High-Throughput Experimentation for Biocompatibility Testing
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HTE Biocompatibility Testing Background and Objectives
High-Throughput Experimentation (HTE) for biocompatibility testing represents a paradigm shift in the evaluation of materials and devices intended for medical applications. The field has evolved significantly from traditional single-sample testing methodologies that dominated the 20th century to advanced parallel processing techniques that emerged in the early 2000s. This evolution has been driven by increasing regulatory demands, growing complexity of medical devices, and the need for more efficient development cycles in the healthcare industry.
The technological trajectory of HTE in biocompatibility assessment has accelerated particularly in the past decade, with significant advancements in microfluidic platforms, automated cell culture systems, and high-content imaging technologies. These developments have enabled researchers to simultaneously evaluate multiple parameters across numerous samples, dramatically reducing the time and resources required for comprehensive biocompatibility profiling.
Current trends indicate a convergence of HTE with artificial intelligence and machine learning algorithms, enabling predictive modeling of biocompatibility outcomes based on material properties and structural characteristics. This integration represents the next frontier in biocompatibility testing, potentially allowing for in silico screening prior to physical testing.
The primary objective of HTE for biocompatibility testing is to establish rapid, reliable, and reproducible methodologies for assessing the biological safety of materials, devices, and pharmaceutical formulations. This includes the development of standardized protocols that can be validated across different laboratories and regulatory environments.
Secondary objectives include reducing animal testing through the implementation of advanced in vitro models that more accurately replicate human physiological responses, thereby addressing both ethical concerns and the limitations of traditional animal models in predicting human outcomes.
A critical goal is the establishment of correlations between high-throughput screening results and long-term clinical performance, enabling more accurate prediction of material behavior in vivo. This requires the development of sophisticated bioinformatics tools capable of integrating diverse data sets and identifying meaningful patterns.
The technical objectives also encompass the miniaturization of testing platforms to reduce sample requirements and increase throughput, the development of non-destructive testing methodologies for continuous monitoring, and the integration of real-time data analysis capabilities for immediate feedback during experimentation.
Ultimately, the field aims to create a comprehensive framework for biocompatibility assessment that combines speed, accuracy, and predictive power, thereby accelerating the development of safe and effective medical technologies while reducing development costs and time-to-market.
The technological trajectory of HTE in biocompatibility assessment has accelerated particularly in the past decade, with significant advancements in microfluidic platforms, automated cell culture systems, and high-content imaging technologies. These developments have enabled researchers to simultaneously evaluate multiple parameters across numerous samples, dramatically reducing the time and resources required for comprehensive biocompatibility profiling.
Current trends indicate a convergence of HTE with artificial intelligence and machine learning algorithms, enabling predictive modeling of biocompatibility outcomes based on material properties and structural characteristics. This integration represents the next frontier in biocompatibility testing, potentially allowing for in silico screening prior to physical testing.
The primary objective of HTE for biocompatibility testing is to establish rapid, reliable, and reproducible methodologies for assessing the biological safety of materials, devices, and pharmaceutical formulations. This includes the development of standardized protocols that can be validated across different laboratories and regulatory environments.
Secondary objectives include reducing animal testing through the implementation of advanced in vitro models that more accurately replicate human physiological responses, thereby addressing both ethical concerns and the limitations of traditional animal models in predicting human outcomes.
A critical goal is the establishment of correlations between high-throughput screening results and long-term clinical performance, enabling more accurate prediction of material behavior in vivo. This requires the development of sophisticated bioinformatics tools capable of integrating diverse data sets and identifying meaningful patterns.
The technical objectives also encompass the miniaturization of testing platforms to reduce sample requirements and increase throughput, the development of non-destructive testing methodologies for continuous monitoring, and the integration of real-time data analysis capabilities for immediate feedback during experimentation.
Ultimately, the field aims to create a comprehensive framework for biocompatibility assessment that combines speed, accuracy, and predictive power, thereby accelerating the development of safe and effective medical technologies while reducing development costs and time-to-market.
Market Analysis for Accelerated Biocompatibility Screening
The global market for biocompatibility testing is experiencing significant growth, driven by increasing regulatory requirements and the expanding medical device and pharmaceutical industries. Currently valued at approximately $1.5 billion, this market is projected to reach $2.3 billion by 2027, representing a compound annual growth rate of 8.9%. High-throughput experimentation (HTE) for biocompatibility testing represents a particularly dynamic segment within this broader market.
