How Alkylations Are Transforming the Petrochemical Industry?
JUL 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkylation Evolution
Alkylation has undergone a significant evolution in the petrochemical industry, transforming from a niche process to a cornerstone technology. The journey began in the 1930s when the first commercial alkylation units were introduced, primarily for the production of high-octane aviation fuel during World War II. This marked the beginning of a technological revolution that would reshape the industry.
In the post-war era, alkylation found new applications in the production of motor gasoline, becoming an essential process for improving fuel quality. The 1950s and 1960s saw rapid advancements in catalyst technology, with the introduction of hydrofluoric acid (HF) and sulfuric acid (H2SO4) as catalysts, each offering unique advantages in terms of reaction efficiency and product quality.
The 1970s brought about a shift towards environmental consciousness, prompting refineries to adapt their alkylation processes to meet stricter regulations. This period saw the development of improved safety measures and more efficient reactor designs, addressing concerns about the handling of hazardous catalysts.
The 1980s and 1990s witnessed a surge in research focused on solid acid catalysts, aiming to replace the liquid acid catalysts that posed environmental and safety risks. While these efforts showed promise, liquid acid catalysts remained dominant due to their superior performance and cost-effectiveness.
The turn of the millennium brought renewed interest in alkylation technology, driven by the increasing demand for cleaner-burning fuels and the need for more efficient refinery operations. This period saw the emergence of novel reactor designs, such as the stratified bed reactor, which offered improved mixing and reaction control.
In recent years, the focus has shifted towards sustainability and process intensification. Advanced control systems and real-time monitoring have been implemented to optimize alkylation units, reducing energy consumption and improving product yields. Additionally, there has been a resurgence of interest in solid acid catalysts, with new materials and manufacturing techniques showing potential for commercial viability.
The latest frontier in alkylation evolution is the development of ionic liquid catalysts. These novel materials promise to combine the efficiency of liquid acid catalysts with improved safety and environmental profiles. While still in the early stages of commercialization, ionic liquid catalysts represent a potentially transformative technology for the future of alkylation.
Throughout its evolution, alkylation has consistently adapted to meet the changing needs of the petrochemical industry, from wartime fuel production to modern environmental standards. This ongoing transformation underscores the critical role of alkylation in shaping the industry's future, driving innovation in catalyst technology, reactor design, and process optimization.
In the post-war era, alkylation found new applications in the production of motor gasoline, becoming an essential process for improving fuel quality. The 1950s and 1960s saw rapid advancements in catalyst technology, with the introduction of hydrofluoric acid (HF) and sulfuric acid (H2SO4) as catalysts, each offering unique advantages in terms of reaction efficiency and product quality.
The 1970s brought about a shift towards environmental consciousness, prompting refineries to adapt their alkylation processes to meet stricter regulations. This period saw the development of improved safety measures and more efficient reactor designs, addressing concerns about the handling of hazardous catalysts.
The 1980s and 1990s witnessed a surge in research focused on solid acid catalysts, aiming to replace the liquid acid catalysts that posed environmental and safety risks. While these efforts showed promise, liquid acid catalysts remained dominant due to their superior performance and cost-effectiveness.
The turn of the millennium brought renewed interest in alkylation technology, driven by the increasing demand for cleaner-burning fuels and the need for more efficient refinery operations. This period saw the emergence of novel reactor designs, such as the stratified bed reactor, which offered improved mixing and reaction control.
In recent years, the focus has shifted towards sustainability and process intensification. Advanced control systems and real-time monitoring have been implemented to optimize alkylation units, reducing energy consumption and improving product yields. Additionally, there has been a resurgence of interest in solid acid catalysts, with new materials and manufacturing techniques showing potential for commercial viability.
The latest frontier in alkylation evolution is the development of ionic liquid catalysts. These novel materials promise to combine the efficiency of liquid acid catalysts with improved safety and environmental profiles. While still in the early stages of commercialization, ionic liquid catalysts represent a potentially transformative technology for the future of alkylation.
Throughout its evolution, alkylation has consistently adapted to meet the changing needs of the petrochemical industry, from wartime fuel production to modern environmental standards. This ongoing transformation underscores the critical role of alkylation in shaping the industry's future, driving innovation in catalyst technology, reactor design, and process optimization.
