How High-Throughput Experimentation Facilitates New Drug Discoveries
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HTE Background and Objectives
High-throughput experimentation (HTE) represents a paradigm shift in pharmaceutical research, evolving from traditional one-reaction-at-a-time approaches to parallel processing methodologies capable of conducting thousands of experiments simultaneously. This technological advancement emerged in the late 1990s and early 2000s, initially in materials science before being adapted for pharmaceutical applications. The fundamental principle behind HTE is the miniaturization and parallelization of experiments, enabling researchers to explore vast chemical spaces with unprecedented efficiency.
The evolution of HTE has been closely tied to advancements in automation, robotics, and data analytics. Early systems focused primarily on reaction optimization, while contemporary platforms integrate sophisticated machine learning algorithms to predict outcomes and guide experimental design. This progression reflects the industry's growing recognition of HTE's potential to address the declining productivity in pharmaceutical research and development.
Current HTE technologies encompass a diverse array of capabilities, including automated synthesis platforms, rapid analytical techniques, and integrated informatics systems. These technologies have collectively transformed the drug discovery process by accelerating lead identification and optimization phases. The integration of microfluidic systems has further enhanced throughput capabilities while reducing reagent consumption, addressing both efficiency and sustainability concerns.
The primary objective of HTE in drug discovery is to compress development timelines while expanding the chemical diversity explored. By enabling researchers to rapidly test multiple reaction conditions, catalysts, and substrates, HTE facilitates the identification of optimal synthetic routes for complex pharmaceutical compounds. This capability is particularly valuable for developing drugs targeting previously undruggable proteins or addressing emerging therapeutic areas.
Another critical goal is to enhance decision-making quality through data-rich experimentation. By generating comprehensive datasets across multiple parameters, HTE provides researchers with robust statistical foundations for advancing promising candidates while early termination of unpromising pathways. This data-driven approach represents a significant departure from traditional intuition-based research methodologies.
Looking forward, HTE aims to evolve beyond its current capabilities through deeper integration with computational methods, particularly artificial intelligence and machine learning. The convergence of experimental and computational approaches promises to create predictive frameworks that can further accelerate discovery processes while reducing resource requirements. The ultimate vision is a seamless integration of high-throughput experimentation with computational modeling to create truly predictive drug discovery platforms.
The evolution of HTE has been closely tied to advancements in automation, robotics, and data analytics. Early systems focused primarily on reaction optimization, while contemporary platforms integrate sophisticated machine learning algorithms to predict outcomes and guide experimental design. This progression reflects the industry's growing recognition of HTE's potential to address the declining productivity in pharmaceutical research and development.
Current HTE technologies encompass a diverse array of capabilities, including automated synthesis platforms, rapid analytical techniques, and integrated informatics systems. These technologies have collectively transformed the drug discovery process by accelerating lead identification and optimization phases. The integration of microfluidic systems has further enhanced throughput capabilities while reducing reagent consumption, addressing both efficiency and sustainability concerns.
The primary objective of HTE in drug discovery is to compress development timelines while expanding the chemical diversity explored. By enabling researchers to rapidly test multiple reaction conditions, catalysts, and substrates, HTE facilitates the identification of optimal synthetic routes for complex pharmaceutical compounds. This capability is particularly valuable for developing drugs targeting previously undruggable proteins or addressing emerging therapeutic areas.
Another critical goal is to enhance decision-making quality through data-rich experimentation. By generating comprehensive datasets across multiple parameters, HTE provides researchers with robust statistical foundations for advancing promising candidates while early termination of unpromising pathways. This data-driven approach represents a significant departure from traditional intuition-based research methodologies.
Looking forward, HTE aims to evolve beyond its current capabilities through deeper integration with computational methods, particularly artificial intelligence and machine learning. The convergence of experimental and computational approaches promises to create predictive frameworks that can further accelerate discovery processes while reducing resource requirements. The ultimate vision is a seamless integration of high-throughput experimentation with computational modeling to create truly predictive drug discovery platforms.
Pharmaceutical Market Demand Analysis
The pharmaceutical industry is experiencing a significant shift in drug discovery methodologies, with high-throughput experimentation (HTE) emerging as a critical driver of innovation. The global pharmaceutical market, valued at approximately $1.4 trillion in 2022, is projected to reach $1.9 trillion by 2027, with research and development investments constituting about 20% of revenue for major pharmaceutical companies.