Demand for accelerated biocompatibility screening solutions stems primarily from medical device manufacturers, pharmaceutical companies, and contract research organizations (CROs). These stakeholders face mounting pressure to reduce time-to-market while ensuring compliance with increasingly stringent regulatory standards such as ISO 10993 and FDA guidelines. Traditional biocompatibility testing methods typically require 4-6 months to complete, creating a substantial bottleneck in product development pipelines.
The North American market currently dominates with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 10.5% annually, driven by expanding healthcare infrastructure and increasing outsourcing of biocompatibility testing to countries like China and India.
Key market segments for high-throughput biocompatibility testing include cytotoxicity (largest segment at 32%), sensitization (18%), irritation (15%), systemic toxicity (12%), and other specialized tests (23%). The in vitro testing segment is growing particularly rapidly as regulatory bodies increasingly accept alternative testing methods that reduce animal testing requirements.
Industry surveys indicate that 76% of medical device manufacturers consider accelerated biocompatibility testing a high priority for their R&D investments. The potential for cost savings is substantial, with high-throughput methods potentially reducing testing expenses by 30-40% while decreasing development timelines by up to 60%.
Market challenges include the high initial investment required for advanced high-throughput systems, regulatory uncertainty regarding the acceptance of novel testing methodologies, and technical limitations in replicating complex biological interactions in accelerated testing environments. Despite these challenges, venture capital investment in biocompatibility testing technologies has increased by 45% over the past three years.
The subscription-based testing services model is gaining traction, with an estimated market size of $320 million and projected annual growth of 12.3%. This model allows smaller companies to access advanced testing capabilities without significant capital expenditure, further driving market expansion and technology adoption across the industry.
Demand for accelerated biocompatibility screening solutions stems primarily from medical device manufacturers, pharmaceutical companies, and contract research organizations (CROs). These stakeholders face mounting pressure to reduce time-to-market while ensuring compliance with increasingly stringent regulatory standards such as ISO 10993 and FDA guidelines. Traditional biocompatibility testing methods typically require 4-6 months to complete, creating a substantial bottleneck in product development pipelines.
The North American market currently dominates with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 10.5% annually, driven by expanding healthcare infrastructure and increasing outsourcing of biocompatibility testing to countries like China and India.
Key market segments for high-throughput biocompatibility testing include cytotoxicity (largest segment at 32%), sensitization (18%), irritation (15%), systemic toxicity (12%), and other specialized tests (23%). The in vitro testing segment is growing particularly rapidly as regulatory bodies increasingly accept alternative testing methods that reduce animal testing requirements.
Industry surveys indicate that 76% of medical device manufacturers consider accelerated biocompatibility testing a high priority for their R&D investments. The potential for cost savings is substantial, with high-throughput methods potentially reducing testing expenses by 30-40% while decreasing development timelines by up to 60%.
Market challenges include the high initial investment required for advanced high-throughput systems, regulatory uncertainty regarding the acceptance of novel testing methodologies, and technical limitations in replicating complex biological interactions in accelerated testing environments. Despite these challenges, venture capital investment in biocompatibility testing technologies has increased by 45% over the past three years.
The subscription-based testing services model is gaining traction, with an estimated market size of $320 million and projected annual growth of 12.3%. This model allows smaller companies to access advanced testing capabilities without significant capital expenditure, further driving market expansion and technology adoption across the industry.
Current Challenges in High-Throughput Biocompatibility Assessment
Despite significant advancements in high-throughput experimentation (HTE) for biocompatibility testing, several critical challenges continue to impede widespread implementation and reliability. The primary obstacle remains the complexity of biological systems, which are inherently variable and difficult to model accurately in high-throughput formats. This biological variability often leads to inconsistent results across different testing platforms and laboratories, undermining standardization efforts.
Scale-up challenges present another significant hurdle, as many high-throughput methods developed in research settings fail to translate effectively to industrial-scale applications. The miniaturization required for HTE can introduce artifacts that do not manifest in traditional testing formats, potentially leading to false positives or negatives when scaled to clinical applications.
