and cooling systems.
Environmental and safety considerations further complicate the synthesis process. The potential for release of highly toxic and corrosive substances demands robust containment strategies and emergency response protocols. Waste management and disposal of byproducts or unreacted materials pose significant environmental challenges, requiring specialized treatment and neutralization procedures.
In the context of comparative studies between fluoroantimonic acid and other superacids, researchers must navigate the varying synthesis requirements for each compound. Different superacids may demand unique precursors, reaction conditions, and handling protocols. This diversity complicates standardization efforts and makes direct comparisons of synthetic routes challenging. Researchers must develop innovative approaches to ensure consistent quality and purity across different superacid samples for meaningful comparative analyses.
Current Handling Methods
01 Synthesis and properties of fluoroantimonic acid
Fluoroantimonic acid is a superacid formed by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5). It is considered one of the strongest known superacids, with exceptional proton-donating ability. The synthesis and characterization of fluoroantimonic acid involve careful handling due to its extreme reactivity and corrosive nature.- Synthesis and properties of fluoroantimonic acid: Fluoroantimonic acid is a superacid formed by combining hydrogen fluoride (HF) and antimony pentafluoride (SbF5). It is considered one of the strongest known superacids, with exceptional acidity and reactivity. The synthesis and characterization of fluoroantimonic acid involve specialized techniques due to its highly corrosive and reactive nature.
- Applications of superacids in chemical processes: Superacids, including fluoroantimonic acid, find applications in various chemical processes such as catalysis, isomerization, and alkylation reactions. They are particularly useful in the petrochemical industry for the production of high-octane gasoline components and in the synthesis of specialty chemicals. The extreme acidity of these compounds enables reactions that are difficult or impossible with conventional acids.
- Handling and safety considerations for superacids: Due to their extreme reactivity and corrosive nature, superacids require specialized handling techniques and safety precautions. This includes the use of specialized materials for containment, personal protective equipment, and controlled environments. Proper disposal and neutralization methods are crucial to prevent environmental contamination and ensure worker safety when dealing with these powerful acids.
- Measurement and characterization of superacid strength: The strength of superacids, including fluoroantimonic acid, is often measured using specialized techniques such as the Hammett acidity function. These methods allow for the comparison and classification of extremely strong acids that cannot be measured by conventional pH scales. Understanding the acidity and reactivity of superacids is crucial for their effective application in research and industry.
- Development of novel superacid systems: Research continues in the development of new superacid systems, including modifications to fluoroantimonic acid and the creation of novel superacid combinations. These efforts aim to create even stronger acids, improve stability, or tailor the properties for specific applications. This includes the exploration of different metal fluorides, mixed superacid systems, and supported superacids for heterogeneous catalysis.
02 Applications of superacids in chemical processes
Superacids, including fluoroantimonic acid, find applications in various chemical processes such as catalysis, isomerization, and alkylation reactions. They are particularly useful in the petrochemical industry for the production of high-octane gasoline components and in the synthesis of specialty chemicals. The extreme acidity of these compounds enables reactions that are difficult or impossible with conventional acids.Expand Specific Solutions03 Handling and safety considerations for superacids
Working with superacids like fluoroantimonic acid requires stringent safety measures due to their highly corrosive and reactive nature. Special equipment, such as fluoropolymer-lined vessels and protective gear, is necessary. Proper storage, handling, and disposal protocols must be followed to prevent accidents and environmental contamination. Safety training and risk assessment are crucial for personnel working with these materials.Expand Specific Solutions04 Measurement and characterization of superacid strength
The strength of superacids is often measured using the Hammett acidity function or other specialized techniques. Characterization methods may include NMR spectroscopy, conductivity measurements, and computational studies. Understanding the acidity and proton-donating ability of superacids is crucial for their effective application in various chemical processes and for comparing different superacid systems.Expand Specific Solutions05 Development of novel superacid systems
Research continues on developing new superacid systems with enhanced properties or specific applications. This includes the exploration of different combinations of Lewis acids and Brønsted acids, solid superacids, and supported superacid catalysts. Novel superacid systems aim to improve reactivity, selectivity, or ease of handling compared to traditional superacids like fluoroantimonic acid.Expand Specific Solutions
Key Superacid Producers
The field of fluoroantimonic acid and superacids research is in a mature stage, with established players and ongoing innovation. The global superacids market is projected to grow steadily, driven by applications in petrochemicals, pharmaceuticals, and materials science. Technologically, companies like BASF Corp., Air Products & Chemicals, Inc., and China Petroleum & Chemical Corp. are at the forefront, developing advanced synthesis methods and exploring novel applications. Academic institutions such as the University of Freiburg and Centre National de la Recherche Scientifique contribute significantly to fundamental research, while pharmaceutical giants like Pfizer Inc. and Bayer AG leverage superacids in drug development processes, indicating a high level of technological maturity across various sectors.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has invested in research on superacids, particularly focusing on their application in the petroleum refining industry. They have developed a series of solid superacid catalysts based on sulfated zirconia and tungstated zirconia for use in isomerization and alkylation processes. Sinopec's approach emphasizes the development of heterogeneous catalysts that can withstand the harsh conditions of industrial processes while providing high activity and selectivity. Their research also extends to the use of ionic liquids as alternatives to traditional superacids, aiming to improve process efficiency and reduce environmental impact in petrochemical operations.
