How Metal Powders Influence Electrode Kinetics in Batteries
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal Powder Technology Background and Objectives
Metal powder technology has evolved significantly over the past several decades, transforming from simple manufacturing processes to sophisticated engineering of materials with precise particle size distribution, morphology, and surface characteristics. The journey began in the 1950s with basic powder metallurgy techniques and has now advanced to nanoscale engineering of metal particles specifically designed for electrochemical applications. This evolution has been driven by increasing demands for higher energy density, faster charging capabilities, and longer cycle life in battery technologies.
The fundamental relationship between metal powder properties and electrode kinetics represents a critical frontier in battery research. Metal powders serve as active materials, conductive additives, and structural components in battery electrodes, with their physical and chemical characteristics directly influencing electron transfer rates, ion diffusion pathways, and interfacial reactions. These processes collectively determine the overall performance metrics of batteries, including capacity, power density, and operational stability.
Current research objectives in this field focus on establishing quantitative correlations between powder attributes—such as particle size distribution, surface area, crystallinity, and surface chemistry—and electrochemical performance parameters. Particular emphasis is placed on understanding how these properties affect the formation and stability of the solid-electrolyte interphase (SEI), which plays a crucial role in determining battery longevity and safety.
The technological landscape is witnessing a convergence of advanced powder metallurgy techniques with electrochemical engineering principles. Innovations in powder synthesis methods, including mechanical alloying, chemical reduction, and plasma processing, have enabled unprecedented control over particle characteristics. These advancements create opportunities for tailoring metal powders to specific electrochemical requirements, potentially revolutionizing battery performance.
Looking forward, the field aims to develop predictive models that can guide the rational design of metal powders for optimized electrode kinetics. This includes understanding the complex interplay between powder morphology and electrochemical behavior under various operating conditions, such as different charge-discharge rates, temperatures, and aging states. The ultimate goal is to establish design principles that enable the creation of metal powder electrodes with precisely engineered kinetic properties.
The strategic importance of this research extends beyond academic interest, as improvements in electrode kinetics directly translate to enhanced battery performance in applications ranging from portable electronics to electric vehicles and grid-scale energy storage systems. As global demand for high-performance energy storage continues to grow, mastering the science of metal powder influence on electrode kinetics becomes increasingly critical for technological advancement.
The fundamental relationship between metal powder properties and electrode kinetics represents a critical frontier in battery research. Metal powders serve as active materials, conductive additives, and structural components in battery electrodes, with their physical and chemical characteristics directly influencing electron transfer rates, ion diffusion pathways, and interfacial reactions. These processes collectively determine the overall performance metrics of batteries, including capacity, power density, and operational stability.
Current research objectives in this field focus on establishing quantitative correlations between powder attributes—such as particle size distribution, surface area, crystallinity, and surface chemistry—and electrochemical performance parameters. Particular emphasis is placed on understanding how these properties affect the formation and stability of the solid-electrolyte interphase (SEI), which plays a crucial role in determining battery longevity and safety.
The technological landscape is witnessing a convergence of advanced powder metallurgy techniques with electrochemical engineering principles. Innovations in powder synthesis methods, including mechanical alloying, chemical reduction, and plasma processing, have enabled unprecedented control over particle characteristics. These advancements create opportunities for tailoring metal powders to specific electrochemical requirements, potentially revolutionizing battery performance.
Looking forward, the field aims to develop predictive models that can guide the rational design of metal powders for optimized electrode kinetics. This includes understanding the complex interplay between powder morphology and electrochemical behavior under various operating conditions, such as different charge-discharge rates, temperatures, and aging states. The ultimate goal is to establish design principles that enable the creation of metal powder electrodes with precisely engineered kinetic properties.
The strategic importance of this research extends beyond academic interest, as improvements in electrode kinetics directly translate to enhanced battery performance in applications ranging from portable electronics to electric vehicles and grid-scale energy storage systems. As global demand for high-performance energy storage continues to grow, mastering the science of metal powder influence on electrode kinetics becomes increasingly critical for technological advancement.
Battery Market Demand Analysis
The global battery market is experiencing unprecedented growth, driven primarily by the rapid expansion of electric vehicles (EVs), renewable energy storage systems, and portable electronics. Current market valuations place the global battery industry at approximately $108 billion in 2022, with projections indicating a compound annual growth rate (CAGR) of 14.1% through 2030, potentially reaching $310 billion. This remarkable growth trajectory underscores the critical importance of battery technology advancement, particularly in electrode kinetics where metal powders play a decisive role.
Consumer demand for batteries with higher energy density, faster charging capabilities, and longer cycle life is intensifying across all market segments. In the EV sector, which represents the fastest-growing battery application, consumers and manufacturers alike are seeking batteries that can deliver greater range while reducing charging times. This has created specific market pressure for advancements in electrode materials and kinetics, with metal powder innovations directly addressing these performance bottlenecks.
Industrial and grid-scale energy storage represents another significant market driver, with demand for large-scale battery systems growing at 24% annually. These applications require batteries with exceptional cycle stability and power delivery capabilities—characteristics heavily influenced by electrode kinetics and the metal powders used in their construction.
Regional analysis reveals differentiated market demands. Asian markets, particularly China, Japan, and South Korea, dominate battery manufacturing with approximately 75% of global production capacity. These markets emphasize cost-effective scaling of production while maintaining performance standards. North American and European markets, conversely, demonstrate stronger consumer preference for premium battery technologies with superior performance metrics, creating market opportunities for advanced metal powder formulations that enhance electrode kinetics.