Market demand for accelerated drug discovery processes has intensified due to several converging factors. The COVID-19 pandemic dramatically highlighted the need for rapid therapeutic development, creating unprecedented public and regulatory pressure to compress traditional decade-long development timelines. This catalytic event has permanently altered market expectations regarding development speed.
Rising healthcare costs globally have simultaneously created demand for more cost-effective drug discovery approaches. Traditional methods typically require $2.6 billion and 10-15 years to bring a single drug to market, with failure rates exceeding 90%. HTE technologies promise to reduce both time and financial investments while improving success rates, addressing a critical market pain point.
Demographic shifts are further driving demand for novel therapeutics. An aging global population has increased the prevalence of chronic conditions including cancer, cardiovascular disease, and neurodegenerative disorders. These complex diseases require sophisticated treatment approaches, creating market pull for advanced discovery technologies capable of identifying effective compounds for challenging targets.
Personalized medicine represents another significant market driver, with the segment growing at 11.5% annually. As treatment paradigms shift toward targeted therapies based on genetic profiles, the need for efficient screening of compound libraries against specific molecular targets has intensified. HTE provides the technological foundation for this precision approach.
Regulatory environments worldwide have evolved to accommodate accelerated approval pathways for breakthrough therapies, creating market incentives for technologies that can rapidly identify promising drug candidates. The FDA's Breakthrough Therapy designation and similar programs internationally have established regulatory frameworks supporting faster development timelines.
Venture capital and private equity investments in drug discovery technologies have reached record levels, with over $20 billion invested in 2021 alone. This influx of capital reflects market confidence in the transformative potential of HTE and related technologies. Pharmaceutical companies increasingly view HTE capabilities as essential competitive advantages rather than optional research tools.
Contract research organizations (CROs) have responded to this demand by expanding HTE service offerings, creating a robust market subsegment estimated at $5.7 billion annually and growing at 14% per year. This service model has democratized access to advanced discovery technologies, allowing smaller biotechnology companies to leverage HTE capabilities without significant capital investments.
Market demand for accelerated drug discovery processes has intensified due to several converging factors. The COVID-19 pandemic dramatically highlighted the need for rapid therapeutic development, creating unprecedented public and regulatory pressure to compress traditional decade-long development timelines. This catalytic event has permanently altered market expectations regarding development speed.
Rising healthcare costs globally have simultaneously created demand for more cost-effective drug discovery approaches. Traditional methods typically require $2.6 billion and 10-15 years to bring a single drug to market, with failure rates exceeding 90%. HTE technologies promise to reduce both time and financial investments while improving success rates, addressing a critical market pain point.
Demographic shifts are further driving demand for novel therapeutics. An aging global population has increased the prevalence of chronic conditions including cancer, cardiovascular disease, and neurodegenerative disorders. These complex diseases require sophisticated treatment approaches, creating market pull for advanced discovery technologies capable of identifying effective compounds for challenging targets.
Personalized medicine represents another significant market driver, with the segment growing at 11.5% annually. As treatment paradigms shift toward targeted therapies based on genetic profiles, the need for efficient screening of compound libraries against specific molecular targets has intensified. HTE provides the technological foundation for this precision approach.
Regulatory environments worldwide have evolved to accommodate accelerated approval pathways for breakthrough therapies, creating market incentives for technologies that can rapidly identify promising drug candidates. The FDA's Breakthrough Therapy designation and similar programs internationally have established regulatory frameworks supporting faster development timelines.
Venture capital and private equity investments in drug discovery technologies have reached record levels, with over $20 billion invested in 2021 alone. This influx of capital reflects market confidence in the transformative potential of HTE and related technologies. Pharmaceutical companies increasingly view HTE capabilities as essential competitive advantages rather than optional research tools.
Contract research organizations (CROs) have responded to this demand by expanding HTE service offerings, creating a robust market subsegment estimated at $5.7 billion annually and growing at 14% per year. This service model has democratized access to advanced discovery technologies, allowing smaller biotechnology companies to leverage HTE capabilities without significant capital investments.
Current HTE Technologies and Challenges
High-throughput experimentation (HTE) has revolutionized the drug discovery landscape by enabling researchers to conduct thousands of experiments simultaneously. Current HTE technologies encompass a diverse array of platforms and methodologies that significantly accelerate the drug discovery process.
