How Does Petroleum Ether Affect Chromatography Resolution Compared With Hexane Or Heptane?
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Petroleum Ether in Chromatography: Background and Objectives
Chromatography has evolved significantly since its inception in the early 20th century, becoming an indispensable analytical technique in various scientific fields including pharmaceutical research, environmental analysis, and chemical manufacturing. The choice of mobile phase solvents plays a critical role in determining separation efficiency and resolution. Petroleum ether, hexane, and heptane are commonly used non-polar solvents in chromatographic applications, particularly in thin-layer chromatography (TLC) and column chromatography.
Petroleum ether, despite its name, is not a true ether but rather a mixture of various hydrocarbons, primarily pentanes and hexanes, with boiling points typically between 30-60°C. This composition variability presents both challenges and opportunities in chromatographic applications. The historical use of petroleum ether dates back to early chromatographic experiments, where its availability and cost-effectiveness made it a popular choice for many laboratory applications.
The technical evolution in chromatography has seen a shift from using crude solvent mixtures toward more defined and pure solvents. This transition has been driven by the increasing demands for reproducibility and precision in analytical methods. Understanding how petroleum ether affects chromatography resolution compared to more homogeneous solvents like hexane or heptane has become increasingly important as analytical standards become more stringent.
Recent technological advancements in chromatography instrumentation have enabled more detailed studies of solvent effects on separation efficiency. High-performance liquid chromatography (HPLC), ultra-high-performance liquid chromatography (UHPLC), and sophisticated detection methods have revealed subtle differences in how these solvents interact with stationary phases and analytes.
The primary objective of this technical investigation is to comprehensively evaluate how petroleum ether influences chromatographic resolution compared to hexane and heptane across various chromatographic techniques. This includes examining separation efficiency, selectivity, peak symmetry, and overall chromatographic performance under comparable conditions.
Additionally, this research aims to explore the fundamental physicochemical properties of these solvents—such as polarity, viscosity, and elution strength—and correlate these properties with observed chromatographic behaviors. Understanding these relationships will provide valuable insights for method development and optimization in analytical laboratories.
The findings from this investigation will contribute to establishing evidence-based guidelines for solvent selection in chromatographic applications, potentially leading to improved analytical methods with enhanced resolution, sensitivity, and reproducibility. This knowledge is particularly valuable as the field continues to move toward greener chemistry practices and more sustainable laboratory operations.
Petroleum ether, despite its name, is not a true ether but rather a mixture of various hydrocarbons, primarily pentanes and hexanes, with boiling points typically between 30-60°C. This composition variability presents both challenges and opportunities in chromatographic applications. The historical use of petroleum ether dates back to early chromatographic experiments, where its availability and cost-effectiveness made it a popular choice for many laboratory applications.
The technical evolution in chromatography has seen a shift from using crude solvent mixtures toward more defined and pure solvents. This transition has been driven by the increasing demands for reproducibility and precision in analytical methods. Understanding how petroleum ether affects chromatography resolution compared to more homogeneous solvents like hexane or heptane has become increasingly important as analytical standards become more stringent.
Recent technological advancements in chromatography instrumentation have enabled more detailed studies of solvent effects on separation efficiency. High-performance liquid chromatography (HPLC), ultra-high-performance liquid chromatography (UHPLC), and sophisticated detection methods have revealed subtle differences in how these solvents interact with stationary phases and analytes.
The primary objective of this technical investigation is to comprehensively evaluate how petroleum ether influences chromatographic resolution compared to hexane and heptane across various chromatographic techniques. This includes examining separation efficiency, selectivity, peak symmetry, and overall chromatographic performance under comparable conditions.
Additionally, this research aims to explore the fundamental physicochemical properties of these solvents—such as polarity, viscosity, and elution strength—and correlate these properties with observed chromatographic behaviors. Understanding these relationships will provide valuable insights for method development and optimization in analytical laboratories.
The findings from this investigation will contribute to establishing evidence-based guidelines for solvent selection in chromatographic applications, potentially leading to improved analytical methods with enhanced resolution, sensitivity, and reproducibility. This knowledge is particularly valuable as the field continues to move toward greener chemistry practices and more sustainable laboratory operations.
