Fluoroantimonic Acid: Understanding Its Unique Reactivity
JUN 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Background and Objectives
Fluoroantimonic acid, often referred to as the world's strongest superacid, has been a subject of intense scientific interest since its discovery in the mid-20th century. This compound, formed by mixing hydrogen fluoride (HF) and antimony pentafluoride (SbF5), exhibits unprecedented levels of acidity and reactivity, far surpassing conventional strong acids like sulfuric acid.
The development of fluoroantimonic acid can be traced back to the broader field of superacid chemistry, which gained momentum in the 1960s and 1970s. Pioneering work by chemists such as George A. Olah significantly contributed to our understanding of these extremely acidic substances and their potential applications.
As research in this area progressed, scientists began to explore the unique properties of fluoroantimonic acid, particularly its ability to protonate even extremely weak bases and its capacity to stabilize highly reactive carbocations. These characteristics opened up new possibilities in organic synthesis and catalysis, prompting further investigation into its fundamental chemistry and potential industrial applications.
The evolution of fluoroantimonic acid research has been closely tied to advancements in analytical techniques and safety protocols. Due to its extreme reactivity and corrosive nature, handling and studying this superacid has required the development of specialized equipment and methodologies, driving innovation in materials science and laboratory safety practices.
In recent years, the focus of fluoroantimonic acid research has shifted towards understanding its molecular structure, reaction mechanisms, and potential applications in various fields. Scientists are particularly interested in harnessing its unique reactivity for challenging chemical transformations, such as the activation of typically inert compounds like alkanes.
The primary objectives of current fluoroantimonic acid research include elucidating its precise structure in solution, exploring its potential as a catalyst in industrial processes, and investigating its role in generating novel reactive intermediates. Additionally, researchers aim to develop safer handling methods and to explore potential applications in materials science, such as the creation of super-strong materials or advanced etching processes for semiconductors.
As we continue to push the boundaries of chemical reactivity, fluoroantimonic acid stands as a testament to the ongoing quest for understanding extreme chemical environments. Its study not only advances our fundamental knowledge of acid-base chemistry but also holds promise for revolutionary applications across multiple scientific disciplines.
The development of fluoroantimonic acid can be traced back to the broader field of superacid chemistry, which gained momentum in the 1960s and 1970s. Pioneering work by chemists such as George A. Olah significantly contributed to our understanding of these extremely acidic substances and their potential applications.
As research in this area progressed, scientists began to explore the unique properties of fluoroantimonic acid, particularly its ability to protonate even extremely weak bases and its capacity to stabilize highly reactive carbocations. These characteristics opened up new possibilities in organic synthesis and catalysis, prompting further investigation into its fundamental chemistry and potential industrial applications.
The evolution of fluoroantimonic acid research has been closely tied to advancements in analytical techniques and safety protocols. Due to its extreme reactivity and corrosive nature, handling and studying this superacid has required the development of specialized equipment and methodologies, driving innovation in materials science and laboratory safety practices.
In recent years, the focus of fluoroantimonic acid research has shifted towards understanding its molecular structure, reaction mechanisms, and potential applications in various fields. Scientists are particularly interested in harnessing its unique reactivity for challenging chemical transformations, such as the activation of typically inert compounds like alkanes.
The primary objectives of current fluoroantimonic acid research include elucidating its precise structure in solution, exploring its potential as a catalyst in industrial processes, and investigating its role in generating novel reactive intermediates. Additionally, researchers aim to develop safer handling methods and to explore potential applications in materials science, such as the creation of super-strong materials or advanced etching processes for semiconductors.
As we continue to push the boundaries of chemical reactivity, fluoroantimonic acid stands as a testament to the ongoing quest for understanding extreme chemical environments. Its study not only advances our fundamental knowledge of acid-base chemistry but also holds promise for revolutionary applications across multiple scientific disciplines.
Industrial Applications and Market Demand
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in various industrial sectors due to its unique reactivity. The market demand for this powerful compound is primarily driven by its applications in petrochemical processing, particularly in the isomerization of hydrocarbons and alkylation reactions. These processes are crucial for producing high-octane gasoline and other valuable petroleum products, making fluoroantimonic acid an essential component in the oil refining industry.
