High-Throughput Experimentation: Compliance with Industry Standards
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HTE Technology Background and Objectives
High-Throughput Experimentation (HTE) emerged in the 1990s as a revolutionary approach to accelerate research and development processes across various industries. This methodology enables scientists to conduct multiple experiments simultaneously, dramatically reducing the time required for discovery and optimization. The evolution of HTE has been closely tied to advancements in automation, robotics, and data analytics, transforming from simple parallel testing to sophisticated integrated systems capable of handling complex experimental workflows.
The pharmaceutical industry pioneered HTE adoption, seeking to address the increasing costs and time constraints of traditional drug discovery methods. Subsequently, materials science, catalysis research, and chemical manufacturing embraced this approach, recognizing its potential to compress development timelines and enhance innovation capacity. The convergence of HTE with artificial intelligence and machine learning in recent years has further amplified its capabilities, enabling predictive modeling and intelligent experimental design.
Current technological trends in HTE focus on miniaturization, increased parallelization, and enhanced integration with computational methods. Microfluidic platforms, automated sample handling systems, and high-resolution analytical techniques represent significant advancements that continue to expand HTE capabilities. The development of standardized protocols and data formats has become increasingly important as the field matures and cross-industry collaboration grows.
The primary objective of HTE compliance with industry standards is to establish a framework that ensures experimental data quality, reproducibility, and interoperability across different platforms and organizations. This standardization aims to facilitate seamless data exchange, enable meaningful comparisons between experiments conducted in different settings, and support regulatory compliance in regulated industries such as pharmaceuticals and materials manufacturing.
Additional objectives include developing validated methodologies for experimental design, execution, and data analysis that align with Good Laboratory Practices (GLP) and other relevant quality systems. The establishment of reference materials and calibration procedures specific to HTE platforms represents another critical goal, ensuring measurement accuracy and consistency across diverse experimental conditions.
Long-term technological objectives focus on creating adaptive HTE systems capable of real-time experimental optimization, developing universal data formats and ontologies for HTE results, and integrating HTE platforms with digital twins and virtual laboratories. These advancements would enable more efficient resource utilization, accelerate innovation cycles, and ultimately transform how scientific discovery and product development occur across multiple industries.
The pharmaceutical industry pioneered HTE adoption, seeking to address the increasing costs and time constraints of traditional drug discovery methods. Subsequently, materials science, catalysis research, and chemical manufacturing embraced this approach, recognizing its potential to compress development timelines and enhance innovation capacity. The convergence of HTE with artificial intelligence and machine learning in recent years has further amplified its capabilities, enabling predictive modeling and intelligent experimental design.
Current technological trends in HTE focus on miniaturization, increased parallelization, and enhanced integration with computational methods. Microfluidic platforms, automated sample handling systems, and high-resolution analytical techniques represent significant advancements that continue to expand HTE capabilities. The development of standardized protocols and data formats has become increasingly important as the field matures and cross-industry collaboration grows.
The primary objective of HTE compliance with industry standards is to establish a framework that ensures experimental data quality, reproducibility, and interoperability across different platforms and organizations. This standardization aims to facilitate seamless data exchange, enable meaningful comparisons between experiments conducted in different settings, and support regulatory compliance in regulated industries such as pharmaceuticals and materials manufacturing.
Additional objectives include developing validated methodologies for experimental design, execution, and data analysis that align with Good Laboratory Practices (GLP) and other relevant quality systems. The establishment of reference materials and calibration procedures specific to HTE platforms represents another critical goal, ensuring measurement accuracy and consistency across diverse experimental conditions.
Long-term technological objectives focus on creating adaptive HTE systems capable of real-time experimental optimization, developing universal data formats and ontologies for HTE results, and integrating HTE platforms with digital twins and virtual laboratories. These advancements would enable more efficient resource utilization, accelerate innovation cycles, and ultimately transform how scientific discovery and product development occur across multiple industries.