Data management and analysis represent increasingly formidable challenges as HTE generates massive datasets that exceed traditional analytical capabilities. Current computational infrastructure often struggles with the volume, velocity, and variety of data produced, while sophisticated algorithms for pattern recognition and predictive modeling remain in developmental stages. The lack of standardized data formats and reporting protocols further complicates cross-platform comparisons and meta-analyses.
Regulatory acceptance constitutes a major bottleneck in the adoption of HTE methodologies. Regulatory agencies worldwide have been cautious in accepting high-throughput data as replacements for established testing protocols, citing concerns about validation, reproducibility, and clinical relevance. This regulatory uncertainty discourages investment in novel HTE technologies and slows their integration into standard testing workflows.
The correlation between in vitro high-throughput results and in vivo outcomes remains problematic. Many HTE platforms fail to adequately recapitulate the complex microenvironments and systemic interactions present in living organisms, limiting their predictive power for actual biocompatibility in clinical settings. This translation gap undermines confidence in HTE-derived safety assessments.
Cost considerations present ongoing challenges, particularly for smaller organizations. While HTE promises long-term cost reductions through increased efficiency, the initial investment in specialized equipment, software, and expertise can be prohibitive. Additionally, the rapid pace of technological advancement often renders systems obsolete before organizations can fully recoup their investments.
Workforce training represents an underappreciated challenge, as the interdisciplinary nature of HTE requires expertise spanning biology, materials science, engineering, data science, and regulatory affairs. Educational institutions and professional development programs have not kept pace with industry needs, creating a significant skills gap that hampers effective implementation and interpretation of high-throughput biocompatibility assessments.
Scale-up challenges present another significant hurdle, as many high-throughput methods developed in research settings fail to translate effectively to industrial-scale applications. The miniaturization required for HTE can introduce artifacts that do not manifest in traditional testing formats, potentially leading to false positives or negatives when scaled to clinical applications.
Data management and analysis represent increasingly formidable challenges as HTE generates massive datasets that exceed traditional analytical capabilities. Current computational infrastructure often struggles with the volume, velocity, and variety of data produced, while sophisticated algorithms for pattern recognition and predictive modeling remain in developmental stages. The lack of standardized data formats and reporting protocols further complicates cross-platform comparisons and meta-analyses.
Regulatory acceptance constitutes a major bottleneck in the adoption of HTE methodologies. Regulatory agencies worldwide have been cautious in accepting high-throughput data as replacements for established testing protocols, citing concerns about validation, reproducibility, and clinical relevance. This regulatory uncertainty discourages investment in novel HTE technologies and slows their integration into standard testing workflows.
The correlation between in vitro high-throughput results and in vivo outcomes remains problematic. Many HTE platforms fail to adequately recapitulate the complex microenvironments and systemic interactions present in living organisms, limiting their predictive power for actual biocompatibility in clinical settings. This translation gap undermines confidence in HTE-derived safety assessments.
Cost considerations present ongoing challenges, particularly for smaller organizations. While HTE promises long-term cost reductions through increased efficiency, the initial investment in specialized equipment, software, and expertise can be prohibitive. Additionally, the rapid pace of technological advancement often renders systems obsolete before organizations can fully recoup their investments.
Workforce training represents an underappreciated challenge, as the interdisciplinary nature of HTE requires expertise spanning biology, materials science, engineering, data science, and regulatory affairs. Educational institutions and professional development programs have not kept pace with industry needs, creating a significant skills gap that hampers effective implementation and interpretation of high-throughput biocompatibility assessments.
Current HTE Platforms for Biocompatibility Evaluation
01 High-throughput screening methods for biocompatibility assessment
Advanced screening platforms enable rapid evaluation of material biocompatibility through parallel testing of multiple samples. These systems incorporate automated sample handling, data acquisition, and analysis to efficiently assess cellular responses to biomaterials. The methods typically involve cell culture systems with various detection technologies to measure cell viability, proliferation, and functional responses when exposed to test materials, significantly accelerating the biocompatibility evaluation process.- High-throughput screening methods for biocompatibility assessment: Various high-throughput screening methods can be employed to assess the biocompatibility of materials and compounds. These methods involve automated testing of multiple samples simultaneously, allowing for rapid evaluation of biological responses. Such approaches typically utilize cell-based assays, tissue models, or molecular interaction studies to determine potential toxicity, inflammatory responses, or other biological effects that indicate biocompatibility.