Petrochemical Demand
The petrochemical industry has witnessed a significant surge in demand over the past decade, driven by global economic growth and increasing consumption of plastic products. This trend is particularly evident in emerging markets, where rapid industrialization and urbanization have fueled the need for petrochemical-based materials. The alkylation process, a key technology in petrochemical production, has played a crucial role in meeting this growing demand.
Alkylation products, such as high-octane gasoline components and various chemical intermediates, have seen a steady increase in demand across multiple sectors. The automotive industry, for instance, has been a major consumer of alkylate gasoline due to its superior performance characteristics and lower emissions. As environmental regulations become more stringent worldwide, the demand for cleaner-burning fuels has further boosted the alkylation market.
In the plastics and polymers sector, alkylation-derived products serve as essential building blocks for a wide range of applications. The packaging industry, in particular, has been a significant driver of demand, with alkylated materials finding extensive use in food packaging, beverage containers, and consumer goods packaging. The versatility and cost-effectiveness of these materials have contributed to their widespread adoption across various industries.
The construction and infrastructure sectors have also contributed to the growing demand for alkylation products. Alkylated materials are used in the production of insulation, sealants, and adhesives, which are essential components in modern construction practices. As urbanization continues to accelerate globally, the demand for these materials is expected to maintain its upward trajectory.
Furthermore, the textile industry has increasingly incorporated alkylation-derived products in the manufacturing of synthetic fibers and fabrics. The superior properties of these materials, such as durability and resistance to chemicals, have made them popular choices in both consumer and industrial applications. This trend has been particularly pronounced in developing economies, where the textile sector plays a significant role in economic growth.
The pharmaceutical and personal care industries have also contributed to the rising demand for alkylation products. These sectors utilize alkylated compounds in the production of various drugs, cosmetics, and personal care items. As consumer awareness of health and wellness grows, the demand for specialized pharmaceutical and personal care products has increased, further driving the need for alkylation technologies.
Alkylation products, such as high-octane gasoline components and various chemical intermediates, have seen a steady increase in demand across multiple sectors. The automotive industry, for instance, has been a major consumer of alkylate gasoline due to its superior performance characteristics and lower emissions. As environmental regulations become more stringent worldwide, the demand for cleaner-burning fuels has further boosted the alkylation market.
In the plastics and polymers sector, alkylation-derived products serve as essential building blocks for a wide range of applications. The packaging industry, in particular, has been a significant driver of demand, with alkylated materials finding extensive use in food packaging, beverage containers, and consumer goods packaging. The versatility and cost-effectiveness of these materials have contributed to their widespread adoption across various industries.
The construction and infrastructure sectors have also contributed to the growing demand for alkylation products. Alkylated materials are used in the production of insulation, sealants, and adhesives, which are essential components in modern construction practices. As urbanization continues to accelerate globally, the demand for these materials is expected to maintain its upward trajectory.
Furthermore, the textile industry has increasingly incorporated alkylation-derived products in the manufacturing of synthetic fibers and fabrics. The superior properties of these materials, such as durability and resistance to chemicals, have made them popular choices in both consumer and industrial applications. This trend has been particularly pronounced in developing economies, where the textile sector plays a significant role in economic growth.
The pharmaceutical and personal care industries have also contributed to the rising demand for alkylation products. These sectors utilize alkylated compounds in the production of various drugs, cosmetics, and personal care items. As consumer awareness of health and wellness grows, the demand for specialized pharmaceutical and personal care products has increased, further driving the need for alkylation technologies.
