High-Throughput Experimentation and Global Pharmaceutical Patents
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HTE Technology Evolution and Objectives
High-throughput experimentation (HTE) has evolved significantly since its inception in the 1990s, transforming from simple parallel synthesis techniques to sophisticated automated platforms capable of conducting thousands of experiments simultaneously. The initial development focused primarily on combinatorial chemistry for drug discovery, allowing pharmaceutical researchers to rapidly synthesize and test large libraries of compounds.
By the early 2000s, HTE technologies expanded beyond synthesis to include screening and characterization capabilities, incorporating advanced robotics, microfluidics, and miniaturization techniques. This evolution enabled significant reductions in reagent consumption and experimental timeframes while dramatically increasing data output.
The integration of artificial intelligence and machine learning represents a pivotal advancement in HTE development during the 2010s. These computational tools enhanced experimental design, data analysis, and predictive modeling, creating a synergistic relationship between experimental throughput and computational power that continues to drive innovation in pharmaceutical research.
Current HTE platforms incorporate sophisticated laboratory automation systems, including robotic liquid handling, automated sample preparation, and integrated analytical instrumentation. These systems operate with minimal human intervention, maintaining precise control over experimental conditions while generating standardized, high-quality data suitable for computational analysis.
The primary objective of modern HTE technology is to accelerate the drug discovery and development process by enabling rapid exploration of chemical space, optimization of lead compounds, and identification of structure-activity relationships. This acceleration directly addresses the pharmaceutical industry's need to reduce time-to-market and development costs while improving success rates.
Additional objectives include enhancing reproducibility through standardized protocols and automated documentation, reducing material consumption through miniaturization, and generating comprehensive datasets that support more robust decision-making in pharmaceutical development pipelines.
Looking forward, HTE technology aims to achieve seamless integration with computational methods, creating closed-loop systems where AI algorithms can autonomously design, execute, and interpret experiments. This self-driving laboratory concept represents the frontier of pharmaceutical research, potentially revolutionizing how intellectual property is generated and protected in the global pharmaceutical landscape.
By the early 2000s, HTE technologies expanded beyond synthesis to include screening and characterization capabilities, incorporating advanced robotics, microfluidics, and miniaturization techniques. This evolution enabled significant reductions in reagent consumption and experimental timeframes while dramatically increasing data output.
The integration of artificial intelligence and machine learning represents a pivotal advancement in HTE development during the 2010s. These computational tools enhanced experimental design, data analysis, and predictive modeling, creating a synergistic relationship between experimental throughput and computational power that continues to drive innovation in pharmaceutical research.
Current HTE platforms incorporate sophisticated laboratory automation systems, including robotic liquid handling, automated sample preparation, and integrated analytical instrumentation. These systems operate with minimal human intervention, maintaining precise control over experimental conditions while generating standardized, high-quality data suitable for computational analysis.
The primary objective of modern HTE technology is to accelerate the drug discovery and development process by enabling rapid exploration of chemical space, optimization of lead compounds, and identification of structure-activity relationships. This acceleration directly addresses the pharmaceutical industry's need to reduce time-to-market and development costs while improving success rates.
Additional objectives include enhancing reproducibility through standardized protocols and automated documentation, reducing material consumption through miniaturization, and generating comprehensive datasets that support more robust decision-making in pharmaceutical development pipelines.
Looking forward, HTE technology aims to achieve seamless integration with computational methods, creating closed-loop systems where AI algorithms can autonomously design, execute, and interpret experiments. This self-driving laboratory concept represents the frontier of pharmaceutical research, potentially revolutionizing how intellectual property is generated and protected in the global pharmaceutical landscape.
Pharmaceutical Market Demand Analysis
The global pharmaceutical market demonstrates a robust and growing demand for high-throughput experimentation (HTE) technologies, driven by several key factors. The pharmaceutical industry faces increasing pressure to accelerate drug discovery processes while simultaneously reducing costs. Traditional drug development pathways typically require 10-15 years and investments exceeding $2.6 billion per successful compound, creating substantial economic incentives for technologies that can streamline this process.