Strengths: Strong focus on practical applications in the petroleum industry, development of robust heterogeneous catalysts. Weaknesses: Limited research on extremely strong superacids like fluoroantimonic acid.
Air Products & Chemicals, Inc.
Technical Solution: Air Products & Chemicals has developed a proprietary process for the production and handling of fluoroantimonic acid, one of the strongest known superacids. Their method involves the controlled reaction of hydrogen fluoride with antimony pentafluoride under strictly anhydrous conditions. The company has engineered specialized containment systems using materials resistant to extreme acidity, such as fluoropolymers and certain alloys. They have also implemented advanced safety protocols for the storage and transportation of this highly reactive substance.
Strengths: Expertise in handling extremely corrosive materials, advanced safety protocols, and specialized containment systems. Weaknesses: High production costs and limited commercial applications due to the extreme reactivity of fluoroantimonic acid.
Fluoroantimonic Innovations
Process For The Fluorination of Boron Hydrides
PatentInactiveEP1930336A1
Innovation
- A method involving the reaction of borohydride salts with anhydrous hydrogen fluoride and a superacid additive, such as boron trifluoride or tantalum pentafluoride, to selectively and controllably convert B-H bonds to B-F bonds, allowing for high-yielding synthesis of fluorinated boron compounds with narrow degrees of fluorination under practicable conditions.
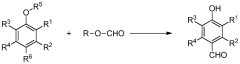
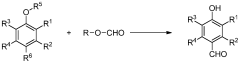
Process for production of vanillin and vanillin derivatives
PatentWO2013166946A1
Innovation
- A one-step process using superacids to perform regio- and chemoselective formylation of guaiacol derivatives, achieving high yields of vanillin or vanillin derivatives under mild conditions without significant isomer production, utilizing superacids like trifluoromethanesulfonic acid and fluoroalkanesulfonic acid as catalysts.
Safety and Regulations
The handling and use of fluoroantimonic acid and other superacids require stringent safety measures and regulatory compliance due to their extreme corrosiveness and reactivity. Proper personal protective equipment (PPE) is essential, including chemical-resistant suits, gloves, and full-face respirators with appropriate filters. All work with these substances must be conducted in well-ventilated fume hoods or specialized containment systems to prevent exposure to vapors or aerosols.
Storage and transportation of superacids are subject to strict regulations. They must be kept in specially designed containers made of materials resistant to their corrosive effects, such as fluoropolymers or certain alloys. Proper labeling and documentation are crucial for compliance with hazardous materials regulations during transport and storage.
Waste disposal of superacids and related materials is heavily regulated to prevent environmental contamination. Neutralization procedures must be carefully followed, and disposal should only be carried out by licensed hazardous waste management facilities. Comprehensive emergency response plans must be in place to address potential spills or accidents involving these substances.
Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) have established specific guidelines for the handling of superacids. These include exposure limits, required safety equipment, and protocols for accidental release. Compliance with these regulations is mandatory for any facility working with fluoroantimonic acid or other superacids.
Research institutions and industrial facilities using superacids must obtain proper permits and licenses, which often involve regular inspections and audits to ensure ongoing compliance. Staff training is a critical component of regulatory compliance, with all personnel involved in handling these substances required to undergo comprehensive safety training and periodic refresher courses.
The use of superacids in research and industry is subject to strict record-keeping requirements. Detailed logs of usage, storage, and disposal must be maintained and made available for regulatory review. Additionally, any incidents or near-misses involving these substances must be promptly reported to the appropriate authorities.