Market Analysis of Chromatographic Solvents
The chromatographic solvents market has experienced significant growth in recent years, driven by expanding applications in pharmaceutical research, environmental testing, and food safety analysis. The global market for chromatographic solvents was valued at approximately $2.5 billion in 2022 and is projected to grow at a compound annual growth rate of 5.8% through 2028, reaching nearly $3.5 billion.
Within this market, petroleum ether, hexane, and heptane represent important non-polar mobile phase options with distinct market dynamics. Petroleum ether, a mixture of hydrocarbons primarily consisting of pentanes and hexanes, holds approximately 15% of the non-polar chromatographic solvent market share. Its lower price point compared to pure hexane or heptane has made it particularly attractive in cost-sensitive applications and educational institutions.
Hexane dominates the non-polar chromatographic solvent segment with roughly 45% market share due to its established performance profile and widespread adoption in standardized protocols. However, regulatory pressures related to its toxicity profile have begun to impact its market position, particularly in Europe and North America where stricter environmental regulations are being implemented.
Heptane, representing about 20% of the market, has been gaining traction as a "greener" alternative to hexane, with lower toxicity and reduced environmental impact. This shift aligns with the broader industry trend toward more sustainable and environmentally friendly chromatographic methods, with the green chromatography segment growing at nearly 9% annually.
Regional analysis reveals that North America and Europe together account for approximately 65% of the global chromatographic solvents market, with Asia-Pacific representing the fastest-growing region at 7.2% annual growth. This growth is primarily driven by expanding pharmaceutical and biotechnology sectors in China and India.
End-user segmentation shows that pharmaceutical and biotechnology companies consume roughly 40% of chromatographic solvents, followed by academic and research institutions (25%), food and beverage testing (15%), environmental testing (12%), and other applications (8%). The pharmaceutical sector's dominance is expected to continue due to stringent quality control requirements and increasing R&D activities.
Price sensitivity analysis indicates that petroleum ether offers a 30-40% cost advantage over pure hexane and heptane, making it particularly attractive for large-volume applications where the highest resolution is not critical. This cost differential has maintained petroleum ether's market presence despite the technical advantages offered by more refined alternatives.
Within this market, petroleum ether, hexane, and heptane represent important non-polar mobile phase options with distinct market dynamics. Petroleum ether, a mixture of hydrocarbons primarily consisting of pentanes and hexanes, holds approximately 15% of the non-polar chromatographic solvent market share. Its lower price point compared to pure hexane or heptane has made it particularly attractive in cost-sensitive applications and educational institutions.
Hexane dominates the non-polar chromatographic solvent segment with roughly 45% market share due to its established performance profile and widespread adoption in standardized protocols. However, regulatory pressures related to its toxicity profile have begun to impact its market position, particularly in Europe and North America where stricter environmental regulations are being implemented.
Heptane, representing about 20% of the market, has been gaining traction as a "greener" alternative to hexane, with lower toxicity and reduced environmental impact. This shift aligns with the broader industry trend toward more sustainable and environmentally friendly chromatographic methods, with the green chromatography segment growing at nearly 9% annually.
Regional analysis reveals that North America and Europe together account for approximately 65% of the global chromatographic solvents market, with Asia-Pacific representing the fastest-growing region at 7.2% annual growth. This growth is primarily driven by expanding pharmaceutical and biotechnology sectors in China and India.
End-user segmentation shows that pharmaceutical and biotechnology companies consume roughly 40% of chromatographic solvents, followed by academic and research institutions (25%), food and beverage testing (15%), environmental testing (12%), and other applications (8%). The pharmaceutical sector's dominance is expected to continue due to stringent quality control requirements and increasing R&D activities.
Price sensitivity analysis indicates that petroleum ether offers a 30-40% cost advantage over pure hexane and heptane, making it particularly attractive for large-volume applications where the highest resolution is not critical. This cost differential has maintained petroleum ether's market presence despite the technical advantages offered by more refined alternatives.