The electronics industry also represents a growing market for fluoroantimonic acid. Its exceptional ability to etch silicon and other semiconductors makes it valuable in the production of microchips and integrated circuits. As the demand for smaller, more powerful electronic devices continues to rise, the need for advanced etching agents like fluoroantimonic acid is expected to increase correspondingly.
In the field of materials science, fluoroantimonic acid plays a role in the synthesis of novel compounds and materials. Its extreme acidity allows for reactions that are not possible with conventional acids, opening up new avenues for material development. This application has potential implications for industries ranging from aerospace to energy storage, where new materials with enhanced properties are constantly sought after.
The pharmaceutical industry has shown interest in fluoroantimonic acid for its potential in organic synthesis. While its use is limited due to its extreme reactivity, it has been explored for specific reactions in the production of certain drug precursors. This niche application could expand as new synthetic routes are discovered and refined.
Despite its industrial potential, the market for fluoroantimonic acid remains relatively specialized due to its hazardous nature and the stringent safety requirements associated with its handling and use. This has led to a focus on developing safer alternatives or containment methods that could potentially broaden its application scope and increase market demand.
The global market for superacids, including fluoroantimonic acid, is projected to grow steadily in the coming years. This growth is attributed to increasing research and development activities in both academic and industrial settings, as well as the ongoing expansion of industries that rely on advanced chemical processes. However, the exact market size remains difficult to quantify due to the specialized nature of the product and the confidentiality surrounding its industrial applications.
The electronics industry also represents a growing market for fluoroantimonic acid. Its exceptional ability to etch silicon and other semiconductors makes it valuable in the production of microchips and integrated circuits. As the demand for smaller, more powerful electronic devices continues to rise, the need for advanced etching agents like fluoroantimonic acid is expected to increase correspondingly.
In the field of materials science, fluoroantimonic acid plays a role in the synthesis of novel compounds and materials. Its extreme acidity allows for reactions that are not possible with conventional acids, opening up new avenues for material development. This application has potential implications for industries ranging from aerospace to energy storage, where new materials with enhanced properties are constantly sought after.
The pharmaceutical industry has shown interest in fluoroantimonic acid for its potential in organic synthesis. While its use is limited due to its extreme reactivity, it has been explored for specific reactions in the production of certain drug precursors. This niche application could expand as new synthetic routes are discovered and refined.
Despite its industrial potential, the market for fluoroantimonic acid remains relatively specialized due to its hazardous nature and the stringent safety requirements associated with its handling and use. This has led to a focus on developing safer alternatives or containment methods that could potentially broaden its application scope and increase market demand.
The global market for superacids, including fluoroantimonic acid, is projected to grow steadily in the coming years. This growth is attributed to increasing research and development activities in both academic and industrial settings, as well as the ongoing expansion of industries that rely on advanced chemical processes. However, the exact market size remains difficult to quantify due to the specialized nature of the product and the confidentiality surrounding its industrial applications.
Current State and Challenges in Synthesis
The synthesis of fluoroantimonic acid presents significant challenges due to its extreme reactivity and corrosive nature. Currently, the most common method involves the reaction of hydrogen fluoride (HF) with antimony pentafluoride (SbF5) in a 2:1 molar ratio. This process requires specialized equipment and stringent safety measures, as both reactants are highly dangerous.
One of the primary challenges in the synthesis of fluoroantimonic acid is the handling and containment of the reactants and the product. Traditional glassware and most metals are unsuitable due to the acid's extreme corrosiveness. Researchers typically use fluoropolymer containers, such as those made from polytetrafluoroethylene (PTFE), to handle and store the acid. However, even these materials can degrade over time, necessitating frequent replacement and careful monitoring.
The synthesis process itself must be conducted under anhydrous conditions, as fluoroantimonic acid reacts violently with water. This requirement adds complexity to the production process, demanding the use of dry boxes or inert atmosphere techniques. The hygroscopic nature of the acid also complicates its storage and handling, requiring specialized moisture-free environments.