Alkylation Challenges
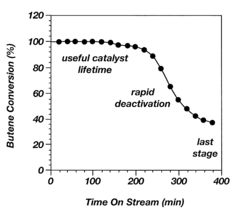
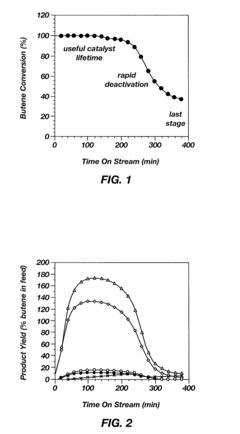
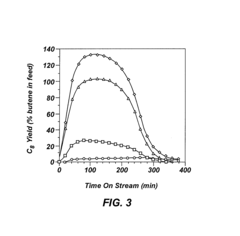
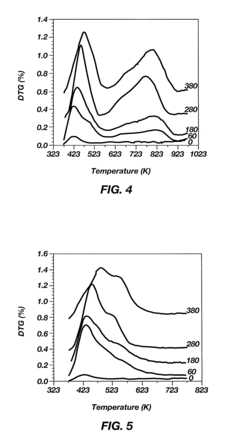
Alkylation processes in the petrochemical industry face several significant challenges that impact their efficiency, cost-effectiveness, and environmental sustainability. One of the primary concerns is catalyst deactivation, which occurs due to the formation of heavy hydrocarbon deposits on the catalyst surface. This phenomenon, known as coking, reduces catalyst activity and selectivity, leading to decreased product quality and increased operational costs.
Another major challenge is the corrosive nature of the acids used in traditional alkylation processes. Hydrofluoric acid (HF) and sulfuric acid (H2SO4) are commonly employed catalysts, but they pose severe safety and environmental risks. HF, in particular, is extremely hazardous, requiring stringent safety measures and specialized handling procedures. The potential for acid releases or leaks presents a constant threat to worker safety and surrounding communities.
Temperature control during alkylation reactions is crucial yet challenging. The highly exothermic nature of these reactions necessitates efficient heat removal systems to prevent runaway reactions and maintain optimal product selectivity. Inadequate temperature control can lead to the formation of undesired byproducts, reducing overall process efficiency and product quality.
The petrochemical industry also grapples with feedstock quality and variability. Alkylation processes are sensitive to impurities in the feedstock, which can adversely affect catalyst performance and product specifications. Fluctuations in feedstock composition require constant process adjustments, adding complexity to operations and potentially impacting product consistency.
Water management presents another significant challenge in alkylation units. The presence of water can lead to acid dilution, corrosion of equipment, and the formation of emulsions that are difficult to separate. Effective water removal and management systems are essential to maintain process efficiency and equipment integrity.
Environmental regulations pose increasing challenges for alkylation processes. Stringent emissions standards and waste disposal regulations require petrochemical companies to invest in advanced pollution control technologies and develop more environmentally friendly alkylation methods. This includes efforts to reduce volatile organic compound (VOC) emissions and minimize the generation of hazardous waste streams.
Lastly, the industry faces the challenge of improving energy efficiency in alkylation processes. These operations are energy-intensive, contributing significantly to the overall carbon footprint of petrochemical facilities. Developing more energy-efficient technologies and integrating heat recovery systems are crucial steps towards reducing energy consumption and operational costs while meeting sustainability goals.
Another major challenge is the corrosive nature of the acids used in traditional alkylation processes. Hydrofluoric acid (HF) and sulfuric acid (H2SO4) are commonly employed catalysts, but they pose severe safety and environmental risks. HF, in particular, is extremely hazardous, requiring stringent safety measures and specialized handling procedures. The potential for acid releases or leaks presents a constant threat to worker safety and surrounding communities.
Temperature control during alkylation reactions is crucial yet challenging. The highly exothermic nature of these reactions necessitates efficient heat removal systems to prevent runaway reactions and maintain optimal product selectivity. Inadequate temperature control can lead to the formation of undesired byproducts, reducing overall process efficiency and product quality.
The petrochemical industry also grapples with feedstock quality and variability. Alkylation processes are sensitive to impurities in the feedstock, which can adversely affect catalyst performance and product specifications. Fluctuations in feedstock composition require constant process adjustments, adding complexity to operations and potentially impacting product consistency.
Water management presents another significant challenge in alkylation units. The presence of water can lead to acid dilution, corrosion of equipment, and the formation of emulsions that are difficult to separate. Effective water removal and management systems are essential to maintain process efficiency and equipment integrity.
Environmental regulations pose increasing challenges for alkylation processes. Stringent emissions standards and waste disposal regulations require petrochemical companies to invest in advanced pollution control technologies and develop more environmentally friendly alkylation methods. This includes efforts to reduce volatile organic compound (VOC) emissions and minimize the generation of hazardous waste streams.