- Microfluidic platforms for biocompatibility testing: Microfluidic systems provide efficient platforms for high-throughput biocompatibility testing by enabling precise control over experimental conditions in miniaturized environments. These platforms allow for the simultaneous testing of multiple parameters with minimal sample volumes, making them ideal for screening biomaterials and medical devices. The integration of sensors and imaging capabilities within these systems enables real-time monitoring of cellular responses to test materials.
- Automated data analysis systems for biocompatibility experiments: Advanced computational systems and algorithms are utilized to process and analyze the large datasets generated from high-throughput biocompatibility experiments. These systems employ machine learning, artificial intelligence, and statistical methods to identify patterns, correlations, and potential biocompatibility issues. Automated analysis significantly reduces the time required for data interpretation and enables more objective assessment of experimental results.
- Parallel testing of biomaterials with multiple cell lines: High-throughput experimentation for biocompatibility often involves parallel testing of materials against multiple cell lines or tissue types. This approach provides comprehensive information about how different biological systems respond to the test materials. By simultaneously evaluating responses across various cell types, researchers can better predict in vivo biocompatibility and identify potential tissue-specific adverse reactions.
- Integrated biocompatibility testing platforms: Integrated systems combine multiple testing modalities into unified platforms for comprehensive biocompatibility assessment. These platforms incorporate various analytical techniques, cell culture systems, and data processing capabilities to provide holistic evaluation of material-biological interactions. Such integration enables efficient workflow management, reduces experimental variability, and allows for standardized testing protocols that can be applied across different materials and biological systems.
02 Microfluidic platforms for biocompatibility testing
Microfluidic devices provide controlled environments for biocompatibility assessment by mimicking physiological conditions. These platforms enable precise manipulation of small fluid volumes and cellular samples, allowing for dynamic testing of material-tissue interactions. The technology supports real-time monitoring of cellular responses to biomaterials under flow conditions that better represent in vivo environments, offering advantages in terms of reduced sample volumes, increased throughput, and improved physiological relevance compared to traditional static testing methods.Expand Specific Solutions03 Automated image-based analysis for biocompatibility evaluation
Advanced imaging systems coupled with automated analysis algorithms enable high-content screening of biomaterial-cell interactions. These systems capture multiple cellular parameters simultaneously through fluorescence microscopy or other imaging modalities, then process the data using machine learning or other computational approaches to quantify biocompatibility metrics. The technology allows for detailed characterization of cell morphology, distribution, and functional responses when in contact with test materials, providing rich datasets for comprehensive biocompatibility assessment.Expand Specific Solutions04 Integrated data management systems for biocompatibility experiments
Specialized software platforms facilitate the management, analysis, and interpretation of large datasets generated in high-throughput biocompatibility testing. These systems integrate experimental design, data acquisition, storage, and analysis functions to streamline the workflow from experiment planning to results interpretation. The technology enables researchers to efficiently handle complex experimental matrices, track sample histories, and apply advanced statistical methods to extract meaningful patterns from biocompatibility studies, supporting data-driven decision making in biomaterial development.Expand Specific Solutions05 Parallel synthesis and testing platforms for biomaterial development
Integrated systems combine high-throughput material synthesis with immediate biocompatibility assessment capabilities. These platforms enable the rapid creation of material libraries with systematic variations in composition or structure, followed by automated testing of their biological performance. The technology accelerates the discovery and optimization of biocompatible materials by establishing direct relationships between material properties and biological responses, significantly reducing development timelines for medical devices, implants, and tissue engineering scaffolds.Expand Specific Solutions
Leading Organizations in HTE Biocompatibility Research
High-Throughput Experimentation (HTE) for biocompatibility testing is evolving rapidly in a growing market driven by pharmaceutical and medical device development needs. The competitive landscape features established players like Corning and Beckman Coulter providing specialized laboratory equipment, alongside innovative biotechnology companies such as Recursion Pharmaceuticals and Just-Evotec Biologics developing AI-integrated platforms. Academic institutions including Harvard, Tsinghua University, and EPFL contribute significant research advancements. The technology is approaching maturity in standardized applications but remains in development for complex biological systems, with companies like HighRes Biosolutions and MGI Tech leading automation integration. Market growth is accelerated by increasing demand for efficient drug discovery processes and medical device testing protocols.