Market analysis reveals that pharmaceutical companies are increasingly adopting HTE methodologies to address the productivity challenges in R&D. The global pharmaceutical R&D spending reached approximately $200 billion in 2022, with a significant portion allocated to improving discovery efficiency. Companies implementing HTE technologies report up to 100-fold increases in compound screening capacity and 30-50% reductions in early-stage development timelines, translating to substantial competitive advantages.
The aging global population and rising prevalence of chronic diseases have intensified market demand for novel therapeutics. With over 10,000 diseases known to affect humans but effective treatments available for only about 500, there exists an enormous unmet medical need. This gap represents a significant market opportunity for pharmaceutical companies that can efficiently identify and develop new therapeutic compounds through HTE technologies.
Patent analysis indicates growing market recognition of HTE's value, with annual patent filings related to pharmaceutical HTE technologies increasing at a compound annual growth rate of 15% over the past decade. Geographic distribution shows concentration in North America, Europe, and increasingly in Asia, particularly China and Japan, reflecting global market demand.
Regulatory pressures also drive market demand for HTE technologies. Stringent approval requirements from agencies like the FDA and EMA necessitate more comprehensive data packages, which HTE can help generate more efficiently. Additionally, the push for personalized medicine requires testing compounds against diverse genetic backgrounds, creating demand for technologies that can perform parallel experiments across multiple conditions.
The contract research organization (CRO) segment represents a rapidly expanding market for HTE technologies, growing at approximately 8% annually. These organizations leverage HTE platforms to offer cost-effective drug discovery services to pharmaceutical companies of all sizes, democratizing access to advanced screening capabilities and expanding the overall market for these technologies.
Market analysis reveals that pharmaceutical companies are increasingly adopting HTE methodologies to address the productivity challenges in R&D. The global pharmaceutical R&D spending reached approximately $200 billion in 2022, with a significant portion allocated to improving discovery efficiency. Companies implementing HTE technologies report up to 100-fold increases in compound screening capacity and 30-50% reductions in early-stage development timelines, translating to substantial competitive advantages.
The aging global population and rising prevalence of chronic diseases have intensified market demand for novel therapeutics. With over 10,000 diseases known to affect humans but effective treatments available for only about 500, there exists an enormous unmet medical need. This gap represents a significant market opportunity for pharmaceutical companies that can efficiently identify and develop new therapeutic compounds through HTE technologies.
Patent analysis indicates growing market recognition of HTE's value, with annual patent filings related to pharmaceutical HTE technologies increasing at a compound annual growth rate of 15% over the past decade. Geographic distribution shows concentration in North America, Europe, and increasingly in Asia, particularly China and Japan, reflecting global market demand.
Regulatory pressures also drive market demand for HTE technologies. Stringent approval requirements from agencies like the FDA and EMA necessitate more comprehensive data packages, which HTE can help generate more efficiently. Additionally, the push for personalized medicine requires testing compounds against diverse genetic backgrounds, creating demand for technologies that can perform parallel experiments across multiple conditions.
The contract research organization (CRO) segment represents a rapidly expanding market for HTE technologies, growing at approximately 8% annually. These organizations leverage HTE platforms to offer cost-effective drug discovery services to pharmaceutical companies of all sizes, democratizing access to advanced screening capabilities and expanding the overall market for these technologies.
Global HTE Development Status and Barriers
High-throughput experimentation (HTE) has evolved significantly across global pharmaceutical research landscapes, with varying levels of adoption and implementation. In North America and Western Europe, HTE platforms are well-established in major pharmaceutical companies, with advanced integration into drug discovery workflows. These regions benefit from mature ecosystems of technology providers, academic partnerships, and specialized expertise.
Asia-Pacific represents the fastest-growing region for HTE adoption, particularly in China, Japan, and South Korea. Chinese pharmaceutical companies have made substantial investments in HTE infrastructure over the past decade, rapidly closing the technological gap with Western counterparts. However, implementation quality and standardization remain inconsistent across different organizations.