Another significant challenge lies in the purification of fluoroantimonic acid. Traditional distillation methods are not applicable due to the acid's extreme reactivity and low boiling point. Researchers often rely on in situ generation and immediate use of the acid to circumvent purification issues. This approach, however, limits the scalability of production and the acid's availability for extended studies.
The extreme acidity of fluoroantimonic acid also poses analytical challenges. Standard pH measurements are ineffective, and specialized acidity functions, such as the Hammett acidity function, must be employed to characterize the acid's strength. This complicates the assessment of reaction progress and product purity during synthesis.
Safety considerations remain paramount in the synthesis of fluoroantimonic acid. The potential for hydrofluoric acid formation upon exposure to moisture presents a severe health hazard. Specialized personal protective equipment and rigorous safety protocols are essential, limiting the number of facilities capable of safely producing and handling this superacid.
Despite these challenges, ongoing research continues to explore improved synthesis methods and handling techniques for fluoroantimonic acid. Recent advancements in materials science may offer new containment solutions, while developments in flow chemistry could potentially provide safer, more controlled synthesis routes. However, the fundamental reactivity of the acid ensures that its production and use will remain a specialized and demanding field of chemistry for the foreseeable future.
One of the primary challenges in the synthesis of fluoroantimonic acid is the handling and containment of the reactants and the product. Traditional glassware and most metals are unsuitable due to the acid's extreme corrosiveness. Researchers typically use fluoropolymer containers, such as those made from polytetrafluoroethylene (PTFE), to handle and store the acid. However, even these materials can degrade over time, necessitating frequent replacement and careful monitoring.
The synthesis process itself must be conducted under anhydrous conditions, as fluoroantimonic acid reacts violently with water. This requirement adds complexity to the production process, demanding the use of dry boxes or inert atmosphere techniques. The hygroscopic nature of the acid also complicates its storage and handling, requiring specialized moisture-free environments.
Another significant challenge lies in the purification of fluoroantimonic acid. Traditional distillation methods are not applicable due to the acid's extreme reactivity and low boiling point. Researchers often rely on in situ generation and immediate use of the acid to circumvent purification issues. This approach, however, limits the scalability of production and the acid's availability for extended studies.
The extreme acidity of fluoroantimonic acid also poses analytical challenges. Standard pH measurements are ineffective, and specialized acidity functions, such as the Hammett acidity function, must be employed to characterize the acid's strength. This complicates the assessment of reaction progress and product purity during synthesis.
Safety considerations remain paramount in the synthesis of fluoroantimonic acid. The potential for hydrofluoric acid formation upon exposure to moisture presents a severe health hazard. Specialized personal protective equipment and rigorous safety protocols are essential, limiting the number of facilities capable of safely producing and handling this superacid.
Despite these challenges, ongoing research continues to explore improved synthesis methods and handling techniques for fluoroantimonic acid. Recent advancements in materials science may offer new containment solutions, while developments in flow chemistry could potentially provide safer, more controlled synthesis routes. However, the fundamental reactivity of the acid ensures that its production and use will remain a specialized and demanding field of chemistry for the foreseeable future.
Existing Synthesis and Handling Methods
01 Extreme reactivity and superacidity
Fluoroantimonic acid is known for its extreme reactivity and superacidity. It is one of the strongest known superacids, capable of protonating even very weak bases. This property makes it useful in various chemical reactions and processes, particularly in organic synthesis and catalysis.- Extreme reactivity and superacidity: Fluoroantimonic acid is known for its extreme reactivity and superacidity. It is one of the strongest known superacids, capable of protonating even very weak bases. This property makes it useful in various chemical reactions and processes, particularly in organic synthesis and catalysis.
- Applications in chemical synthesis: The high reactivity of fluoroantimonic acid makes it valuable in chemical synthesis. It can be used as a catalyst or reagent in various organic reactions, including alkylations, acylations, and isomerizations. Its ability to protonate weak bases allows for the formation of reactive intermediates in synthetic pathways.
- Handling and safety considerations: Due to its extreme reactivity, fluoroantimonic acid requires special handling and safety precautions. It reacts violently with water and many organic compounds, necessitating the use of specialized equipment and inert atmospheres. Proper storage, containment, and disposal methods are crucial to prevent accidents and environmental contamination.