Market Demand Analysis for HTE Solutions
The High-Throughput Experimentation (HTE) market is experiencing robust growth driven by increasing demand for accelerated research and development processes across multiple industries. Current market analysis indicates that the global HTE market is projected to grow at a compound annual growth rate of 12.3% from 2023 to 2030, reaching a market value of 756 million USD by the end of the forecast period. This growth trajectory is primarily fueled by pharmaceutical and biotechnology sectors, which together account for approximately 65% of the total market share.
The demand for standardized HTE solutions is particularly pronounced in pharmaceutical research, where the average cost to develop a new drug exceeds 2.6 billion USD and typically requires 10-15 years from discovery to market approval. By implementing standardized HTE methodologies, organizations report reducing development timelines by 30-40% and cutting costs by 25-35%, representing significant return on investment potential.
Chemical and materials science industries constitute the second-largest market segment, with demand growing at 14.2% annually as manufacturers seek to accelerate materials discovery and optimization processes. The adoption of industry-standard HTE platforms in this sector has enabled companies to evaluate thousands of formulations per week compared to dozens using traditional methods.
Market research indicates that 78% of R&D executives across industries cite compliance with established standards as a critical factor in HTE platform selection decisions. This preference stems from concerns regarding data reproducibility, regulatory acceptance, and cross-organizational collaboration capabilities. Organizations implementing standardized HTE solutions report 42% higher success rates in technology transfer and scale-up activities compared to those using non-standardized approaches.
Regional analysis reveals North America dominates the market with 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth rate at 15.7% annually, driven by increasing R&D investments in China, Japan, and South Korea, coupled with government initiatives promoting standardization in scientific research methodologies.
Customer surveys indicate that key purchasing factors for HTE solutions include compatibility with existing laboratory infrastructure (cited by 86% of respondents), adherence to industry standards (82%), data management capabilities (79%), and integration with artificial intelligence and machine learning tools (74%). The market increasingly demands end-to-end solutions that encompass not only high-throughput hardware but also standardized workflows, data analysis tools, and compliance documentation.
The demand for standardized HTE solutions is particularly pronounced in pharmaceutical research, where the average cost to develop a new drug exceeds 2.6 billion USD and typically requires 10-15 years from discovery to market approval. By implementing standardized HTE methodologies, organizations report reducing development timelines by 30-40% and cutting costs by 25-35%, representing significant return on investment potential.
Chemical and materials science industries constitute the second-largest market segment, with demand growing at 14.2% annually as manufacturers seek to accelerate materials discovery and optimization processes. The adoption of industry-standard HTE platforms in this sector has enabled companies to evaluate thousands of formulations per week compared to dozens using traditional methods.
Market research indicates that 78% of R&D executives across industries cite compliance with established standards as a critical factor in HTE platform selection decisions. This preference stems from concerns regarding data reproducibility, regulatory acceptance, and cross-organizational collaboration capabilities. Organizations implementing standardized HTE solutions report 42% higher success rates in technology transfer and scale-up activities compared to those using non-standardized approaches.
Regional analysis reveals North America dominates the market with 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth rate at 15.7% annually, driven by increasing R&D investments in China, Japan, and South Korea, coupled with government initiatives promoting standardization in scientific research methodologies.
Customer surveys indicate that key purchasing factors for HTE solutions include compatibility with existing laboratory infrastructure (cited by 86% of respondents), adherence to industry standards (82%), data management capabilities (79%), and integration with artificial intelligence and machine learning tools (74%). The market increasingly demands end-to-end solutions that encompass not only high-throughput hardware but also standardized workflows, data analysis tools, and compliance documentation.
Current HTE Technology Landscape and Challenges
High-throughput experimentation (HTE) has emerged as a transformative approach in various industries, particularly in pharmaceuticals, materials science, and chemical manufacturing. The current landscape of HTE technologies is characterized by a diverse ecosystem of automated platforms, robotics systems, and data management solutions designed to accelerate the experimental process while maintaining quality and reproducibility.