Lastly, the industry faces the challenge of improving energy efficiency in alkylation processes. These operations are energy-intensive, contributing significantly to the overall carbon footprint of petrochemical facilities. Developing more energy-efficient technologies and integrating heat recovery systems are crucial steps towards reducing energy consumption and operational costs while meeting sustainability goals.
Current Processes
01 Alkylation processes in organic synthesis
Alkylation is a fundamental process in organic chemistry involving the transfer of an alkyl group from one molecule to another. It is widely used in the synthesis of various organic compounds, including pharmaceuticals, polymers, and fine chemicals. The process typically involves the reaction of an alkyl halide or other alkylating agent with a nucleophile, often under specific reaction conditions to control selectivity and yield.- Alkylation processes in organic synthesis: Alkylation is a fundamental process in organic chemistry involving the transfer of an alkyl group from one molecule to another. It is widely used in the synthesis of various organic compounds, including pharmaceuticals, polymers, and fine chemicals. The process typically involves the reaction of an alkyl halide or other alkylating agent with a nucleophile, often under specific reaction conditions to control selectivity and yield.
- Catalytic alkylation in petrochemical industry: Catalytic alkylation is a crucial process in the petrochemical industry, particularly for the production of high-octane gasoline components. This process typically involves the reaction of isobutane with light olefins in the presence of a strong acid catalyst, such as sulfuric acid or hydrofluoric acid. The resulting alkylate product is a valuable blending component for motor fuels due to its high octane rating and low vapor pressure.
- Alkylation in pharmaceutical synthesis: Alkylation reactions play a significant role in the synthesis of pharmaceutical compounds. These reactions are used to introduce alkyl groups into drug molecules, modifying their properties such as lipophilicity, metabolic stability, and biological activity. Various alkylation strategies, including N-alkylation, O-alkylation, and C-alkylation, are employed in the development of novel drug candidates and the optimization of existing pharmaceuticals.
- Alkylation in polymer chemistry: Alkylation reactions are important in polymer chemistry for the modification of polymer properties and the synthesis of specialized monomers. These reactions can be used to introduce functional groups, alter the solubility or thermal properties of polymers, or create branched and cross-linked structures. Alkylation processes are employed in the production of various types of polymers, including polyethers, polyesters, and specialty resins used in coatings and adhesives.
- Green chemistry approaches to alkylation: Recent developments in alkylation chemistry have focused on more environmentally friendly and sustainable approaches. These include the use of alternative solvents, such as ionic liquids or supercritical fluids, to replace traditional organic solvents. Additionally, researchers are exploring new catalytic systems, including solid acid catalysts and biocatalysts, to improve efficiency and reduce waste in alkylation processes. These green chemistry approaches aim to make alkylation reactions more sustainable and less harmful to the environment.
02 Catalytic alkylation in petrochemical industry
Catalytic alkylation is a crucial process in the petrochemical industry, particularly for the production of high-octane gasoline components. This process typically involves the reaction of light olefins with isobutane in the presence of a strong acid catalyst, such as sulfuric acid or hydrofluoric acid. The resulting alkylate is a valuable blending component for motor fuels due to its high octane rating and low vapor pressure.Expand Specific Solutions03 Alkylation in pharmaceutical synthesis
Alkylation reactions play a crucial role in the synthesis of pharmaceutical compounds. These reactions are used to introduce alkyl groups into drug molecules, modifying their properties such as lipophilicity, binding affinity, and metabolic stability. Various alkylation strategies, including N-alkylation, O-alkylation, and C-alkylation, are employed in the development of new drugs and the optimization of existing ones.Expand Specific Solutions04 Alkylation in polymer chemistry