Despite global progress, several significant barriers impede broader HTE implementation. Cost remains a primary obstacle, with comprehensive HTE platforms requiring investments of $2-10 million for hardware alone, plus ongoing operational expenses. This creates a substantial entry barrier for smaller pharmaceutical companies and research institutions, particularly in emerging markets.
Technical complexity presents another major challenge. HTE systems demand specialized expertise spanning robotics, data science, and pharmaceutical chemistry. The shortage of qualified personnel with cross-disciplinary skills has created a competitive talent market, with companies struggling to build and maintain expert teams capable of maximizing HTE potential.
Data management infrastructure represents a critical bottleneck in many organizations. HTE generates massive datasets requiring sophisticated storage, processing, and analysis capabilities. Many pharmaceutical companies lack the computational infrastructure and data science expertise to effectively leverage the volume of experimental data produced, resulting in underutilization of valuable information.
Regulatory uncertainties also complicate HTE implementation globally. Different jurisdictions maintain varying requirements for experimental validation, documentation standards, and data integrity. This regulatory fragmentation creates compliance challenges for multinational pharmaceutical companies seeking to implement consistent HTE practices across global research networks.
Intellectual property protection presents additional complications. The patentability of HTE-derived innovations varies significantly across jurisdictions, with some countries offering stronger protection for computational models and algorithm-assisted discoveries than others. This inconsistency creates strategic challenges for companies determining where to conduct HTE research and how to protect resulting innovations.
Asia-Pacific represents the fastest-growing region for HTE adoption, particularly in China, Japan, and South Korea. Chinese pharmaceutical companies have made substantial investments in HTE infrastructure over the past decade, rapidly closing the technological gap with Western counterparts. However, implementation quality and standardization remain inconsistent across different organizations.
Despite global progress, several significant barriers impede broader HTE implementation. Cost remains a primary obstacle, with comprehensive HTE platforms requiring investments of $2-10 million for hardware alone, plus ongoing operational expenses. This creates a substantial entry barrier for smaller pharmaceutical companies and research institutions, particularly in emerging markets.
Technical complexity presents another major challenge. HTE systems demand specialized expertise spanning robotics, data science, and pharmaceutical chemistry. The shortage of qualified personnel with cross-disciplinary skills has created a competitive talent market, with companies struggling to build and maintain expert teams capable of maximizing HTE potential.
Data management infrastructure represents a critical bottleneck in many organizations. HTE generates massive datasets requiring sophisticated storage, processing, and analysis capabilities. Many pharmaceutical companies lack the computational infrastructure and data science expertise to effectively leverage the volume of experimental data produced, resulting in underutilization of valuable information.
Regulatory uncertainties also complicate HTE implementation globally. Different jurisdictions maintain varying requirements for experimental validation, documentation standards, and data integrity. This regulatory fragmentation creates compliance challenges for multinational pharmaceutical companies seeking to implement consistent HTE practices across global research networks.
Intellectual property protection presents additional complications. The patentability of HTE-derived innovations varies significantly across jurisdictions, with some countries offering stronger protection for computational models and algorithm-assisted discoveries than others. This inconsistency creates strategic challenges for companies determining where to conduct HTE research and how to protect resulting innovations.
Current HTE Methodologies and Implementations
01 Automated laboratory systems for high-throughput experimentation
Automated laboratory systems enable rapid and efficient execution of multiple experiments simultaneously. These systems incorporate robotics, liquid handling devices, and integrated software to streamline experimental workflows. By automating repetitive tasks, researchers can significantly increase experimental throughput while reducing human error and variability. These systems are particularly valuable in drug discovery, materials science, and biochemical research where large numbers of samples need to be processed.- Automated laboratory systems for high-throughput screening: Automated laboratory systems enable rapid and efficient screening of multiple samples simultaneously. These systems incorporate robotics, liquid handling devices, and integrated software to streamline experimental workflows. By automating repetitive tasks, researchers can significantly increase the number of experiments conducted in parallel, accelerating the discovery process while maintaining consistency and reducing human error.