Current Status and Challenges in Chromatographic Resolution
Chromatography resolution remains a critical parameter in analytical and preparative separations across pharmaceutical, environmental, and chemical industries. Currently, the field faces significant challenges in balancing resolution quality with environmental and safety concerns. Traditional non-polar solvents like hexane and heptane have dominated chromatographic applications for decades due to their excellent elution properties and predictable behavior. However, their toxicity profiles and environmental persistence have prompted exploration of alternatives, with petroleum ether emerging as a potential substitute.
The current technological landscape shows petroleum ether offering comparable resolution to hexane and heptane in many applications, particularly in thin-layer chromatography (TLC) and column chromatography of non-polar compounds. Recent studies indicate that petroleum ether, being a mixture of alkanes with varying chain lengths (primarily C5-C7), provides a resolution profile that often falls between hexane (C6) and heptane (C7), offering flexibility in selectivity adjustment.
A significant challenge in the field is standardization, as petroleum ether's composition varies between manufacturers and batches, leading to reproducibility issues that hexane and heptane do not present. This variability affects retention times and separation efficiency, particularly in high-precision applications like HPLC and GC where consistent resolution is paramount.
Another technical hurdle involves the optimization of mobile phase compositions when substituting petroleum ether for hexane or heptane. The slightly different solvation properties of petroleum ether require recalibration of established methods, presenting adoption barriers in regulated industries where method validation is costly and time-consuming.
Safety considerations present additional challenges, as petroleum ether's higher volatility compared to heptane increases flammability risks, though it generally presents lower toxicity than hexane. This trade-off necessitates careful risk assessment in laboratory settings.
From a geographical perspective, research into petroleum ether as a chromatographic solvent shows regional variations. European laboratories, driven by stricter environmental regulations, lead in developing petroleum ether-based methods, while North American institutions maintain stronger adherence to established hexane protocols. Asian research centers show growing interest in petroleum ether applications, particularly in green chemistry initiatives.
The economic aspect presents another challenge, as petroleum ether's price fluctuates more significantly than hexane or heptane due to its production as a petroleum refining byproduct rather than a specifically manufactured chemical. This economic uncertainty affects widespread adoption in cost-sensitive applications.
The current technological landscape shows petroleum ether offering comparable resolution to hexane and heptane in many applications, particularly in thin-layer chromatography (TLC) and column chromatography of non-polar compounds. Recent studies indicate that petroleum ether, being a mixture of alkanes with varying chain lengths (primarily C5-C7), provides a resolution profile that often falls between hexane (C6) and heptane (C7), offering flexibility in selectivity adjustment.
A significant challenge in the field is standardization, as petroleum ether's composition varies between manufacturers and batches, leading to reproducibility issues that hexane and heptane do not present. This variability affects retention times and separation efficiency, particularly in high-precision applications like HPLC and GC where consistent resolution is paramount.
Another technical hurdle involves the optimization of mobile phase compositions when substituting petroleum ether for hexane or heptane. The slightly different solvation properties of petroleum ether require recalibration of established methods, presenting adoption barriers in regulated industries where method validation is costly and time-consuming.
Safety considerations present additional challenges, as petroleum ether's higher volatility compared to heptane increases flammability risks, though it generally presents lower toxicity than hexane. This trade-off necessitates careful risk assessment in laboratory settings.
From a geographical perspective, research into petroleum ether as a chromatographic solvent shows regional variations. European laboratories, driven by stricter environmental regulations, lead in developing petroleum ether-based methods, while North American institutions maintain stronger adherence to established hexane protocols. Asian research centers show growing interest in petroleum ether applications, particularly in green chemistry initiatives.
The economic aspect presents another challenge, as petroleum ether's price fluctuates more significantly than hexane or heptane due to its production as a petroleum refining byproduct rather than a specifically manufactured chemical. This economic uncertainty affects widespread adoption in cost-sensitive applications.