Miniaturization technologies represent a cornerstone of modern HTE systems. Microfluidic devices, lab-on-a-chip platforms, and nanoliter dispensing systems have dramatically reduced sample volumes from milliliters to nanoliters, enabling conservation of precious compounds while increasing experimental density. These technologies allow for up to 1536-well plate formats, representing a 40-fold increase in throughput compared to traditional 96-well formats.
Automation systems form the backbone of contemporary HTE platforms. Robotic liquid handling stations, automated sample preparation systems, and integrated workflow solutions have minimized human intervention, reducing experimental variability while increasing reproducibility. Advanced systems now incorporate machine learning algorithms that optimize experimental parameters in real-time, further enhancing efficiency.
Detection technologies have similarly evolved to match the high-throughput paradigm. Label-free detection methods, high-content imaging systems, and multiplexed assay technologies enable researchers to capture multiple data points from single experiments. Mass spectrometry integration with HTE platforms has particularly transformed metabolomic and proteomic analyses in drug discovery.
Despite these advances, significant challenges persist in the HTE landscape. Data management represents a primary bottleneck, as HTE platforms generate terabytes of complex, multidimensional data that exceed traditional analysis capabilities. Current bioinformatics tools struggle to efficiently process, integrate, and extract meaningful insights from these massive datasets.
Standardization issues present another major challenge. The diversity of HTE platforms, protocols, and data formats complicates cross-laboratory validation and results comparison. Industry-wide standards for experimental design, data reporting, and quality control remain inconsistent, hampering collaborative efforts.
Technical limitations also constrain HTE applications. Many complex biological assays, particularly those involving 3D cell cultures, organoids, or patient-derived samples, remain difficult to adapt to high-throughput formats. Additionally, the correlation between simplified HTE models and in vivo efficacy continues to be problematic, with many promising compounds failing in later development stages.
Cost barriers represent a significant challenge for smaller research organizations. State-of-the-art HTE platforms require substantial capital investment and specialized expertise, creating inequitable access to these transformative technologies across the research ecosystem.
Miniaturization technologies represent a cornerstone of modern HTE systems. Microfluidic devices, lab-on-a-chip platforms, and nanoliter dispensing systems have dramatically reduced sample volumes from milliliters to nanoliters, enabling conservation of precious compounds while increasing experimental density. These technologies allow for up to 1536-well plate formats, representing a 40-fold increase in throughput compared to traditional 96-well formats.
Automation systems form the backbone of contemporary HTE platforms. Robotic liquid handling stations, automated sample preparation systems, and integrated workflow solutions have minimized human intervention, reducing experimental variability while increasing reproducibility. Advanced systems now incorporate machine learning algorithms that optimize experimental parameters in real-time, further enhancing efficiency.
Detection technologies have similarly evolved to match the high-throughput paradigm. Label-free detection methods, high-content imaging systems, and multiplexed assay technologies enable researchers to capture multiple data points from single experiments. Mass spectrometry integration with HTE platforms has particularly transformed metabolomic and proteomic analyses in drug discovery.
Despite these advances, significant challenges persist in the HTE landscape. Data management represents a primary bottleneck, as HTE platforms generate terabytes of complex, multidimensional data that exceed traditional analysis capabilities. Current bioinformatics tools struggle to efficiently process, integrate, and extract meaningful insights from these massive datasets.
Standardization issues present another major challenge. The diversity of HTE platforms, protocols, and data formats complicates cross-laboratory validation and results comparison. Industry-wide standards for experimental design, data reporting, and quality control remain inconsistent, hampering collaborative efforts.
Technical limitations also constrain HTE applications. Many complex biological assays, particularly those involving 3D cell cultures, organoids, or patient-derived samples, remain difficult to adapt to high-throughput formats. Additionally, the correlation between simplified HTE models and in vivo efficacy continues to be problematic, with many promising compounds failing in later development stages.
Cost barriers represent a significant challenge for smaller research organizations. State-of-the-art HTE platforms require substantial capital investment and specialized expertise, creating inequitable access to these transformative technologies across the research ecosystem.