Corning, Inc.
Technical Solution: Corning has developed an advanced high-throughput experimentation platform for biocompatibility testing that leverages their expertise in materials science and cell culture technologies. Their system centers around specialized microplate formats with proprietary surface modifications that enable controlled presentation of test materials to cellular systems. The platform incorporates Corning's Epic® label-free detection technology, which uses optical biosensors to monitor cellular responses to biomaterials in real-time without requiring fluorescent or radioactive labels. This approach allows for continuous monitoring of subtle cellular changes including adhesion, spreading, and morphological alterations that indicate biocompatibility issues. Corning's system integrates with automated liquid handling and high-content imaging systems to enable parallel testing of hundreds of material formulations simultaneously. Their platform includes specialized 3D cell culture capabilities using Corning Matrigel® and other hydrogel systems that better mimic in vivo tissue environments for more physiologically relevant biocompatibility assessment. The company has validated this approach through extensive internal testing and collaborations with medical device manufacturers, demonstrating improved correlation between high-throughput in vitro results and subsequent in vivo biocompatibility outcomes.
Strengths: Exceptional expertise in surface chemistry and material-cell interactions; proprietary label-free detection technology reduces artifacts; superior 3D culture capabilities for physiologically relevant testing. Weaknesses: Platform components may require significant integration work; higher cost of specialized consumables; more limited throughput compared to fully automated robotic systems.
HighRes Biosolutions, Inc.
Technical Solution: HighRes Biosolutions has developed an integrated high-throughput experimentation platform specifically designed for biocompatibility testing. Their system combines robotic automation, microfluidic technology, and advanced imaging capabilities to enable rapid screening of biomaterials against various cell types and tissues. The platform utilizes modular workstations that can be configured for different biocompatibility assays, including cytotoxicity, inflammatory response, and cell adhesion tests. Their proprietary software, Cellario, orchestrates the entire workflow from sample preparation to data analysis, allowing for testing of hundreds of material-cell interactions simultaneously. The system incorporates real-time monitoring capabilities that track cellular responses over extended periods, providing dynamic biocompatibility profiles rather than single-point measurements. This approach has demonstrated a 10-fold increase in testing throughput compared to traditional methods while maintaining high reproducibility and data quality.
Strengths: Highly flexible modular system that can be adapted to various biocompatibility testing protocols; superior integration of robotics with imaging systems; comprehensive data management. Weaknesses: High initial investment cost; requires specialized technical expertise for operation and maintenance; complex setup may limit adoption in smaller research facilities.
Key Innovations in Rapid Biocompatibility Screening Technologies
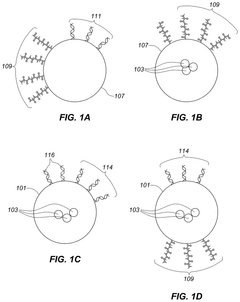
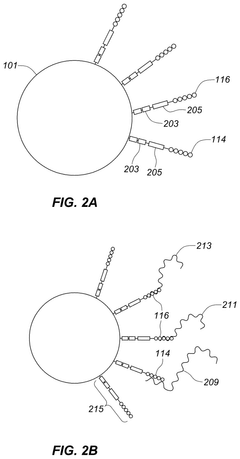
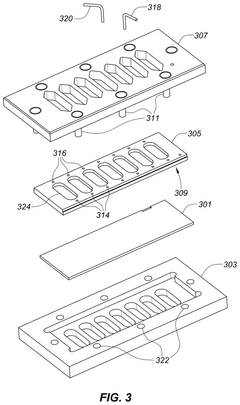
Apparatus for assay, synthesis and storage, and methods of manufacture, use, and manipulation thereof
PatentInactiveEP1920045A2
Innovation
- The development of devices with high-density arrays of through-holes, where reagents can be contained within the holes by capillary action or attached to the walls, allowing for serial or parallel physical, chemical, or biological transformations, and enabling efficient analysis of physical properties of samples.