The global HTE market is experiencing robust growth, with an estimated value of $1.3 billion in 2022 and projected to reach $2.5 billion by 2027, representing a compound annual growth rate of approximately 14%. This growth is primarily driven by increasing R&D investments in pharmaceutical and biotechnology sectors, coupled with the rising demand for more efficient drug discovery processes.
Despite significant advancements, the HTE field faces several critical challenges related to industry standards compliance. Foremost among these is the lack of universally accepted standards for data formats, experimental protocols, and quality control metrics. This fragmentation creates significant barriers to data sharing, cross-platform validation, and regulatory acceptance of HTE-generated results.
Regulatory frameworks governing HTE implementation vary considerably across regions and industries. While pharmaceutical applications must adhere to Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) guidelines, materials science and chemical applications often operate under different regulatory paradigms. This regulatory heterogeneity complicates compliance efforts, particularly for multinational organizations operating across multiple jurisdictions.
Technical challenges further compound compliance issues. Integration of diverse analytical instruments, each with proprietary data formats and control systems, creates interoperability problems that impede standardization efforts. Additionally, the sheer volume and complexity of data generated through HTE approaches necessitate sophisticated data management systems capable of ensuring data integrity, traceability, and compliance with electronic record requirements such as 21 CFR Part 11.
Quality assurance represents another significant challenge. Traditional quality control approaches designed for conventional experimentation often prove inadequate for HTE workflows, where thousands of experiments may be conducted simultaneously. Developing appropriate statistical methods and quality metrics for high-throughput processes remains an active area of research and development.
The geographical distribution of HTE technology development shows concentration in North America (particularly the United States), Western Europe, and increasingly in East Asia, with Japan, South Korea, and China emerging as significant contributors to technological innovation in this space. This global distribution creates additional complexity for standards harmonization efforts, as different regions may prioritize different aspects of compliance based on local regulatory environments and industry needs.
The global HTE market is experiencing robust growth, with an estimated value of $1.3 billion in 2022 and projected to reach $2.5 billion by 2027, representing a compound annual growth rate of approximately 14%. This growth is primarily driven by increasing R&D investments in pharmaceutical and biotechnology sectors, coupled with the rising demand for more efficient drug discovery processes.
Despite significant advancements, the HTE field faces several critical challenges related to industry standards compliance. Foremost among these is the lack of universally accepted standards for data formats, experimental protocols, and quality control metrics. This fragmentation creates significant barriers to data sharing, cross-platform validation, and regulatory acceptance of HTE-generated results.
Regulatory frameworks governing HTE implementation vary considerably across regions and industries. While pharmaceutical applications must adhere to Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) guidelines, materials science and chemical applications often operate under different regulatory paradigms. This regulatory heterogeneity complicates compliance efforts, particularly for multinational organizations operating across multiple jurisdictions.
Technical challenges further compound compliance issues. Integration of diverse analytical instruments, each with proprietary data formats and control systems, creates interoperability problems that impede standardization efforts. Additionally, the sheer volume and complexity of data generated through HTE approaches necessitate sophisticated data management systems capable of ensuring data integrity, traceability, and compliance with electronic record requirements such as 21 CFR Part 11.
Quality assurance represents another significant challenge. Traditional quality control approaches designed for conventional experimentation often prove inadequate for HTE workflows, where thousands of experiments may be conducted simultaneously. Developing appropriate statistical methods and quality metrics for high-throughput processes remains an active area of research and development.
The geographical distribution of HTE technology development shows concentration in North America (particularly the United States), Western Europe, and increasingly in East Asia, with Japan, South Korea, and China emerging as significant contributors to technological innovation in this space. This global distribution creates additional complexity for standards harmonization efforts, as different regions may prioritize different aspects of compliance based on local regulatory environments and industry needs.