Current HTE Implementation Strategies
01 Automated laboratory systems for high-throughput screening
Automated laboratory systems enable rapid and efficient screening of multiple samples simultaneously. These systems incorporate robotics, liquid handling devices, and integrated software to streamline experimental workflows. By automating repetitive tasks, researchers can significantly increase the number of experiments performed in a given time period, accelerating the discovery process while maintaining consistency and reducing human error.- Automated laboratory systems for high-throughput experimentation: Automated laboratory systems enable high-throughput experimentation by integrating robotics, liquid handling systems, and analytical instruments. These systems can perform multiple experiments simultaneously with minimal human intervention, significantly increasing the speed and efficiency of the discovery process. The automation of experimental workflows allows for standardized procedures, reducing human error and improving reproducibility of results.
- Data management and analysis platforms for high-throughput experiments: Specialized software platforms are designed to manage and analyze the large volumes of data generated by high-throughput experiments. These platforms incorporate advanced algorithms and machine learning techniques to identify patterns, correlations, and insights that might not be apparent through traditional analysis methods. By efficiently processing experimental data, these systems accelerate the discovery process and help researchers make informed decisions about subsequent experiments.
- Parallel processing techniques for experimental design and optimization: Parallel processing techniques enable the simultaneous execution of multiple experimental conditions, significantly reducing the time required for discovery. These methods involve the design of experiment arrays that systematically explore parameter spaces to identify optimal conditions. By testing numerous variables concurrently rather than sequentially, researchers can rapidly converge on promising directions and eliminate unproductive paths, accelerating the overall discovery process.
- Miniaturization technologies for increased experimental throughput: Miniaturization technologies, such as microfluidics and lab-on-a-chip systems, allow for the reduction in scale of experimental setups, enabling more experiments to be conducted with less material and in less space. These technologies facilitate the handling of small sample volumes, reduce reagent consumption, and increase experimental density. The ability to perform thousands of micro-scale reactions simultaneously dramatically enhances the efficiency of the discovery process.
- Collaborative platforms and networked research environments: Collaborative platforms and networked research environments enable researchers from different locations to work together on high-throughput experimentation projects. These systems facilitate the sharing of experimental protocols, data, and insights in real-time, allowing for distributed expertise to be applied to complex problems. By leveraging collective intelligence and resources, these platforms accelerate the discovery process and promote innovation through cross-disciplinary collaboration.
02 Data analysis and machine learning for experimental results
Advanced data analysis techniques and machine learning algorithms help scientists extract meaningful insights from large experimental datasets. These computational methods can identify patterns, correlations, and anomalies that might be missed through traditional analysis approaches. By applying artificial intelligence to experimental data, researchers can accelerate hypothesis generation, optimize experimental conditions, and make predictions that guide future experiments, ultimately facilitating scientific discoveries.Expand Specific Solutions03 Parallel processing and miniaturization technologies
Miniaturization technologies such as microfluidics, lab-on-a-chip devices, and microarray platforms enable researchers to conduct multiple experiments simultaneously using minimal sample volumes. These technologies facilitate parallel processing of samples, allowing for the rapid screening of numerous conditions or compounds. By reducing reagent consumption and increasing experimental throughput, these approaches accelerate the discovery process while conserving valuable resources.Expand Specific Solutions04 Collaborative research platforms and knowledge sharing
Digital platforms that facilitate collaboration and knowledge sharing among researchers enhance the efficiency of high-throughput experimentation. These systems enable real-time data sharing, remote experiment monitoring, and collaborative analysis across different locations and institutions. By breaking down information silos and promoting open science practices, these platforms accelerate discoveries by leveraging collective expertise and preventing duplication of efforts.Expand Specific Solutions05 Integrated experimental design and workflow management
Comprehensive systems for experimental design and workflow management optimize the high-throughput discovery process. These systems incorporate statistical design of experiments, resource allocation algorithms, and scheduling tools to maximize efficiency. By systematically planning experiments, tracking progress, and managing resources, researchers can ensure that high-throughput approaches yield meaningful results while minimizing waste and redundancy.Expand Specific Solutions
Leading Companies in HTE Technology
High-throughput experimentation (HTE) in drug discovery is currently in a growth phase, with the market expanding rapidly as pharmaceutical companies seek to accelerate development timelines and reduce costs. The global HTE market is estimated to reach several billion dollars within the next five years, driven by increasing R&D investments. Technologically, the field is maturing with companies like Recursion Pharmaceuticals leveraging advanced AI integration, while established players such as Merck Sharp & Dohme and Life Technologies provide robust commercial platforms. IBM and HighRes Biosolutions are advancing automation capabilities, while academic institutions like Washington University in St. Louis contribute fundamental research. Emerging players like Kuano are introducing quantum computing approaches, indicating a competitive landscape that spans from established pharmaceutical giants to specialized technology providers and innovative startups.