- Industrial applications: Fluoroantimonic acid finds use in various industrial applications, including the production of certain polymers, the processing of petroleum products, and in some electrochemical processes. Its strong acidic properties make it effective in removing certain contaminants or catalyzing specific industrial reactions.
- Analytical and research applications: In analytical chemistry and research, fluoroantimonic acid is used for studying superacidity and as a reference point for acidity scales. It also has applications in spectroscopy and in the development of new materials with unique properties based on its extreme acidity.
02 Applications in chemical synthesis
The high reactivity of fluoroantimonic acid makes it valuable in chemical synthesis. It is used as a catalyst in various organic reactions, including alkylation, acylation, and isomerization. Its ability to protonate weak bases allows for the formation of reactive intermediates that can facilitate challenging transformations.Expand Specific Solutions03 Handling and safety considerations
Due to its extreme reactivity, fluoroantimonic acid requires special handling and safety precautions. It reacts violently with water and many organic compounds, necessitating the use of specialized equipment and inert atmospheres. Proper storage, containment, and disposal methods are crucial to prevent accidents and environmental contamination.Expand Specific Solutions04 Analytical and characterization techniques
Specialized analytical and characterization techniques are required to study fluoroantimonic acid and its reactions. These may include NMR spectroscopy, mass spectrometry, and computational methods. Understanding the behavior and properties of this superacid is crucial for optimizing its use in various applications.Expand Specific Solutions05 Industrial applications and process improvements
Fluoroantimonic acid finds applications in various industrial processes, including petroleum refining, polymer production, and materials science. Its unique properties can lead to process improvements, such as increased reaction rates, higher yields, or the ability to carry out reactions under milder conditions.Expand Specific Solutions
Key Players in Fluoroantimonic Acid Research
The field of Fluoroantimonic Acid research is in a nascent stage, with significant potential for growth. The market size remains relatively small due to the compound's highly specialized applications. Technologically, it's still in the early development phase, with academic institutions like the University of Manitoba and the University of North Carolina at Chapel Hill leading fundamental research. Industry players such as DuPont de Nemours, Inc. and DAIKIN INDUSTRIES Ltd. are exploring practical applications. The competitive landscape is characterized by a mix of academic and industrial efforts, with companies like Solvay China Co. Ltd. and Central Glass Co., Ltd. also contributing to advancements. The extreme reactivity of Fluoroantimonic Acid presents both challenges and opportunities, driving ongoing research and development efforts across various sectors.
University of Manitoba
Technical Solution: The University of Manitoba has conducted extensive research on fluoroantimonic acid, focusing on its unique reactivity and potential applications. Their approach involves studying the acid's behavior in various organic reactions, particularly in the protonation of weak bases and the activation of C-H bonds. The university's research team has developed specialized containment and handling protocols to work safely with this highly corrosive superacid. They have also explored its potential as a catalyst in certain petrochemical processes, leveraging its extreme acidity to facilitate reactions that are challenging with conventional acid catalysts.
Strengths: Cutting-edge research facilities and expertise in handling superacids. Weaknesses: Limited industrial-scale application experience and potential safety concerns in large-scale use.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed proprietary technology for the synthesis and application of fluoroantimonic acid in industrial processes. Their approach focuses on harnessing the superacid's extreme reactivity for specialized chemical transformations. DuPont's research has led to the development of novel fluoropolymer materials that can withstand the corrosive nature of fluoroantimonic acid, enabling its use in contained systems for industrial applications. They have also explored its potential in the production of high-performance lubricants and as a catalyst in the petrochemical industry, particularly in isomerization and alkylation reactions.
Strengths: Strong industrial application focus and expertise in fluorine chemistry. Weaknesses: High costs associated with specialized containment materials and safety measures.