Current HTE Compliance Solutions
01 Automated compliance monitoring systems for high-throughput experiments
Systems designed to automatically monitor and ensure compliance in high-throughput experimentation environments. These systems track experimental parameters, validate protocols against regulatory requirements, and generate compliance reports. They incorporate real-time monitoring capabilities to detect deviations from approved protocols and can automatically flag non-compliant activities, reducing human error and ensuring consistent adherence to regulatory standards.- Automated compliance monitoring systems for high-throughput experimentation: Systems designed to automatically monitor and ensure compliance in high-throughput experimental environments. These systems incorporate real-time tracking of experimental parameters, automated documentation, and validation checks to ensure that experiments adhere to regulatory requirements and standard operating procedures. The automation reduces human error while maintaining comprehensive audit trails for regulatory inspection.
- Data management and validation for regulatory compliance: Solutions focused on managing the large volumes of data generated during high-throughput experimentation to ensure regulatory compliance. These approaches include secure data storage, automated data validation protocols, electronic signatures, and systems that maintain data integrity throughout the experimental lifecycle. The solutions facilitate compliance with regulations such as 21 CFR Part 11 and GDPR while enabling efficient data retrieval for audits.
- Networked laboratory equipment compliance frameworks: Frameworks that connect laboratory equipment in high-throughput environments to ensure standardized compliance across multiple devices and experimental stations. These systems enable centralized monitoring of equipment calibration status, maintenance schedules, and operational parameters. The networked approach allows for immediate identification of non-compliant equipment and facilitates coordinated updates to compliance protocols across the laboratory infrastructure.
- Compliance documentation and audit trail systems: Specialized systems for generating and maintaining comprehensive documentation and audit trails for high-throughput experimentation. These solutions automatically capture experimental conditions, user actions, and results in tamper-evident formats. They include features for electronic signatures, version control, and automated report generation that satisfy regulatory requirements while minimizing the documentation burden on researchers.
- AI-assisted compliance verification for experimental protocols: Advanced systems that leverage artificial intelligence to verify compliance of high-throughput experimental protocols. These solutions analyze experimental designs, execution parameters, and results to identify potential compliance issues before they become regulatory problems. The AI components can learn from previous compliance audits to improve detection of subtle non-compliance indicators and suggest corrective actions to researchers.
02 Data management and validation for regulatory compliance
Solutions focused on managing and validating experimental data to meet regulatory compliance requirements in high-throughput settings. These approaches include secure data storage, audit trail maintenance, electronic signatures, and data integrity verification. They ensure that experimental data is properly documented, traceable, and protected from unauthorized alterations, which is essential for regulatory submissions and inspections.Expand Specific Solutions03 Standardized protocols and workflow management for compliance
Methods for implementing standardized experimental protocols and workflow management systems to ensure compliance in high-throughput experimentation. These approaches include protocol libraries, version control, approval workflows, and documentation systems. They help maintain consistency across multiple experiments, ensure proper authorization before protocol execution, and provide comprehensive documentation of experimental procedures.Expand Specific Solutions04 Integrated quality control and compliance verification
Systems that integrate quality control measures with compliance verification in high-throughput experimentation. These solutions incorporate automated quality checks, statistical process control, and compliance verification at critical points in the experimental workflow. They help identify and address quality issues that could impact compliance, ensure consistent experimental conditions, and maintain the integrity of results.Expand Specific Solutions05 Network-based compliance management for distributed experimentation
Network-based systems for managing compliance across distributed high-throughput experimentation facilities. These solutions enable centralized oversight of compliance activities across multiple locations, synchronize compliance protocols, and facilitate remote auditing. They support collaborative research while maintaining consistent compliance standards, allowing organizations to efficiently manage regulatory requirements across geographically dispersed research operations.Expand Specific Solutions
Key Industry Players in HTE Field
High-Throughput Experimentation (HTE) in compliance with industry standards is currently in a growth phase, with the market expanding rapidly due to increasing demand for efficient R&D processes. The global HTE market is estimated to reach $1.5-2 billion by 2025, driven by pharmaceutical, semiconductor, and materials science applications. Technologically, the field shows varying maturity levels across industries. Leading players like Agilent Technologies, Teradyne, and IBM have developed advanced automated systems with robust compliance frameworks, while semiconductor companies including Intel, GLOBALFOUNDRIES, and Samsung are integrating HTE into their manufacturing processes. Academic institutions like Shanghai University and Zhejiang University are contributing significant research, while pharmaceutical entities such as Bayer Technology Services and Alembic Pharmaceuticals are implementing HTE to accelerate drug discovery while maintaining regulatory compliance.