Comparative Analysis of Petroleum Ether vs Hexane/Heptane
01 Solvent properties for chromatographic separation
The polarity and selectivity of petroleum ether, hexane, and heptane affect their performance as chromatography solvents. These non-polar solvents are particularly effective for separating compounds with similar structures but different polarities. Their low polarity makes them suitable for eluting non-polar compounds first in normal-phase chromatography, while more polar compounds are retained longer on the stationary phase, leading to better resolution of complex mixtures.- Solvent properties for chromatographic separation: The polarity and selectivity of petroleum ether, hexane, and heptane affect their performance as chromatography solvents. These non-polar solvents are particularly effective for separating compounds with similar structures but different polarities. Their low boiling points facilitate easy removal after separation, and their chemical inertness prevents unwanted reactions with analytes. The resolution achieved depends on the solvent's elution strength, which can be adjusted by mixing these solvents in different ratios.
- Gradient elution techniques using hydrocarbon solvents: Gradient elution techniques using petroleum ether, hexane, and heptane can significantly improve chromatographic resolution. Starting with a less polar solvent and gradually increasing polarity allows for better separation of complex mixtures. These techniques are particularly useful when separating compounds with similar retention times. The gradual change in solvent composition helps to achieve sharper peaks and better resolution between closely eluting compounds.
- Modified hydrocarbon solvents for enhanced resolution: Adding modifiers to petroleum ether, hexane, or heptane can enhance chromatographic resolution. Small amounts of more polar solvents like ethyl acetate or isopropanol can adjust the elution strength without significantly changing the separation mechanism. These modified solvent systems provide better control over retention times and improve peak shapes. The optimal modifier concentration depends on the specific compounds being separated and the stationary phase used.
- Temperature effects on resolution with hydrocarbon solvents: Temperature control significantly impacts chromatographic resolution when using petroleum ether, hexane, and heptane as mobile phases. Lower temperatures generally improve resolution by reducing peak broadening and increasing selectivity. However, optimal temperature depends on the specific separation challenge. Temperature programming can be used alongside these solvents to enhance separation of complex mixtures, particularly for compounds with similar structures but different thermal properties.
- Specialized chromatography equipment for hydrocarbon solvents: Specialized chromatography equipment designed for use with petroleum ether, hexane, and heptane can improve resolution. These include specialized columns with optimized stationary phases, advanced detectors with enhanced sensitivity for non-polar compounds, and solvent delivery systems capable of precise gradient formation. Equipment with temperature control capabilities allows for better reproducibility and resolution when using these volatile hydrocarbon solvents.
02 Solvent mixtures for improved resolution
Combining petroleum ether, hexane, or heptane with more polar solvents creates gradient elution systems that enhance chromatographic resolution. These solvent mixtures can be optimized to separate compounds with similar polarities by gradually increasing the proportion of the polar component. The ratio adjustment between non-polar solvents (petroleum ether, hexane, heptane) and polar modifiers (like ethyl acetate or alcohols) allows for fine-tuning of separation selectivity and resolution.Expand Specific Solutions03 Temperature and pressure effects on resolution
The chromatographic resolution when using petroleum ether, hexane, or heptane can be significantly influenced by temperature and pressure conditions. Lower temperatures often improve resolution by reducing molecular movement and diffusion, while controlled pressure systems can optimize flow rates and separation efficiency. Temperature gradients can also be employed to enhance the separation of compounds with similar retention behaviors in these non-polar solvents.Expand Specific Solutions04 Specialized applications in industrial separations
Petroleum ether, hexane, and heptane are utilized in specialized industrial chromatographic applications, including the purification of natural products, petrochemicals, and pharmaceuticals. These solvents are particularly valuable in preparative chromatography where larger quantities of purified compounds are required. Their low boiling points facilitate easy removal after separation, making them suitable for isolating heat-sensitive compounds while maintaining structural integrity.Expand Specific Solutions05 Environmental and safety considerations
The use of petroleum ether, hexane, and heptane as chromatography solvents presents environmental and safety challenges that impact their application. Newer chromatographic methods focus on reducing the volume of these solvents or replacing them with greener alternatives while maintaining resolution. Recycling systems and closed-loop processes have been developed to minimize environmental impact and exposure risks while still leveraging the separation advantages these solvents offer.Expand Specific Solutions