Core Innovations in Superacid Chemistry
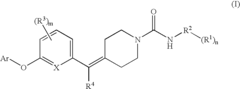
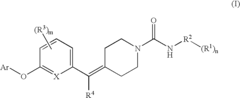
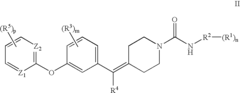
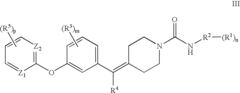
Biaryl Ether Urea Compounds
PatentActiveUS20080261941A1
Innovation
- Development of biaryl ether urea compounds and their pharmaceutically acceptable salts, which act as FAAH inhibitors, providing a therapeutic approach for treating a wide range of disorders including pain, depression, and anxiety by elevating brain anandamide levels.
Patent
Innovation
- Novel synthesis method for producing highly pure fluoroantimonic acid with improved stability.
- Development of specialized containment materials resistant to fluoroantimonic acid's extreme corrosiveness.
- Unique application of fluoroantimonic acid as a superacid catalyst in specific organic reactions.
Safety and Environmental Considerations
Fluoroantimonic acid, known as the world's strongest superacid, poses significant safety and environmental risks that demand careful consideration and stringent handling protocols. The extreme corrosiveness and reactivity of this compound necessitate specialized containment measures to prevent accidental release or exposure.
In laboratory settings, fluoroantimonic acid must be handled exclusively in highly resistant fluoropolymer containers, as it rapidly corrodes glass, metal, and most other materials. Personal protective equipment (PPE) for researchers working with this acid includes fully encapsulating chemical suits, multiple layers of chemically resistant gloves, and self-contained breathing apparatus. Even minor exposure can result in severe chemical burns and potentially life-threatening injuries.
The environmental impact of fluoroantimonic acid is a critical concern. Any release into the environment could have catastrophic consequences due to its ability to react violently with water and organic matter. This reactivity could lead to rapid acidification of soil and water bodies, causing extensive damage to ecosystems and potentially long-lasting environmental degradation.
Proper disposal of fluoroantimonic acid and related waste products is crucial. Neutralization procedures must be conducted with extreme caution, typically involving controlled reactions with bases in specialized facilities. The resulting neutralized compounds must then be treated as hazardous waste and disposed of according to strict regulatory guidelines.
Emergency response planning for facilities handling fluoroantimonic acid is essential. This includes developing detailed spill containment and cleanup procedures, as well as training personnel in rapid response techniques. Specialized neutralizing agents and absorbents must be readily available, and evacuation protocols should be established for surrounding areas in case of a major release.
The transportation of fluoroantimonic acid is heavily regulated due to its hazardous nature. It is classified as a dangerous good and subject to strict packaging, labeling, and documentation requirements. Only specially trained and certified personnel are permitted to handle its transport, with routes carefully planned to minimize risks to populated areas and sensitive environments.
Given these significant safety and environmental concerns, research into safer alternatives or methods to mitigate the risks associated with fluoroantimonic acid is ongoing. This includes exploring less hazardous superacids for similar applications and developing more robust containment technologies. As understanding of its unique reactivity advances, so too must the strategies for ensuring its safe use and minimizing its potential environmental impact.
In laboratory settings, fluoroantimonic acid must be handled exclusively in highly resistant fluoropolymer containers, as it rapidly corrodes glass, metal, and most other materials. Personal protective equipment (PPE) for researchers working with this acid includes fully encapsulating chemical suits, multiple layers of chemically resistant gloves, and self-contained breathing apparatus. Even minor exposure can result in severe chemical burns and potentially life-threatening injuries.
The environmental impact of fluoroantimonic acid is a critical concern. Any release into the environment could have catastrophic consequences due to its ability to react violently with water and organic matter. This reactivity could lead to rapid acidification of soil and water bodies, causing extensive damage to ecosystems and potentially long-lasting environmental degradation.
Proper disposal of fluoroantimonic acid and related waste products is crucial. Neutralization procedures must be conducted with extreme caution, typically involving controlled reactions with bases in specialized facilities. The resulting neutralized compounds must then be treated as hazardous waste and disposed of according to strict regulatory guidelines.
Emergency response planning for facilities handling fluoroantimonic acid is essential. This includes developing detailed spill containment and cleanup procedures, as well as training personnel in rapid response techniques. Specialized neutralizing agents and absorbents must be readily available, and evacuation protocols should be established for surrounding areas in case of a major release.