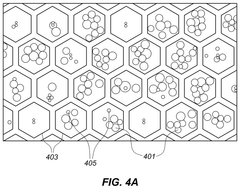
High Throughput Assays for Identifying the Biological Response of a Cell
PatentPendingUS20250236863A1
Innovation
- A method involving microbeads with multiple biological components, including perturbation microbeads for agents and barcodes, spatial-index microbeads for optical labels, and capture microbeads for analytes, allowing for high-throughput analysis by imaging and sequencing to identify cellular responses to perturbations.
Regulatory Framework for HTE Biocompatibility Testing
The regulatory landscape for High-Throughput Experimentation (HTE) in biocompatibility testing is complex and evolving, with frameworks varying significantly across global regions. In the United States, the FDA has established guidelines under the 21 CFR Part 58 (Good Laboratory Practices) that govern preclinical testing, though specific provisions for HTE methodologies remain limited. The agency's recent initiatives, including the Predictive Toxicology Roadmap (2017), signal increasing openness to alternative testing methods that reduce animal testing while maintaining safety standards.
The European Union operates under the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746), which emphasize risk-based approaches to biocompatibility assessment. The European Medicines Agency (EMA) has shown progressive acceptance of HTE data through its Innovation Task Force, particularly when such methods demonstrate superior predictive value compared to traditional testing paradigms.
International harmonization efforts are primarily coordinated through ISO 10993 standards, with the recent ISO 10993-23:2021 specifically addressing in vitro irritation testing methodologies. These standards increasingly acknowledge the validity of high-throughput screening approaches when properly validated, though full regulatory acceptance remains a work in progress.
Validation requirements present significant hurdles for HTE implementation in regulatory submissions. Regulatory bodies typically require extensive correlation studies between high-throughput methods and traditional endpoints, with demonstration of reproducibility across multiple laboratories. The OECD Guidelines for the Testing of Chemicals provide frameworks for validation that increasingly incorporate alternative testing strategies.
Recent regulatory milestones include the FDA's Modernization Act 2.0 (2022), which explicitly encourages non-animal testing methods, and the EU's commitment to phasing out animal testing under the European Green Deal. These developments create favorable conditions for HTE adoption in biocompatibility assessment.
Challenges remain in standardizing data interpretation across the diverse array of HTE platforms. Regulatory agencies increasingly request comprehensive data management plans that address how high-dimensional data will be analyzed, interpreted, and translated into safety assessments. The FDA's Biocompatibility Guidance Document (updated 2020) acknowledges computational approaches but emphasizes the need for transparent methodology documentation.
For companies implementing HTE in biocompatibility testing, early engagement with regulatory authorities through pre-submission consultations has proven effective in establishing acceptable protocols. Successful regulatory submissions typically include bridging studies that demonstrate concordance between traditional and high-throughput methodologies.
The European Union operates under the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746), which emphasize risk-based approaches to biocompatibility assessment. The European Medicines Agency (EMA) has shown progressive acceptance of HTE data through its Innovation Task Force, particularly when such methods demonstrate superior predictive value compared to traditional testing paradigms.
International harmonization efforts are primarily coordinated through ISO 10993 standards, with the recent ISO 10993-23:2021 specifically addressing in vitro irritation testing methodologies. These standards increasingly acknowledge the validity of high-throughput screening approaches when properly validated, though full regulatory acceptance remains a work in progress.
Validation requirements present significant hurdles for HTE implementation in regulatory submissions. Regulatory bodies typically require extensive correlation studies between high-throughput methods and traditional endpoints, with demonstration of reproducibility across multiple laboratories. The OECD Guidelines for the Testing of Chemicals provide frameworks for validation that increasingly incorporate alternative testing strategies.
Recent regulatory milestones include the FDA's Modernization Act 2.0 (2022), which explicitly encourages non-animal testing methods, and the EU's commitment to phasing out animal testing under the European Green Deal. These developments create favorable conditions for HTE adoption in biocompatibility assessment.
Challenges remain in standardizing data interpretation across the diverse array of HTE platforms. Regulatory agencies increasingly request comprehensive data management plans that address how high-dimensional data will be analyzed, interpreted, and translated into safety assessments. The FDA's Biocompatibility Guidance Document (updated 2020) acknowledges computational approaches but emphasizes the need for transparent methodology documentation.
For companies implementing HTE in biocompatibility testing, early engagement with regulatory authorities through pre-submission consultations has proven effective in establishing acceptable protocols. Successful regulatory submissions typically include bridging studies that demonstrate concordance between traditional and high-throughput methodologies.