Alkylation reactions are important in polymer chemistry for the modification of polymer properties and the synthesis of specialized monomers. These reactions can be used to introduce functional groups, alter the solubility or thermal properties of polymers, or create branched and cross-linked structures. Controlled alkylation of polymers can lead to materials with tailored characteristics for specific applications in areas such as coatings, adhesives, and biomaterials.Expand Specific Solutions05 Green chemistry approaches to alkylation
Recent developments in alkylation chemistry have focused on more environmentally friendly and sustainable approaches. These include the use of recyclable catalysts, ionic liquids as reaction media, and the development of solvent-free or aqueous-phase alkylation processes. Biocatalytic alkylation using enzymes and the application of flow chemistry for continuous alkylation processes are also emerging as promising green chemistry alternatives to traditional alkylation methods.Expand Specific Solutions
Industry Leaders
The alkylation technology in the petrochemical industry is currently in a mature stage, with a growing market driven by increasing demand for high-octane gasoline and petrochemical products. The global alkylation market size is projected to expand significantly in the coming years, fueled by rising energy consumption and stringent fuel quality regulations. Key players like China Petroleum & Chemical Corp., ExxonMobil, and Saudi Aramco are at the forefront of technological advancements, investing heavily in research and development to improve process efficiency and product quality. These companies, along with UOP LLC and Sinopec Research Institute, are continuously innovating to enhance catalytic processes, reduce environmental impact, and optimize production costs, solidifying their positions in this competitive landscape.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced alkylation technologies to transform the petrochemical industry. Their solid acid alkylation technology uses a proprietary solid catalyst, eliminating the need for liquid acids. This process operates at lower temperatures and pressures, reducing energy consumption by up to 75% compared to traditional methods[1]. Sinopec's alkylation units can produce high-octane gasoline blending components with over 96% alkylate yield[2]. The company has also implemented digital twin technology to optimize alkylation processes, improving product quality and reducing operational costs by approximately 10%[3].
Strengths: Environmentally friendly, energy-efficient, and safer than traditional acid-based processes. Weaknesses: Higher initial capital investment and potential catalyst deactivation issues.
UOP LLC
Technical Solution: UOP LLC, a Honeywell company, has revolutionized alkylation processes with its AlkyClean® solid-bed alkylation technology. This innovative approach uses a proprietary solid catalyst that eliminates the need for liquid acids, significantly improving safety and environmental performance. The AlkyClean process operates at mild conditions, with temperatures below 100°C and pressures under 3.5 MPa[4]. It achieves alkylate yields of up to 97%, with a research octane number (RON) typically above 96[5]. UOP's technology also incorporates advanced process control systems, which optimize reaction conditions in real-time, leading to a 5-8% increase in overall process efficiency[6].
Strengths: Excellent safety profile, high-quality alkylate production, and reduced environmental impact. Weaknesses: Potential higher initial costs and the need for specialized operator training.
Key Innovations
Enhancement of alkylation catalysts for improved supercritical fluid regeneration
PatentInactiveUS7858069B2
Innovation
- The method involves modifying the alkylation catalyst by selectively poisoning strongly acidic active sites, adjusting the pore size distribution, and reducing the number of strongly acidic sites to prevent the formation of condensed hydrocarbon species, thereby enhancing regeneration efficiency through supercritical fluid regeneration.
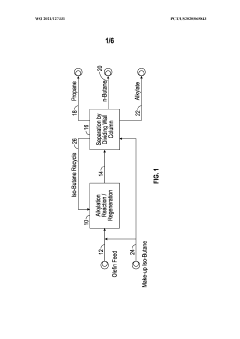
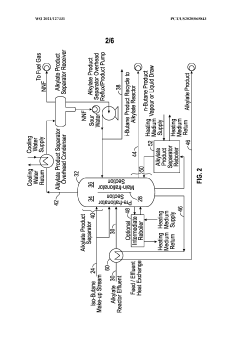
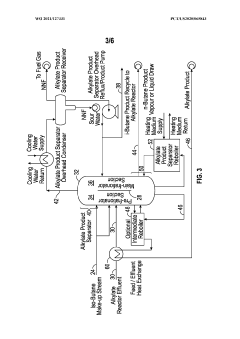
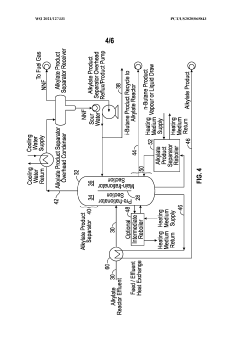
Diving wall column in alkylation process for reactor recycle and product separation
PatentWO2021127331A1
Innovation
- The implementation of a dividing wall column (DWC) in the alkylation process to separate alkylate reactor effluent into iso-butane, n-butane, and alkylate product streams, allowing for efficient recycling of iso-butane and optimizing energy usage through heat integration and reboiler configuration.