The transportation of fluoroantimonic acid is heavily regulated due to its hazardous nature. It is classified as a dangerous good and subject to strict packaging, labeling, and documentation requirements. Only specially trained and certified personnel are permitted to handle its transport, with routes carefully planned to minimize risks to populated areas and sensitive environments.
Given these significant safety and environmental concerns, research into safer alternatives or methods to mitigate the risks associated with fluoroantimonic acid is ongoing. This includes exploring less hazardous superacids for similar applications and developing more robust containment technologies. As understanding of its unique reactivity advances, so too must the strategies for ensuring its safe use and minimizing its potential environmental impact.
Regulatory Framework for Superacid Usage
The regulatory framework for superacid usage, particularly concerning fluoroantimonic acid, is a complex and evolving landscape. Given the extreme reactivity and potential hazards associated with this superacid, stringent regulations are in place to govern its production, handling, storage, and disposal. At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a foundation for standardizing safety information and handling procedures for highly corrosive substances like fluoroantimonic acid.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific guidelines for working with highly corrosive materials. These regulations mandate the use of appropriate personal protective equipment (PPE), proper ventilation systems, and strict protocols for handling and storage. The Environmental Protection Agency (EPA) also plays a crucial role in regulating the environmental impact of superacids, particularly under the Toxic Substances Control Act (TSCA) and the Resource Conservation and Recovery Act (RCRA).
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation imposes additional requirements on the registration and assessment of highly reactive substances. Under REACH, manufacturers and importers must provide detailed safety information and risk assessments for fluoroantimonic acid and other superacids. The Classification, Labelling, and Packaging (CLP) Regulation further ensures that the hazards of such chemicals are clearly communicated to workers and consumers.
In Asia, countries like Japan and South Korea have implemented their own versions of chemical control laws, which include specific provisions for highly corrosive and reactive substances. These regulations often align with international standards but may include additional local requirements for registration, labeling, and handling.
The transportation of fluoroantimonic acid is subject to strict international regulations, including the International Air Transport Association (IATA) Dangerous Goods Regulations and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify packaging requirements, labeling standards, and documentation procedures for the safe transport of superacids.
Research institutions and industrial facilities working with fluoroantimonic acid must adhere to these regulatory frameworks and often implement additional safety measures. This includes specialized training programs for personnel, regular safety audits, and the development of emergency response plans. The unique reactivity of fluoroantimonic acid necessitates constant vigilance and ongoing review of safety protocols to ensure compliance with evolving regulations and best practices in chemical safety management.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific guidelines for working with highly corrosive materials. These regulations mandate the use of appropriate personal protective equipment (PPE), proper ventilation systems, and strict protocols for handling and storage. The Environmental Protection Agency (EPA) also plays a crucial role in regulating the environmental impact of superacids, particularly under the Toxic Substances Control Act (TSCA) and the Resource Conservation and Recovery Act (RCRA).
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation imposes additional requirements on the registration and assessment of highly reactive substances. Under REACH, manufacturers and importers must provide detailed safety information and risk assessments for fluoroantimonic acid and other superacids. The Classification, Labelling, and Packaging (CLP) Regulation further ensures that the hazards of such chemicals are clearly communicated to workers and consumers.
In Asia, countries like Japan and South Korea have implemented their own versions of chemical control laws, which include specific provisions for highly corrosive and reactive substances. These regulations often align with international standards but may include additional local requirements for registration, labeling, and handling.
The transportation of fluoroantimonic acid is subject to strict international regulations, including the International Air Transport Association (IATA) Dangerous Goods Regulations and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify packaging requirements, labeling standards, and documentation procedures for the safe transport of superacids.
Research institutions and industrial facilities working with fluoroantimonic acid must adhere to these regulatory frameworks and often implement additional safety measures. This includes specialized training programs for personnel, regular safety audits, and the development of emergency response plans. The unique reactivity of fluoroantimonic acid necessitates constant vigilance and ongoing review of safety protocols to ensure compliance with evolving regulations and best practices in chemical safety management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!