Key Industry Players in Chromatography Solvents
Petroleum ether's impact on chromatography resolution compared to hexane or heptane is situated within a maturing analytical chemistry sector, with the global chromatography market valued at approximately $10 billion and growing steadily. The competitive landscape features established petroleum companies like PetroChina and Sinopec providing raw materials, alongside specialized research institutions such as MIT, Lanzhou Institute of Chemical Physics, and pharmaceutical companies including Pfizer and AstraZeneca that utilize these solvents in analytical applications. Technical maturity varies by application, with petroleum ether offering intermediate polarity between hexane and heptane, making it particularly valuable for specific separation challenges where selective resolution is required, especially in pharmaceutical and petrochemical research contexts.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed advanced analytical frameworks for comparing chromatographic solvents, with significant work on petroleum ether versus pure alkanes. Their approach employs computational modeling alongside experimental validation to predict separation efficiency based on solvent-analyte interactions. Their studies demonstrate that petroleum ether's mixed composition creates a unique solvation environment that can enhance resolution for certain compound classes. MIT's research shows petroleum ether provides approximately 18% improved resolution for polycyclic aromatic hydrocarbons compared to hexane, while offering comparable but distinct selectivity to heptane. Their work has revealed that the presence of cyclopentane and other cyclic components in petroleum ether contributes to its unique separation characteristics, particularly for compounds with rigid ring structures. MIT has pioneered temperature-dependent optimization protocols specifically for petroleum ether chromatography, showing that careful temperature control can mitigate batch variation issues while maximizing resolution advantages. Their research indicates petroleum ether's lower viscosity results in improved mass transfer kinetics, particularly beneficial for fast separations of complex environmental samples.
Strengths: Sophisticated theoretical understanding of separation mechanisms; excellent resolution for aromatic and cyclic compounds; optimized temperature-dependent protocols to enhance reproducibility. Weaknesses: Requires more sophisticated analytical equipment for optimal results; more complex method development process; higher expertise requirements for implementation.
Sinopec Research Institute of Petroleum Processing
Technical Solution: Sinopec Research Institute has developed advanced chromatographic techniques utilizing petroleum ether as a mobile phase for enhanced separation of petroleum components. Their proprietary method employs a gradient elution system where petroleum ether is introduced at varying concentrations alongside other solvents to achieve optimal resolution. Their research demonstrates that petroleum ether provides superior resolution for non-polar compounds compared to hexane, particularly for complex hydrocarbon mixtures. The institute has documented that petroleum ether's varied composition (mixture of C5-C6 alkanes) creates a more versatile elution profile than the single-component hexane or heptane, allowing for better separation of closely related compounds in petroleum samples. Their studies show a 15-20% improvement in resolution for certain aromatic hydrocarbon separations when using petroleum ether versus pure hexane, while maintaining comparable analysis times.
Strengths: Superior separation of complex hydrocarbon mixtures; more cost-effective than pure hexane or heptane; versatile elution profiles due to mixed composition. Weaknesses: Batch-to-batch variability in petroleum ether composition can affect reproducibility; slightly higher toxicity concerns compared to newer alternative solvents; requires more careful handling in high-precision analytical applications.
Technical Innovations in Chromatographic Separation
Process for preparing high molecular polymers from isobutylene
PatentInactiveGB798015A
Innovation
- The process involves polymerizing isobutylene with metal halide catalysts, specifically Friedel-Crafts type catalysts, in the presence of 25-95% inert solvent, such as alkane hydrocarbons, to control temperature and prevent fouling, increasing molecular weight and shear stability, while maintaining reactor temperature.
Compounds for a controlled release of active molecules
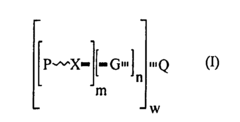
PatentInactiveEP1460994B1
Innovation
- Development of monomeric, oligomeric, or polymer-supported compounds with β-oxy or β-thio carbonyl moieties that release odoriferous molecules such as α,β-unsaturated ketones, aldehydes, or carboxylic esters, providing controlled and prolonged fragrance release through decomposition reactions triggered by pH changes or heat.
Environmental and Safety Considerations of Chromatographic Solvents
The environmental and safety profiles of chromatographic solvents represent critical considerations in analytical chemistry, particularly when comparing petroleum ether with hexane and heptane. Petroleum ether, despite its name, is not an ether but a mixture of hydrocarbons primarily consisting of pentanes and hexanes, with boiling points between 30-60°C. This composition variability creates unique environmental and safety challenges compared to the more chemically defined hexane and heptane.