- Data management and analysis platforms for large-scale experiments: Specialized software platforms are essential for managing and analyzing the vast amounts of data generated by high-throughput experiments. These platforms incorporate advanced algorithms for data processing, visualization tools, and statistical analysis capabilities. They enable researchers to identify patterns, correlations, and significant results from complex datasets, facilitating knowledge discovery and decision-making in research and development processes.
- Microfluidic and miniaturized assay technologies: Microfluidic technologies enable the miniaturization of experimental setups, allowing for parallel processing of numerous samples with minimal reagent consumption. These systems utilize microscale channels, chambers, and sensors to perform complex biochemical assays. The reduced volumes not only conserve valuable materials but also enhance reaction kinetics, improve sensitivity, and enable higher experimental throughput compared to conventional methods.
- Parallel synthesis and formulation techniques: Advanced parallel synthesis methods allow for the simultaneous creation of multiple compounds or formulations. These techniques employ arrays of reaction vessels, specialized equipment for dispensing reagents, and automated monitoring systems. By conducting numerous reactions in parallel under controlled conditions, researchers can rapidly explore chemical space, optimize formulations, and accelerate the development of new materials and compounds.
- Machine learning integration for experimental design and optimization: Machine learning algorithms are increasingly integrated into high-throughput experimentation workflows to optimize experimental design and predict outcomes. These computational approaches can analyze historical data, identify promising experimental conditions, and suggest optimal parameters. By incorporating artificial intelligence, researchers can reduce the number of experiments needed, focus on the most promising directions, and accelerate the discovery process through intelligent experimental planning.
02 Data management and analysis platforms for high-throughput experiments
Specialized software platforms are essential for managing and analyzing the large volumes of data generated by high-throughput experimentation. These platforms incorporate advanced algorithms for data processing, visualization, and statistical analysis. They enable researchers to identify patterns, correlations, and insights from complex datasets. Features often include automated quality control, data normalization, and integration with laboratory information management systems to create a seamless workflow from experiment design to results interpretation.Expand Specific Solutions03 Parallel synthesis and screening methodologies
Parallel synthesis and screening methodologies allow for the simultaneous creation and evaluation of multiple compounds or materials. These approaches utilize array-based formats, microfluidic systems, or combinatorial techniques to rapidly generate diverse chemical or biological libraries. Screening these libraries against specific targets or for desired properties enables accelerated discovery of novel compounds with potential applications in pharmaceuticals, catalysis, and materials science. This approach dramatically reduces the time and resources required compared to traditional sequential methods.Expand Specific Solutions04 Miniaturization technologies for high-throughput experimentation
Miniaturization technologies reduce sample volumes and increase experimental density, enabling more experiments to be conducted with less material. These technologies include microfluidic devices, microwell plates, and lab-on-a-chip systems that operate at microscale or nanoscale dimensions. By reducing reaction volumes from milliliters to microliters or nanoliters, researchers can perform thousands of experiments in the space previously required for a single experiment. This approach conserves valuable reagents, reduces waste, and accelerates the experimental cycle.Expand Specific Solutions05 Machine learning integration with high-throughput experimentation
Machine learning algorithms are increasingly integrated with high-throughput experimentation to optimize experimental design and predict outcomes. These computational approaches can identify patterns in experimental data that might be missed by human analysis, suggest promising experimental conditions, and reduce the number of experiments needed to achieve desired results. Active learning frameworks continuously refine experimental parameters based on real-time results, creating a feedback loop that accelerates discovery. This integration is transforming research in materials science, drug discovery, and chemical synthesis.Expand Specific Solutions
Key Pharmaceutical and HTE Industry Players
High-Throughput Experimentation (HTE) in pharmaceutical research is currently in a growth phase, with the global market expanding rapidly due to increasing R&D investments. The competitive landscape features established pharmaceutical companies alongside specialized technology providers, creating a dynamic ecosystem. Key players like Recursion Pharmaceuticals and Nanobiosym are leveraging AI and automation to accelerate drug discovery, while academic institutions (Duke University, Peking University) contribute significant research. Major pharmaceutical entities such as Taisho Pharmaceutical and Bio-Rad Laboratories are investing heavily in HTE technologies. Google's entry signals the growing intersection of data science and pharmaceutical research. The patent landscape is increasingly complex, with companies like DH Technologies and Suven Life Sciences developing proprietary HTE methodologies to secure competitive advantages in this evolving field.