AGILENT TECHNOLOGIES INC
Technical Solution: Agilent's high-throughput experimentation platform integrates automated laboratory systems with sophisticated data management software to ensure compliance with industry standards. Their solution combines robotic sample handling, parallel synthesis reactors, and high-speed analytical instruments with a comprehensive Laboratory Information Management System (LIMS). This system incorporates built-in compliance features for GLP, GMP, and ISO standards, with automated audit trails and electronic signatures that meet 21 CFR Part 11 requirements. Agilent's platform enables researchers to design and execute thousands of experiments simultaneously while maintaining data integrity through automated calibration, system suitability tests, and quality control samples. Their OpenLab software suite provides end-to-end compliance management with version control, data security protocols, and standardized reporting templates that align with industry regulatory frameworks.
Strengths: Comprehensive integration of hardware and software systems specifically designed for regulatory compliance; extensive experience in analytical instrumentation with proven reliability in regulated environments. Weaknesses: Higher implementation costs compared to modular solutions; potential vendor lock-in due to proprietary integration between system components.
Bayer Technology Services GmbH
Technical Solution: Bayer Technology Services has developed an integrated high-throughput experimentation platform specifically designed for pharmaceutical and chemical research with comprehensive compliance capabilities. Their system combines modular automation hardware with their proprietary HTMS (High-Throughput Management System) software to ensure adherence to GLP, GMP, and ISO standards throughout the experimental workflow. The platform features automated liquid handling systems, parallel reaction blocks, and integrated analytical instruments that can process hundreds of samples daily while maintaining complete data traceability. Bayer's compliance framework includes electronic signatures, time-stamped audit trails, and automated validation checks that meet 21 CFR Part 11 requirements. Their system incorporates standardized protocols for method validation, instrument qualification, and data integrity verification, with built-in quality control procedures that automatically flag deviations from acceptance criteria. The platform also generates compliance-ready documentation for regulatory submissions.
Strengths: Purpose-built for pharmaceutical and chemical applications with strong regulatory focus; extensive validation documentation; seamless integration with existing laboratory systems. Weaknesses: Relatively high implementation and maintenance costs; steeper learning curve for new users compared to simpler systems.
Core HTE Standards and Protocols
High throughput research workflow
PatentInactiveUS20110029439A1
Innovation
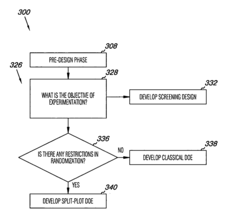
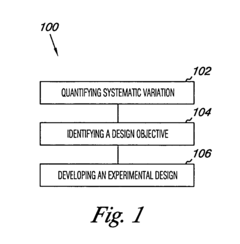
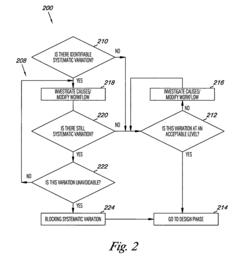
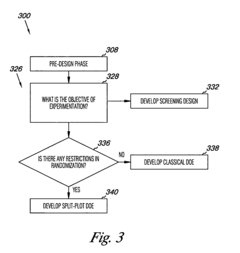
- The method involves quantifying systematic variation through variance component analysis, identifying design objectives, and developing experimental designs such as screening, split-plot, or classical designs to account for systematic variation, and modifying sources of variation to achieve statistically defensible results, using computer-readable mediums and computing devices to implement these steps.