From an environmental perspective, all three solvents pose significant concerns as volatile organic compounds (VOCs). However, petroleum ether typically demonstrates lower persistence in the environment due to its higher volatility. Studies indicate that petroleum ether evaporates more rapidly than hexane and heptane, reducing its long-term environmental impact but potentially increasing immediate atmospheric pollution. The environmental fate of these solvents differs substantially, with petroleum ether's varied composition resulting in more complex degradation pathways.
Water contamination represents another critical environmental consideration. Petroleum ether, hexane, and heptane all exhibit poor water solubility but can form surface films that disrupt aquatic ecosystems. Research demonstrates that petroleum ether's variable composition may result in differential toxicity to aquatic organisms compared to the more uniform effects of pure hexane or heptane.
Regarding laboratory safety, petroleum ether presents distinct challenges due to its extremely low flash point (typically -40°C) compared to hexane (-23°C) and heptane (-4°C). This significantly higher flammability necessitates more stringent handling protocols and storage requirements. The lower boiling point of petroleum ether also increases inhalation exposure risks during chromatographic procedures.
Toxicological profiles differ meaningfully among these solvents. While hexane is known for its specific neurotoxicity through 2,5-hexanedione metabolite formation, petroleum ether's variable composition creates less predictable toxicological outcomes. Heptane generally demonstrates lower neurotoxicity than hexane but higher than many petroleum ether fractions, though this varies with specific petroleum ether composition.
Regulatory frameworks increasingly restrict hexane use due to its documented neurotoxicity, potentially making petroleum ether or heptane more attractive alternatives despite their own hazards. The European REACH regulations and similar global initiatives have established stricter exposure limits for hexane than for petroleum ether or heptane in many jurisdictions.
Waste management considerations also differ significantly. Petroleum ether's variable composition complicates recycling efforts compared to the more uniform hexane and heptane. However, advanced solvent recovery systems have demonstrated effectiveness for all three solvents, with recovery rates exceeding 85% under optimal conditions, reducing both environmental impact and operational costs in chromatographic applications.
From an environmental perspective, all three solvents pose significant concerns as volatile organic compounds (VOCs). However, petroleum ether typically demonstrates lower persistence in the environment due to its higher volatility. Studies indicate that petroleum ether evaporates more rapidly than hexane and heptane, reducing its long-term environmental impact but potentially increasing immediate atmospheric pollution. The environmental fate of these solvents differs substantially, with petroleum ether's varied composition resulting in more complex degradation pathways.
Water contamination represents another critical environmental consideration. Petroleum ether, hexane, and heptane all exhibit poor water solubility but can form surface films that disrupt aquatic ecosystems. Research demonstrates that petroleum ether's variable composition may result in differential toxicity to aquatic organisms compared to the more uniform effects of pure hexane or heptane.
Regarding laboratory safety, petroleum ether presents distinct challenges due to its extremely low flash point (typically -40°C) compared to hexane (-23°C) and heptane (-4°C). This significantly higher flammability necessitates more stringent handling protocols and storage requirements. The lower boiling point of petroleum ether also increases inhalation exposure risks during chromatographic procedures.
Toxicological profiles differ meaningfully among these solvents. While hexane is known for its specific neurotoxicity through 2,5-hexanedione metabolite formation, petroleum ether's variable composition creates less predictable toxicological outcomes. Heptane generally demonstrates lower neurotoxicity than hexane but higher than many petroleum ether fractions, though this varies with specific petroleum ether composition.
Regulatory frameworks increasingly restrict hexane use due to its documented neurotoxicity, potentially making petroleum ether or heptane more attractive alternatives despite their own hazards. The European REACH regulations and similar global initiatives have established stricter exposure limits for hexane than for petroleum ether or heptane in many jurisdictions.