Ethical Considerations in Accelerated Biocompatibility Assessment
The acceleration of biocompatibility testing through high-throughput experimentation raises significant ethical considerations that must be addressed before widespread implementation. The primary concern revolves around the potential compromise of safety standards in pursuit of efficiency. While traditional biocompatibility testing methodologies have established protocols developed over decades, accelerated approaches may inadvertently bypass critical safety checkpoints, potentially exposing patients to unforeseen risks.
The balance between innovation speed and human safety presents a fundamental ethical dilemma. Regulatory frameworks worldwide emphasize the primacy of patient safety, yet market pressures and competitive advantages often push for faster development cycles. This tension requires careful navigation to ensure that high-throughput methodologies maintain or enhance safety profiles rather than diminishing them.
Informed consent represents another critical ethical dimension. When accelerated testing methods are employed, research participants and eventual patients must be fully informed about the novel nature of the testing protocols used to validate the materials they encounter. Transparency regarding the limitations and potential unknowns of high-throughput testing is essential for maintaining ethical integrity in the biomedical field.
Data privacy concerns emerge as high-throughput experimentation generates vast datasets of biological responses. These datasets may contain sensitive information about genetic predispositions or individual biological responses that require robust protection. The ethical management of this data, including appropriate anonymization, secure storage, and controlled access, becomes increasingly important as testing scales up.
Animal welfare considerations also factor prominently in ethical discussions. While high-throughput methods often aim to reduce animal testing through in vitro alternatives, the validation of these alternatives frequently requires comparative animal studies. Ensuring that these studies adhere to the 3Rs principle (Replacement, Reduction, Refinement) remains an ethical imperative even as testing throughput increases.
Global equity in access to advanced testing technologies presents another ethical challenge. If high-throughput biocompatibility testing becomes the new standard, ensuring that these technologies are accessible to researchers and manufacturers in developing regions becomes crucial to prevent widening the global healthcare divide. Ethical frameworks must consider how these technologies can be deployed equitably across different economic contexts.
Finally, the ethical responsibility for long-term monitoring cannot be overlooked. Accelerated testing inherently compresses the timeline for observing potential adverse effects, necessitating robust post-market surveillance systems. The ethical obligation to monitor outcomes extends beyond regulatory approval and must be integrated into the entire product lifecycle when high-throughput testing methodologies are employed.
The balance between innovation speed and human safety presents a fundamental ethical dilemma. Regulatory frameworks worldwide emphasize the primacy of patient safety, yet market pressures and competitive advantages often push for faster development cycles. This tension requires careful navigation to ensure that high-throughput methodologies maintain or enhance safety profiles rather than diminishing them.
Informed consent represents another critical ethical dimension. When accelerated testing methods are employed, research participants and eventual patients must be fully informed about the novel nature of the testing protocols used to validate the materials they encounter. Transparency regarding the limitations and potential unknowns of high-throughput testing is essential for maintaining ethical integrity in the biomedical field.
Data privacy concerns emerge as high-throughput experimentation generates vast datasets of biological responses. These datasets may contain sensitive information about genetic predispositions or individual biological responses that require robust protection. The ethical management of this data, including appropriate anonymization, secure storage, and controlled access, becomes increasingly important as testing scales up.
Animal welfare considerations also factor prominently in ethical discussions. While high-throughput methods often aim to reduce animal testing through in vitro alternatives, the validation of these alternatives frequently requires comparative animal studies. Ensuring that these studies adhere to the 3Rs principle (Replacement, Reduction, Refinement) remains an ethical imperative even as testing throughput increases.
Global equity in access to advanced testing technologies presents another ethical challenge. If high-throughput biocompatibility testing becomes the new standard, ensuring that these technologies are accessible to researchers and manufacturers in developing regions becomes crucial to prevent widening the global healthcare divide. Ethical frameworks must consider how these technologies can be deployed equitably across different economic contexts.
Finally, the ethical responsibility for long-term monitoring cannot be overlooked. Accelerated testing inherently compresses the timeline for observing potential adverse effects, necessitating robust post-market surveillance systems. The ethical obligation to monitor outcomes extends beyond regulatory approval and must be integrated into the entire product lifecycle when high-throughput testing methodologies are employed.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!