Environmental Impact
Alkylation processes in the petrochemical industry have significant environmental implications that warrant careful consideration. The primary environmental concerns associated with alkylation revolve around emissions, waste generation, and resource consumption. During the alkylation process, volatile organic compounds (VOCs) and other potentially harmful gases may be released into the atmosphere. These emissions can contribute to air pollution and the formation of ground-level ozone, which poses risks to human health and ecosystems.
Furthermore, the use of strong acids as catalysts in traditional alkylation processes presents environmental challenges. Sulfuric acid and hydrofluoric acid, commonly employed in alkylation units, are highly corrosive and toxic substances. Their handling, storage, and disposal require stringent safety measures to prevent accidental releases that could harm workers, surrounding communities, and the environment. Spent acid from the process must be carefully managed and regenerated or neutralized, which can result in the generation of hazardous waste.
Water consumption and potential contamination are additional environmental concerns associated with alkylation processes. The production of high-octane gasoline components through alkylation requires substantial amounts of water for cooling and process operations. This water usage can strain local water resources, particularly in water-scarce regions. Moreover, there is a risk of water pollution if proper containment and treatment measures are not implemented to handle process effluents and potential spills.
However, it is important to note that the petrochemical industry has been making strides in addressing these environmental challenges. The development of solid acid catalysts for alkylation has the potential to significantly reduce the environmental impact of the process. These catalysts eliminate the need for liquid acids, thereby minimizing the risks associated with acid handling and disposal. Additionally, solid acid catalysts can operate at lower temperatures, reducing energy consumption and associated greenhouse gas emissions.
Advancements in process design and control have also contributed to improved environmental performance. Modern alkylation units incorporate sophisticated emission control systems, such as scrubbers and thermal oxidizers, to minimize air pollutant releases. Closed-loop cooling systems and water recycling technologies are being implemented to reduce water consumption and minimize the risk of contamination.
As the petrochemical industry continues to evolve, there is a growing emphasis on sustainability and circular economy principles. This shift is driving research into more environmentally friendly alkylation processes, including the exploration of bio-based feedstocks and the development of catalysts that enable milder reaction conditions. These innovations have the potential to further reduce the environmental footprint of alkylation processes while maintaining their crucial role in producing high-quality fuels and petrochemical products.
Furthermore, the use of strong acids as catalysts in traditional alkylation processes presents environmental challenges. Sulfuric acid and hydrofluoric acid, commonly employed in alkylation units, are highly corrosive and toxic substances. Their handling, storage, and disposal require stringent safety measures to prevent accidental releases that could harm workers, surrounding communities, and the environment. Spent acid from the process must be carefully managed and regenerated or neutralized, which can result in the generation of hazardous waste.
Water consumption and potential contamination are additional environmental concerns associated with alkylation processes. The production of high-octane gasoline components through alkylation requires substantial amounts of water for cooling and process operations. This water usage can strain local water resources, particularly in water-scarce regions. Moreover, there is a risk of water pollution if proper containment and treatment measures are not implemented to handle process effluents and potential spills.
However, it is important to note that the petrochemical industry has been making strides in addressing these environmental challenges. The development of solid acid catalysts for alkylation has the potential to significantly reduce the environmental impact of the process. These catalysts eliminate the need for liquid acids, thereby minimizing the risks associated with acid handling and disposal. Additionally, solid acid catalysts can operate at lower temperatures, reducing energy consumption and associated greenhouse gas emissions.
Advancements in process design and control have also contributed to improved environmental performance. Modern alkylation units incorporate sophisticated emission control systems, such as scrubbers and thermal oxidizers, to minimize air pollutant releases. Closed-loop cooling systems and water recycling technologies are being implemented to reduce water consumption and minimize the risk of contamination.