Storage and transportation of superacids are subject to strict regulations. They must be kept in specially designed containers made of materials resistant to their corrosive effects, such as fluoropolymers or certain alloys. Proper labeling and documentation are crucial for compliance with hazardous materials regulations during transport and storage.
Waste disposal of superacids and related materials is heavily regulated to prevent environmental contamination. Neutralization procedures must be carefully followed, and disposal should only be carried out by licensed hazardous waste management facilities. Comprehensive emergency response plans must be in place to address potential spills or accidents involving these substances.
Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) have established specific guidelines for the handling of superacids. These include exposure limits, required safety equipment, and protocols for accidental release. Compliance with these regulations is mandatory for any facility working with fluoroantimonic acid or other superacids.
Research institutions and industrial facilities using superacids must obtain proper permits and licenses, which often involve regular inspections and audits to ensure ongoing compliance. Staff training is a critical component of regulatory compliance, with all personnel involved in handling these substances required to undergo comprehensive safety training and periodic refresher courses.
The use of superacids in research and industry is subject to strict record-keeping requirements. Detailed logs of usage, storage, and disposal must be maintained and made available for regulatory review. Additionally, any incidents or near-misses involving these substances must be promptly reported to the appropriate authorities.
Environmental Impact
The environmental impact of fluoroantimonic acid and other superacids is a critical consideration in their study and application. These highly corrosive substances pose significant risks to ecosystems and human health if not properly managed. Fluoroantimonic acid, being one of the strongest known superacids, is particularly hazardous due to its extreme reactivity and ability to dissolve many materials.
When released into the environment, superacids can cause severe damage to soil and water systems. They rapidly lower the pH of their surroundings, leading to acidification of soil and water bodies. This acidification can have far-reaching consequences on flora and fauna, disrupting delicate ecological balances. Aquatic life is especially vulnerable, as even small changes in water pH can be lethal to many species.
The production and handling of superacids also contribute to air pollution. Volatile compounds released during manufacturing or accidental spills can lead to the formation of acid rain, further exacerbating environmental degradation. The potential for these substances to react with atmospheric moisture and create harmful aerosols is a significant concern for air quality and public health.
Waste management of superacids presents another environmental challenge. Improper disposal can lead to long-term contamination of groundwater and soil. The extreme reactivity of these substances makes them difficult to neutralize safely, requiring specialized treatment facilities and protocols. This complexity in waste handling increases the risk of environmental exposure through accidents or mismanagement.
The manufacturing processes for superacids, particularly fluoroantimonic acid, often involve the use of other hazardous materials and energy-intensive procedures. This indirect environmental impact, including greenhouse gas emissions and resource depletion, must be considered in the overall environmental assessment of these substances.
Given these environmental concerns, research into safer alternatives and improved containment methods is crucial. Developing more environmentally friendly synthesis routes and exploring less hazardous substitutes for superacids in various applications are active areas of study. Additionally, enhancing safety protocols and containment technologies is essential to minimize the risk of environmental contamination during production, transport, and use of these powerful acids.
When released into the environment, superacids can cause severe damage to soil and water systems. They rapidly lower the pH of their surroundings, leading to acidification of soil and water bodies. This acidification can have far-reaching consequences on flora and fauna, disrupting delicate ecological balances. Aquatic life is especially vulnerable, as even small changes in water pH can be lethal to many species.
The production and handling of superacids also contribute to air pollution. Volatile compounds released during manufacturing or accidental spills can lead to the formation of acid rain, further exacerbating environmental degradation. The potential for these substances to react with atmospheric moisture and create harmful aerosols is a significant concern for air quality and public health.
Waste management of superacids presents another environmental challenge. Improper disposal can lead to long-term contamination of groundwater and soil. The extreme reactivity of these substances makes them difficult to neutralize safely, requiring specialized treatment facilities and protocols. This complexity in waste handling increases the risk of environmental exposure through accidents or mismanagement.
The manufacturing processes for superacids, particularly fluoroantimonic acid, often involve the use of other hazardous materials and energy-intensive procedures. This indirect environmental impact, including greenhouse gas emissions and resource depletion, must be considered in the overall environmental assessment of these substances.
Given these environmental concerns, research into safer alternatives and improved containment methods is crucial. Developing more environmentally friendly synthesis routes and exploring less hazardous substitutes for superacids in various applications are active areas of study. Additionally, enhancing safety protocols and containment technologies is essential to minimize the risk of environmental contamination during production, transport, and use of these powerful acids.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!