Method for carrying out high throughput experiments
PatentInactiveEP1725326A1
Innovation
- A fast and targeted evaluation method using frequency statistics to rank influencing variables based on their impact on output variables, allowing for efficient experiment planning and optimization with minimal training and application effort, capable of handling large datasets and identifying interaction effects.
Regulatory Framework for HTE Implementation
The implementation of High-Throughput Experimentation (HTE) technologies necessitates adherence to a complex regulatory framework that varies across industries and geographical regions. In pharmaceutical applications, HTE must comply with Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) guidelines established by regulatory bodies such as the FDA, EMA, and ICH. These frameworks ensure data integrity, reproducibility, and traceability throughout the experimental process.
For chemical industries, HTE implementations must align with REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulations in Europe and TSCA (Toxic Substances Control Act) in the United States. These regulations govern the handling, testing, and documentation of chemical substances, requiring comprehensive safety assessments and environmental impact evaluations.
Quality management systems such as ISO 9001 provide overarching standards for HTE operations, while specialized standards like ISO 17025 specifically address requirements for testing and calibration laboratories. Compliance with these standards ensures that HTE methodologies produce reliable, consistent, and internationally recognized results.
Data management within HTE systems must conform to data integrity principles outlined in ALCOA+ (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available). Additionally, electronic record-keeping systems must comply with 21 CFR Part 11 in the US and Annex 11 in the EU, which establish criteria for trustworthy electronic records and signatures.
Risk management frameworks, including ICH Q9 for pharmaceuticals and ISO 31000 for general applications, provide structured approaches for identifying, assessing, and mitigating risks associated with HTE implementation. These frameworks help organizations balance innovation with compliance requirements.
Environmental regulations, including waste management directives and emissions standards, impose additional compliance requirements on HTE operations. Sustainable laboratory practices and green chemistry principles are increasingly becoming regulatory expectations rather than optional considerations.
The validation and qualification of HTE equipment and methodologies represent another critical regulatory aspect. This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols that verify systems function as intended within specified parameters.
Cross-border considerations add complexity to the regulatory landscape, as organizations implementing HTE across multiple jurisdictions must navigate varying requirements while maintaining consistent quality standards and operational efficiency.
For chemical industries, HTE implementations must align with REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulations in Europe and TSCA (Toxic Substances Control Act) in the United States. These regulations govern the handling, testing, and documentation of chemical substances, requiring comprehensive safety assessments and environmental impact evaluations.
Quality management systems such as ISO 9001 provide overarching standards for HTE operations, while specialized standards like ISO 17025 specifically address requirements for testing and calibration laboratories. Compliance with these standards ensures that HTE methodologies produce reliable, consistent, and internationally recognized results.
Data management within HTE systems must conform to data integrity principles outlined in ALCOA+ (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available). Additionally, electronic record-keeping systems must comply with 21 CFR Part 11 in the US and Annex 11 in the EU, which establish criteria for trustworthy electronic records and signatures.
Risk management frameworks, including ICH Q9 for pharmaceuticals and ISO 31000 for general applications, provide structured approaches for identifying, assessing, and mitigating risks associated with HTE implementation. These frameworks help organizations balance innovation with compliance requirements.
Environmental regulations, including waste management directives and emissions standards, impose additional compliance requirements on HTE operations. Sustainable laboratory practices and green chemistry principles are increasingly becoming regulatory expectations rather than optional considerations.
The validation and qualification of HTE equipment and methodologies represent another critical regulatory aspect. This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols that verify systems function as intended within specified parameters.