Recursion Pharmaceuticals, Inc.
Technical Solution: Recursion has developed a proprietary AI-enabled high-throughput experimentation platform that combines automated wet lab biology with computational tools to accelerate drug discovery. Their platform can test over 1.5 million experiments weekly, generating petabytes of biological and chemical data. The company employs a unique approach called "Phenomics" that captures cellular changes across thousands of biological features simultaneously. This system integrates robotic automation, high-content microscopy, and deep learning algorithms to identify novel therapeutic candidates from their proprietary dataset containing over 15 petabytes of biological images. Recursion's platform has enabled them to build a pipeline of over 12 programs across diverse therapeutic areas, with several compounds advancing to clinical trials.
Strengths: Unprecedented scale of biological data generation; integration of AI with experimental biology; ability to repurpose existing compounds rapidly. Weaknesses: Heavily dependent on computational infrastructure; potential challenges in translating in vitro findings to clinical success; high operational costs for maintaining robotic systems and data storage.
Dow Global Technologies LLC
Technical Solution: Dow has implemented an advanced high-throughput experimentation (HTE) platform focused on materials science and chemical formulations for pharmaceutical applications. Their system employs parallel synthesis reactors capable of conducting up to 96 reactions simultaneously under precisely controlled conditions. Dow's technology integrates automated sampling, rapid analytical techniques including HPLC-MS and NMR, and proprietary data management software that tracks experimental parameters and results. The company has developed specialized microfluidic devices for rapid screening of formulation properties relevant to drug delivery systems. Their patent portfolio includes innovations in catalyst discovery, polymer excipient development, and controlled-release technologies. Dow's HTE capabilities have been particularly valuable in developing novel pharmaceutical excipients and drug delivery systems with optimized properties.
Strengths: Extensive experience in materials science applicable to drug formulation; robust integration of analytical technologies; strong industrial-scale implementation capabilities. Weaknesses: Less focused on biological aspects of drug discovery; primarily oriented toward formulation rather than novel compound identification; dependent on partnerships for clinical development expertise.
Critical Patents and Innovations in HTE
High throughput research workflow
PatentInactiveUS20110029439A1
Innovation
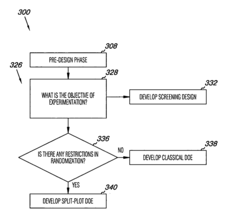
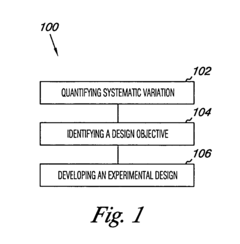
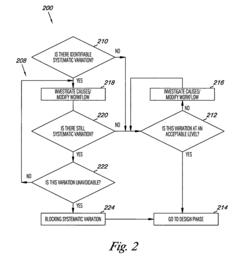
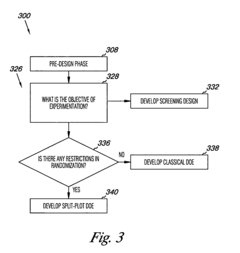
- The method involves quantifying systematic variation through variance component analysis, identifying design objectives, and developing experimental designs such as screening, split-plot, or classical designs to account for systematic variation, and modifying sources of variation to achieve statistically defensible results, using computer-readable mediums and computing devices to implement these steps.
Systems and methods for pairwise inference of drug-gene interaction networks
PatentWO2021050760A1
Innovation
- The use of automated biology and artificial intelligence in cell-based assays to measure high-dimensional sub-cellular structural changes, allowing for the identification of interactions between biological agents like genes, compounds, and toxins through featurized vectors and dimension reduction models, enabling the detection of interactions not mediated by physical interactions.