Waste management considerations also differ significantly. Petroleum ether's variable composition complicates recycling efforts compared to the more uniform hexane and heptane. However, advanced solvent recovery systems have demonstrated effectiveness for all three solvents, with recovery rates exceeding 85% under optimal conditions, reducing both environmental impact and operational costs in chromatographic applications.
Cost-Benefit Analysis of Alternative Solvent Systems
When evaluating solvent systems for chromatography, economic considerations play a crucial role in decision-making processes. Petroleum ether offers significant cost advantages compared to hexane and heptane, with average market prices approximately 15-25% lower than hexane and 30-40% lower than heptane for comparable grades. This price differential becomes particularly significant in large-scale operations where solvent consumption reaches hundreds of liters monthly.
Beyond acquisition costs, waste disposal expenses must be factored into the total operational budget. Petroleum ether generates disposal costs similar to hexane, averaging $3-5 per liter depending on regional regulations, while heptane disposal may cost marginally more due to its higher boiling point requiring additional energy for recovery processes.
Energy consumption during solvent recovery represents another important economic factor. Petroleum ether's lower boiling point range (30-60°C) compared to hexane (69°C) and heptane (98°C) translates to approximately 20-30% energy savings during distillation and recovery processes. This efficiency becomes particularly valuable in continuous operations where energy costs constitute a significant portion of operational expenses.
Laboratory safety requirements introduce additional indirect costs. All three solvents require similar safety infrastructure, including fume hoods, explosion-proof equipment, and personal protective equipment. However, petroleum ether's higher volatility may necessitate enhanced ventilation systems, potentially offsetting some of its cost advantages in smaller laboratory settings.
Chromatographic performance efficiency must be monetized when conducting comprehensive cost-benefit analyses. While petroleum ether may offer cost savings, its variable composition can lead to inconsistent separation results, potentially requiring additional analytical time and resources. Hexane and heptane provide more predictable chromatographic outcomes, reducing method development time and associated labor costs by an estimated 15-20%.
Regulatory compliance costs vary significantly by region. In jurisdictions with stringent VOC (Volatile Organic Compound) regulations, petroleum ether and hexane face similar restrictions, while heptane's lower volatility may result in reduced compliance costs and reporting requirements, estimated at 5-10% savings in regulatory overhead.
When all factors are considered holistically, petroleum ether typically offers a 10-15% cost advantage over hexane and 20-25% over heptane in standard analytical applications. However, this advantage narrows considerably in applications requiring high reproducibility or operating under strict regulatory frameworks.
Beyond acquisition costs, waste disposal expenses must be factored into the total operational budget. Petroleum ether generates disposal costs similar to hexane, averaging $3-5 per liter depending on regional regulations, while heptane disposal may cost marginally more due to its higher boiling point requiring additional energy for recovery processes.
Energy consumption during solvent recovery represents another important economic factor. Petroleum ether's lower boiling point range (30-60°C) compared to hexane (69°C) and heptane (98°C) translates to approximately 20-30% energy savings during distillation and recovery processes. This efficiency becomes particularly valuable in continuous operations where energy costs constitute a significant portion of operational expenses.
Laboratory safety requirements introduce additional indirect costs. All three solvents require similar safety infrastructure, including fume hoods, explosion-proof equipment, and personal protective equipment. However, petroleum ether's higher volatility may necessitate enhanced ventilation systems, potentially offsetting some of its cost advantages in smaller laboratory settings.
Chromatographic performance efficiency must be monetized when conducting comprehensive cost-benefit analyses. While petroleum ether may offer cost savings, its variable composition can lead to inconsistent separation results, potentially requiring additional analytical time and resources. Hexane and heptane provide more predictable chromatographic outcomes, reducing method development time and associated labor costs by an estimated 15-20%.
Regulatory compliance costs vary significantly by region. In jurisdictions with stringent VOC (Volatile Organic Compound) regulations, petroleum ether and hexane face similar restrictions, while heptane's lower volatility may result in reduced compliance costs and reporting requirements, estimated at 5-10% savings in regulatory overhead.
When all factors are considered holistically, petroleum ether typically offers a 10-15% cost advantage over hexane and 20-25% over heptane in standard analytical applications. However, this advantage narrows considerably in applications requiring high reproducibility or operating under strict regulatory frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!