Cross-border considerations add complexity to the regulatory landscape, as organizations implementing HTE across multiple jurisdictions must navigate varying requirements while maintaining consistent quality standards and operational efficiency.
Risk Management in HTE Standardization
Risk management in HTE standardization represents a critical component for organizations implementing high-throughput experimentation technologies. The rapid pace of experimentation and data generation inherent to HTE systems introduces unique compliance risks that must be systematically addressed through comprehensive risk assessment frameworks.
Primary risks in HTE standardization include data integrity vulnerabilities, experimental reproducibility challenges, and regulatory compliance gaps. These risks are amplified by the volume and velocity of experiments conducted simultaneously, creating potential blind spots in quality control processes. Organizations must develop risk matrices specifically tailored to HTE workflows, identifying both likelihood and impact factors for each potential compliance deviation.
Effective risk mitigation strategies should incorporate automated validation protocols that can verify experimental parameters against established standards in real-time. This approach enables early detection of non-compliant conditions before they propagate through the experimental pipeline. Implementation of digital audit trails with immutable record-keeping provides essential documentation for regulatory review while establishing accountability throughout the experimental lifecycle.
Cross-functional risk management teams comprising regulatory specialists, data scientists, and laboratory personnel offer the most comprehensive approach to identifying potential compliance vulnerabilities. These teams should conduct regular risk assessments using standardized methodologies such as Failure Mode and Effects Analysis (FMEA) specifically adapted for HTE environments.
Statistical process control methods play a vital role in risk management by establishing control limits for experimental variables. When integrated with machine learning algorithms, these systems can predict potential compliance deviations before they occur, enabling proactive intervention rather than reactive correction.
Organizations must also consider the risk implications of third-party integrations within HTE platforms. Vendor qualification processes should include thorough evaluation of suppliers' compliance with relevant industry standards, with contractual provisions requiring ongoing adherence to evolving regulatory requirements.
Continuous risk monitoring represents the final critical element in effective HTE standardization risk management. This involves establishing key risk indicators (KRIs) that provide early warning signals for potential compliance issues, coupled with regular review cycles to assess the effectiveness of existing risk controls and identify emerging compliance challenges in the rapidly evolving HTE landscape.
Primary risks in HTE standardization include data integrity vulnerabilities, experimental reproducibility challenges, and regulatory compliance gaps. These risks are amplified by the volume and velocity of experiments conducted simultaneously, creating potential blind spots in quality control processes. Organizations must develop risk matrices specifically tailored to HTE workflows, identifying both likelihood and impact factors for each potential compliance deviation.
Effective risk mitigation strategies should incorporate automated validation protocols that can verify experimental parameters against established standards in real-time. This approach enables early detection of non-compliant conditions before they propagate through the experimental pipeline. Implementation of digital audit trails with immutable record-keeping provides essential documentation for regulatory review while establishing accountability throughout the experimental lifecycle.
Cross-functional risk management teams comprising regulatory specialists, data scientists, and laboratory personnel offer the most comprehensive approach to identifying potential compliance vulnerabilities. These teams should conduct regular risk assessments using standardized methodologies such as Failure Mode and Effects Analysis (FMEA) specifically adapted for HTE environments.
Statistical process control methods play a vital role in risk management by establishing control limits for experimental variables. When integrated with machine learning algorithms, these systems can predict potential compliance deviations before they occur, enabling proactive intervention rather than reactive correction.
Organizations must also consider the risk implications of third-party integrations within HTE platforms. Vendor qualification processes should include thorough evaluation of suppliers' compliance with relevant industry standards, with contractual provisions requiring ongoing adherence to evolving regulatory requirements.
Continuous risk monitoring represents the final critical element in effective HTE standardization risk management. This involves establishing key risk indicators (KRIs) that provide early warning signals for potential compliance issues, coupled with regular review cycles to assess the effectiveness of existing risk controls and identify emerging compliance challenges in the rapidly evolving HTE landscape.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!