Recursion Pharmaceuticals, Inc.
Technical Solution: Recursion has pioneered an AI-enabled phenomics platform that combines automated high-throughput microscopy with computer vision and machine learning to identify novel therapeutic candidates. Their platform can screen over 100,000 compounds weekly against hundreds of disease models. The company's approach integrates cellular phenotypic screening with computational tools to map relationships between compounds, targets, and diseases. Their proprietary dataset includes over 8 petabytes of biological images from more than 33 human cell types exposed to various perturbations. Recursion's platform enables the rapid identification of novel drug candidates for both known and previously undruggable targets, significantly accelerating the traditional drug discovery timeline from years to months[1][3]. The company has successfully advanced multiple candidates to clinical trials using this approach, demonstrating the practical application of their high-throughput experimentation methodology.
Strengths: Unprecedented scale of biological image data; integration of AI with phenotypic screening enables target-agnostic drug discovery; significantly faster than traditional approaches. Weaknesses: Reliance on in vitro cellular models may not fully capture in vivo complexity; computational predictions require extensive validation; high infrastructure and computational costs.
HighRes Biosolutions, Inc.
Technical Solution: HighRes Biosolutions has developed a modular and flexible laboratory automation platform specifically designed for high-throughput experimentation in drug discovery. Their technology centers around dynamic scheduling software and robotic systems that can be reconfigured in real-time to adapt to changing experimental needs. The company's flagship product, Cellario, integrates hardware and software components to create fully automated workcells capable of executing complex experimental protocols with minimal human intervention. HighRes has pioneered the development of microplate-based systems that can handle assay volumes as low as 1-2 microliters, significantly reducing reagent costs and sample requirements. Their platforms incorporate precision liquid handling, automated incubation, multimodal detection systems, and integrated data management. The company has implemented machine learning algorithms to optimize experimental parameters and predict outcomes based on historical data. HighRes systems are designed with open architecture principles, allowing integration with third-party instruments and software to create customized workflows for specific drug discovery applications[7][9]. Their technology has been adopted by numerous pharmaceutical companies and research institutions to accelerate screening campaigns and lead optimization efforts.
Strengths: Highly adaptable and reconfigurable systems; excellent integration capabilities with existing laboratory infrastructure; sophisticated scheduling algorithms maximize throughput. Weaknesses: Significant upfront capital investment; requires specialized technical expertise for maintenance and programming; potential for mechanical failures in complex robotic systems.
Key HTE Patents and Technical Innovations
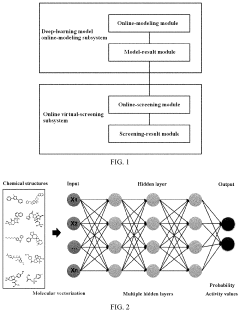
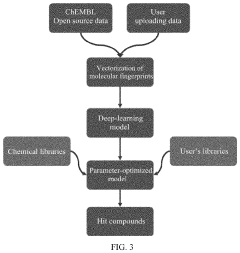
High-throughput virtual drug screening system based on molecular fingerprints and deep learning
PatentActiveUS11581061B2
Innovation
- A high-throughput virtual drug screening system combining molecular fingerprints and deep learning, featuring a deep-learning model online-modeling subsystem and online virtual-screening subsystem for constructing and utilizing models to predict compound activity, enabling rapid and accurate screening of potential drug compounds.