Regulatory Framework for Pharmaceutical HTE
The regulatory landscape governing High-Throughput Experimentation (HTE) in pharmaceutical development presents a complex framework that varies significantly across global jurisdictions. Regulatory bodies such as the FDA in the United States, the EMA in Europe, and the NMPA in China have established distinct approaches to overseeing HTE methodologies within drug discovery and development processes.
In the United States, the FDA has implemented guidance documents specifically addressing high-throughput screening and parallel experimentation under its Critical Path Initiative. These guidelines emphasize validation protocols for HTE data and establish requirements for documentation when submitting HTE-derived evidence in regulatory filings. The agency recognizes HTE as a valuable tool for accelerating drug development while maintaining stringent quality standards.
European regulatory frameworks, coordinated through the EMA, have integrated HTE considerations within broader pharmaceutical innovation policies. The EU Clinical Trials Regulation (No 536/2014) includes provisions that accommodate data generated through high-throughput methodologies, though with additional verification requirements. European regulations particularly emphasize traceability and reproducibility of HTE-generated data.
Asian markets demonstrate varying degrees of regulatory maturity regarding HTE. Japan's PMDA has established specific guidelines for high-throughput technologies in pharmaceutical research, while China's NMPA has recently updated its regulatory framework to better accommodate innovative research methodologies including HTE approaches.
A critical regulatory consideration across all jurisdictions involves data integrity and quality management systems for HTE. Regulatory bodies universally require pharmaceutical companies to demonstrate robust validation of HTE platforms, with particular emphasis on statistical significance given the large data volumes generated. This includes requirements for appropriate controls, calibration procedures, and system suitability tests.
Intellectual property protection intersects significantly with regulatory compliance in the HTE space. Patent applications involving HTE methodologies must navigate both technical regulatory requirements and IP considerations simultaneously. This creates a complex landscape where companies must balance disclosure requirements for regulatory approval against IP protection strategies.
Recent regulatory trends indicate movement toward greater harmonization of HTE standards internationally through initiatives like the International Council for Harmonisation (ICH). These efforts aim to establish consistent quality standards for HTE data across global markets, potentially streamlining multinational drug development programs that leverage high-throughput approaches.
In the United States, the FDA has implemented guidance documents specifically addressing high-throughput screening and parallel experimentation under its Critical Path Initiative. These guidelines emphasize validation protocols for HTE data and establish requirements for documentation when submitting HTE-derived evidence in regulatory filings. The agency recognizes HTE as a valuable tool for accelerating drug development while maintaining stringent quality standards.
European regulatory frameworks, coordinated through the EMA, have integrated HTE considerations within broader pharmaceutical innovation policies. The EU Clinical Trials Regulation (No 536/2014) includes provisions that accommodate data generated through high-throughput methodologies, though with additional verification requirements. European regulations particularly emphasize traceability and reproducibility of HTE-generated data.
Asian markets demonstrate varying degrees of regulatory maturity regarding HTE. Japan's PMDA has established specific guidelines for high-throughput technologies in pharmaceutical research, while China's NMPA has recently updated its regulatory framework to better accommodate innovative research methodologies including HTE approaches.
A critical regulatory consideration across all jurisdictions involves data integrity and quality management systems for HTE. Regulatory bodies universally require pharmaceutical companies to demonstrate robust validation of HTE platforms, with particular emphasis on statistical significance given the large data volumes generated. This includes requirements for appropriate controls, calibration procedures, and system suitability tests.
Intellectual property protection intersects significantly with regulatory compliance in the HTE space. Patent applications involving HTE methodologies must navigate both technical regulatory requirements and IP considerations simultaneously. This creates a complex landscape where companies must balance disclosure requirements for regulatory approval against IP protection strategies.
Recent regulatory trends indicate movement toward greater harmonization of HTE standards internationally through initiatives like the International Council for Harmonisation (ICH). These efforts aim to establish consistent quality standards for HTE data across global markets, potentially streamlining multinational drug development programs that leverage high-throughput approaches.