As the petrochemical industry continues to evolve, there is a growing emphasis on sustainability and circular economy principles. This shift is driving research into more environmentally friendly alkylation processes, including the exploration of bio-based feedstocks and the development of catalysts that enable milder reaction conditions. These innovations have the potential to further reduce the environmental footprint of alkylation processes while maintaining their crucial role in producing high-quality fuels and petrochemical products.
Economic Implications
The alkylation process in the petrochemical industry has significant economic implications, transforming the sector's landscape and driving substantial financial gains. This technology has become a cornerstone in the production of high-octane gasoline components, leading to improved fuel efficiency and reduced emissions. As a result, refineries implementing alkylation processes have seen enhanced profit margins and increased competitiveness in the global market.
The economic impact of alkylation extends beyond individual refineries to influence the entire petrochemical supply chain. By enabling the production of higher-value products from lower-value feedstocks, alkylation has created new revenue streams and optimized resource utilization. This has led to improved overall profitability for integrated petrochemical companies and contributed to the industry's resilience in the face of fluctuating oil prices.
Furthermore, the adoption of alkylation technologies has spurred investment in research and development, driving innovation across the sector. Companies are allocating substantial resources to develop more efficient catalysts, reactor designs, and process control systems, fostering a competitive environment that promotes continuous improvement and technological advancement.
The economic benefits of alkylation have also had ripple effects on related industries. Equipment manufacturers, engineering firms, and technology providers have experienced increased demand for their products and services, contributing to job creation and economic growth in supporting sectors. Additionally, the improved fuel quality resulting from alkylation processes has positively impacted the automotive industry, enabling the development of more efficient and environmentally friendly vehicles.
From a macroeconomic perspective, the widespread implementation of alkylation technologies has contributed to energy security and reduced dependence on imported petroleum products in many countries. This has led to more stable energy prices and improved trade balances, particularly for nations with significant refining capacities.
However, the economic implications of alkylation are not without challenges. The high capital costs associated with implementing and maintaining alkylation units can be a barrier for smaller refineries, potentially leading to industry consolidation. Moreover, the volatility of feedstock prices and changing regulatory landscapes regarding environmental standards can impact the long-term economic viability of alkylation processes.
In conclusion, alkylation technologies have fundamentally transformed the economic dynamics of the petrochemical industry, offering substantial benefits while also presenting new challenges. As the industry continues to evolve, the economic implications of alkylation will likely remain a critical factor in shaping strategic decisions and investment priorities across the sector.
The economic impact of alkylation extends beyond individual refineries to influence the entire petrochemical supply chain. By enabling the production of higher-value products from lower-value feedstocks, alkylation has created new revenue streams and optimized resource utilization. This has led to improved overall profitability for integrated petrochemical companies and contributed to the industry's resilience in the face of fluctuating oil prices.
Furthermore, the adoption of alkylation technologies has spurred investment in research and development, driving innovation across the sector. Companies are allocating substantial resources to develop more efficient catalysts, reactor designs, and process control systems, fostering a competitive environment that promotes continuous improvement and technological advancement.
The economic benefits of alkylation have also had ripple effects on related industries. Equipment manufacturers, engineering firms, and technology providers have experienced increased demand for their products and services, contributing to job creation and economic growth in supporting sectors. Additionally, the improved fuel quality resulting from alkylation processes has positively impacted the automotive industry, enabling the development of more efficient and environmentally friendly vehicles.
From a macroeconomic perspective, the widespread implementation of alkylation technologies has contributed to energy security and reduced dependence on imported petroleum products in many countries. This has led to more stable energy prices and improved trade balances, particularly for nations with significant refining capacities.
However, the economic implications of alkylation are not without challenges. The high capital costs associated with implementing and maintaining alkylation units can be a barrier for smaller refineries, potentially leading to industry consolidation. Moreover, the volatility of feedstock prices and changing regulatory landscapes regarding environmental standards can impact the long-term economic viability of alkylation processes.
In conclusion, alkylation technologies have fundamentally transformed the economic dynamics of the petrochemical industry, offering substantial benefits while also presenting new challenges. As the industry continues to evolve, the economic implications of alkylation will likely remain a critical factor in shaping strategic decisions and investment priorities across the sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!