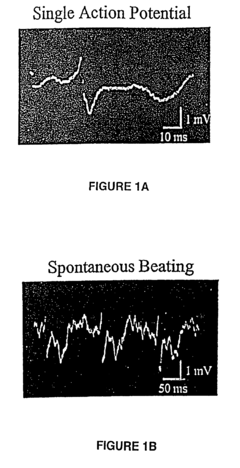
High throughput functional genomics
PatentInactiveUS7734426B2
Innovation
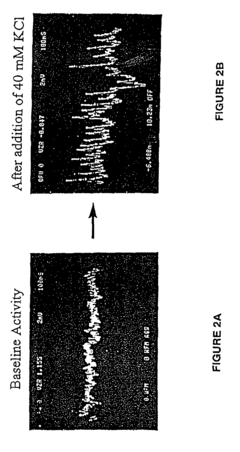
- A system utilizing solid state microelectrodes with a high impedance seal and self-assembled monolayers to record electrical activity from cells, allowing for the measurement of changes in membrane potential and action potential in response to test substances, enabling the identification of affected ion channels and underlying cellular processes without causing cell death.
AI Integration in HTE Workflows
The integration of artificial intelligence (AI) into High-Throughput Experimentation (HTE) workflows represents a transformative advancement in drug discovery processes. Machine learning algorithms now enable researchers to analyze vast experimental datasets generated through HTE platforms with unprecedented speed and accuracy. These AI systems can identify patterns and correlations that might otherwise remain undetected by human researchers, significantly accelerating the identification of promising drug candidates.
Deep learning models have demonstrated particular efficacy in predicting molecular properties and biological activities based on structural information. When incorporated into HTE workflows, these models can prioritize compounds for synthesis and testing, effectively reducing the experimental space that needs to be physically explored. This capability has dramatically shortened the timeline from initial screening to lead compound identification, allowing pharmaceutical companies to bring potentially life-saving medications to market more rapidly.
Natural language processing (NLP) technologies have further enhanced HTE workflows by extracting valuable insights from scientific literature, patents, and clinical trial data. These systems continuously monitor and analyze new publications, providing researchers with up-to-date information that can inform experimental design and interpretation. The ability to rapidly contextualize experimental results within the broader scientific landscape represents a significant competitive advantage in the fast-paced pharmaceutical industry.
Computer vision algorithms have revolutionized image-based assays within HTE platforms. These systems can automatically analyze microscopy images, flow cytometry data, and other visual outputs at scale, detecting subtle phenotypic changes that might indicate therapeutic potential. The automation of image analysis not only increases throughput but also improves consistency and reduces human bias in data interpretation.
Reinforcement learning approaches are increasingly being applied to optimize experimental parameters in real-time. These systems can adaptively modify conditions based on ongoing results, effectively "learning" the optimal experimental space to explore. This dynamic optimization capability represents a significant advancement over traditional design of experiments approaches, particularly for complex biological systems where the parameter space is vast and poorly understood.
Cloud computing infrastructures have become essential for supporting AI-enhanced HTE workflows, providing the computational resources necessary for processing massive datasets and running sophisticated algorithms. These platforms enable seamless collaboration between geographically dispersed research teams and facilitate the integration of diverse data types from multiple experimental sources, creating a truly holistic approach to drug discovery.
Deep learning models have demonstrated particular efficacy in predicting molecular properties and biological activities based on structural information. When incorporated into HTE workflows, these models can prioritize compounds for synthesis and testing, effectively reducing the experimental space that needs to be physically explored. This capability has dramatically shortened the timeline from initial screening to lead compound identification, allowing pharmaceutical companies to bring potentially life-saving medications to market more rapidly.
Natural language processing (NLP) technologies have further enhanced HTE workflows by extracting valuable insights from scientific literature, patents, and clinical trial data. These systems continuously monitor and analyze new publications, providing researchers with up-to-date information that can inform experimental design and interpretation. The ability to rapidly contextualize experimental results within the broader scientific landscape represents a significant competitive advantage in the fast-paced pharmaceutical industry.
Computer vision algorithms have revolutionized image-based assays within HTE platforms. These systems can automatically analyze microscopy images, flow cytometry data, and other visual outputs at scale, detecting subtle phenotypic changes that might indicate therapeutic potential. The automation of image analysis not only increases throughput but also improves consistency and reduces human bias in data interpretation.
Reinforcement learning approaches are increasingly being applied to optimize experimental parameters in real-time. These systems can adaptively modify conditions based on ongoing results, effectively "learning" the optimal experimental space to explore. This dynamic optimization capability represents a significant advancement over traditional design of experiments approaches, particularly for complex biological systems where the parameter space is vast and poorly understood.