IP Strategy and Cross-Border Patent Protection
In the rapidly evolving landscape of pharmaceutical innovation, intellectual property (IP) strategy and cross-border patent protection have become critical components for companies leveraging High-Throughput Experimentation (HTE) technologies. The global nature of pharmaceutical research and development necessitates a comprehensive approach to securing patent rights across multiple jurisdictions.
Pharmaceutical companies employing HTE methodologies must navigate complex international patent frameworks that vary significantly between regions. The Patent Cooperation Treaty (PCT) provides a unified procedure for filing patent applications across its 153 member countries, offering a strategic entry point for global protection of HTE innovations. However, companies must still transition to national phase applications, where substantive examination requirements differ substantially.
Key challenges in cross-border protection of HTE patents include varying standards for patentability, particularly regarding the experimental data requirements. While some jurisdictions like the United States may grant patents based on prophetic examples, others such as China and Europe often demand more robust experimental validation. This creates strategic filing decisions for companies with early-stage HTE data.
Data exclusivity provisions represent another layer of protection that complements patent strategies. The European Union offers up to 11 years of data exclusivity, while the United States provides 5-7 years for small molecules and 12 years for biologics. These provisions can be particularly valuable for protecting HTE-derived pharmaceutical innovations when patent protection might be challenging to secure or maintain.
Strategic considerations for global HTE patent portfolios include timing of disclosures, selective filing in key markets, and tailoring patent claims to meet jurisdiction-specific requirements. Companies often implement tiered approaches, securing broad protection in major pharmaceutical markets while adopting more selective strategies in emerging economies.
Collaborative research arrangements present additional complexity, requiring clear agreements on IP ownership and licensing rights. Cross-border collaborations utilizing HTE technologies must address potential conflicts between national security provisions and export control regulations that may restrict information sharing across borders.
Recent legal developments, including the TRIPS waiver discussions for COVID-19 technologies and China's strengthened pharmaceutical patent enforcement mechanisms, continue to reshape the global landscape for pharmaceutical IP protection. Companies must maintain vigilance regarding these evolving frameworks to optimize protection for their HTE-derived innovations across international boundaries.
Pharmaceutical companies employing HTE methodologies must navigate complex international patent frameworks that vary significantly between regions. The Patent Cooperation Treaty (PCT) provides a unified procedure for filing patent applications across its 153 member countries, offering a strategic entry point for global protection of HTE innovations. However, companies must still transition to national phase applications, where substantive examination requirements differ substantially.
Key challenges in cross-border protection of HTE patents include varying standards for patentability, particularly regarding the experimental data requirements. While some jurisdictions like the United States may grant patents based on prophetic examples, others such as China and Europe often demand more robust experimental validation. This creates strategic filing decisions for companies with early-stage HTE data.
Data exclusivity provisions represent another layer of protection that complements patent strategies. The European Union offers up to 11 years of data exclusivity, while the United States provides 5-7 years for small molecules and 12 years for biologics. These provisions can be particularly valuable for protecting HTE-derived pharmaceutical innovations when patent protection might be challenging to secure or maintain.
Strategic considerations for global HTE patent portfolios include timing of disclosures, selective filing in key markets, and tailoring patent claims to meet jurisdiction-specific requirements. Companies often implement tiered approaches, securing broad protection in major pharmaceutical markets while adopting more selective strategies in emerging economies.
Collaborative research arrangements present additional complexity, requiring clear agreements on IP ownership and licensing rights. Cross-border collaborations utilizing HTE technologies must address potential conflicts between national security provisions and export control regulations that may restrict information sharing across borders.
Recent legal developments, including the TRIPS waiver discussions for COVID-19 technologies and China's strengthened pharmaceutical patent enforcement mechanisms, continue to reshape the global landscape for pharmaceutical IP protection. Companies must maintain vigilance regarding these evolving frameworks to optimize protection for their HTE-derived innovations across international boundaries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!