Cloud computing infrastructures have become essential for supporting AI-enhanced HTE workflows, providing the computational resources necessary for processing massive datasets and running sophisticated algorithms. These platforms enable seamless collaboration between geographically dispersed research teams and facilitate the integration of diverse data types from multiple experimental sources, creating a truly holistic approach to drug discovery.
Regulatory Considerations for HTE-Discovered Drugs
The regulatory landscape for drugs discovered through High-Throughput Experimentation (HTE) presents unique challenges and considerations that pharmaceutical companies must navigate. Regulatory agencies worldwide, including the FDA, EMA, and NMPA, have established specific frameworks for evaluating novel compounds identified through advanced screening technologies. These frameworks typically require comprehensive documentation of the HTE methodologies employed, validation protocols, and quality control measures implemented throughout the discovery process.
Data integrity represents a critical regulatory concern for HTE-discovered drugs. Regulatory bodies increasingly scrutinize the robustness of data management systems, demanding evidence that appropriate controls were in place during the high-volume screening phases. Companies must demonstrate that their HTE platforms incorporate adequate audit trails, data verification mechanisms, and standardized documentation practices that align with regulatory expectations for scientific rigor.
Validation of HTE-derived hits presents another significant regulatory hurdle. Regulatory agencies typically require orthogonal confirmation of activity using traditional assays before accepting HTE-generated data as sufficient evidence for advancing drug candidates. This validation process must be thoroughly documented, with clear explanations of how potential false positives were identified and eliminated during the screening cascade.
The accelerated pace of HTE-driven discovery often creates tension with established regulatory timelines. While HTE can rapidly identify promising compounds, regulatory review processes have not necessarily evolved at the same rate. Forward-thinking pharmaceutical companies are increasingly engaging in early regulatory consultations to establish acceptable parameters for HTE-derived evidence and to develop regulatory strategies that accommodate the unique aspects of HTE-discovered compounds.
Intellectual property considerations intersect with regulatory requirements for HTE-discovered drugs in complex ways. The patentability of compounds identified through algorithmic analysis of massive screening datasets may face different standards of novelty and non-obviousness. Regulatory submissions must carefully address these IP dimensions, particularly when artificial intelligence or machine learning tools have contributed to the identification of novel chemical entities.
International harmonization efforts are gradually addressing the regulatory disparities for HTE-discovered drugs across different jurisdictions. Initiatives like the International Council for Harmonisation (ICH) are working to establish consistent standards for evaluating compounds identified through advanced screening technologies, though significant regional variations persist in how regulatory authorities assess the validity of HTE-derived data.
Data integrity represents a critical regulatory concern for HTE-discovered drugs. Regulatory bodies increasingly scrutinize the robustness of data management systems, demanding evidence that appropriate controls were in place during the high-volume screening phases. Companies must demonstrate that their HTE platforms incorporate adequate audit trails, data verification mechanisms, and standardized documentation practices that align with regulatory expectations for scientific rigor.
Validation of HTE-derived hits presents another significant regulatory hurdle. Regulatory agencies typically require orthogonal confirmation of activity using traditional assays before accepting HTE-generated data as sufficient evidence for advancing drug candidates. This validation process must be thoroughly documented, with clear explanations of how potential false positives were identified and eliminated during the screening cascade.
The accelerated pace of HTE-driven discovery often creates tension with established regulatory timelines. While HTE can rapidly identify promising compounds, regulatory review processes have not necessarily evolved at the same rate. Forward-thinking pharmaceutical companies are increasingly engaging in early regulatory consultations to establish acceptable parameters for HTE-derived evidence and to develop regulatory strategies that accommodate the unique aspects of HTE-discovered compounds.
Intellectual property considerations intersect with regulatory requirements for HTE-discovered drugs in complex ways. The patentability of compounds identified through algorithmic analysis of massive screening datasets may face different standards of novelty and non-obviousness. Regulatory submissions must carefully address these IP dimensions, particularly when artificial intelligence or machine learning tools have contributed to the identification of novel chemical entities.
International harmonization efforts are gradually addressing the regulatory disparities for HTE-discovered drugs across different jurisdictions. Initiatives like the International Council for Harmonisation (ICH) are working to establish consistent standards for evaluating compounds identified through advanced screening technologies, though significant regional variations persist in how regulatory authorities assess the validity of HTE-derived data.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!