The portable electronics segment continues to drive innovation in compact, high-performance batteries. Consumer expectations for devices that charge in minutes rather than hours have created a distinct market segment for batteries with enhanced electrode kinetics. This segment values performance improvements that metal powder innovations can deliver, even at premium price points.
Market research indicates that manufacturers are willing to invest significantly in electrode material innovations that can deliver verifiable performance improvements. A recent industry survey revealed that 68% of battery manufacturers consider advanced electrode materials, including specialized metal powders, as their highest R&D priority for the next five years. This represents a substantial market opportunity for technologies that can optimize electrode kinetics through metal powder engineering.
Consumer demand for batteries with higher energy density, faster charging capabilities, and longer cycle life is intensifying across all market segments. In the EV sector, which represents the fastest-growing battery application, consumers and manufacturers alike are seeking batteries that can deliver greater range while reducing charging times. This has created specific market pressure for advancements in electrode materials and kinetics, with metal powder innovations directly addressing these performance bottlenecks.
Industrial and grid-scale energy storage represents another significant market driver, with demand for large-scale battery systems growing at 24% annually. These applications require batteries with exceptional cycle stability and power delivery capabilities—characteristics heavily influenced by electrode kinetics and the metal powders used in their construction.
Regional analysis reveals differentiated market demands. Asian markets, particularly China, Japan, and South Korea, dominate battery manufacturing with approximately 75% of global production capacity. These markets emphasize cost-effective scaling of production while maintaining performance standards. North American and European markets, conversely, demonstrate stronger consumer preference for premium battery technologies with superior performance metrics, creating market opportunities for advanced metal powder formulations that enhance electrode kinetics.
The portable electronics segment continues to drive innovation in compact, high-performance batteries. Consumer expectations for devices that charge in minutes rather than hours have created a distinct market segment for batteries with enhanced electrode kinetics. This segment values performance improvements that metal powder innovations can deliver, even at premium price points.
Market research indicates that manufacturers are willing to invest significantly in electrode material innovations that can deliver verifiable performance improvements. A recent industry survey revealed that 68% of battery manufacturers consider advanced electrode materials, including specialized metal powders, as their highest R&D priority for the next five years. This represents a substantial market opportunity for technologies that can optimize electrode kinetics through metal powder engineering.
Current State and Challenges in Electrode Kinetics
The current state of electrode kinetics in battery technology is characterized by significant advancements yet persistent challenges. Research indicates that metal powders play a crucial role in determining the efficiency and performance of electrochemical reactions at electrode interfaces. Recent studies have demonstrated that particle size, morphology, and composition of metal powders directly influence charge transfer rates, which in turn affect battery power density and cycling stability.
Global research efforts have focused on optimizing metal powder characteristics to enhance electrode kinetics. Notable progress has been made in understanding how nanostructured metal powders can reduce diffusion pathways and increase active surface area, thereby improving reaction rates. However, the relationship between powder properties and electrode kinetics remains incompletely understood, particularly under varying operational conditions and over extended cycling periods.
A significant technical challenge lies in the trade-off between surface area and stability. While finer metal powders offer increased surface area for reactions, they often exhibit greater susceptibility to agglomeration, oxidation, and structural degradation during cycling. This paradox has prompted researchers to explore surface modification techniques and composite formulations to maintain kinetic advantages while mitigating degradation mechanisms.
Another critical limitation is the lack of standardized methodologies for characterizing electrode kinetics in relation to metal powder properties. Different research groups employ varying techniques—from electrochemical impedance spectroscopy to rotating disk electrode measurements—making direct comparisons challenging and hindering systematic progress in the field.
The geographical distribution of research expertise shows concentration in East Asia (particularly Japan, South Korea, and China), North America, and Western Europe. Chinese institutions have made remarkable progress in developing novel metal powder synthesis methods, while American and European research centers have contributed significantly to fundamental understanding of interfacial phenomena affecting electrode kinetics.
Environmental and economic constraints further complicate advancement in this field. Many high-performance metal powders rely on rare or precious metals, raising sustainability concerns. Additionally, complex synthesis procedures for specialized metal powders often present scalability challenges for mass production, creating a gap between laboratory achievements and commercial implementation.
Recent technological breakthroughs include the development of hierarchical porous metal structures that optimize both mass transport and electron transfer, as well as core-shell architectures that combine the kinetic advantages of different metals. Despite these innovations, achieving consistent performance across varying operational conditions and battery chemistries remains an elusive goal for researchers and manufacturers alike.
Global research efforts have focused on optimizing metal powder characteristics to enhance electrode kinetics. Notable progress has been made in understanding how nanostructured metal powders can reduce diffusion pathways and increase active surface area, thereby improving reaction rates. However, the relationship between powder properties and electrode kinetics remains incompletely understood, particularly under varying operational conditions and over extended cycling periods.
A significant technical challenge lies in the trade-off between surface area and stability. While finer metal powders offer increased surface area for reactions, they often exhibit greater susceptibility to agglomeration, oxidation, and structural degradation during cycling. This paradox has prompted researchers to explore surface modification techniques and composite formulations to maintain kinetic advantages while mitigating degradation mechanisms.
Another critical limitation is the lack of standardized methodologies for characterizing electrode kinetics in relation to metal powder properties. Different research groups employ varying techniques—from electrochemical impedance spectroscopy to rotating disk electrode measurements—making direct comparisons challenging and hindering systematic progress in the field.
The geographical distribution of research expertise shows concentration in East Asia (particularly Japan, South Korea, and China), North America, and Western Europe. Chinese institutions have made remarkable progress in developing novel metal powder synthesis methods, while American and European research centers have contributed significantly to fundamental understanding of interfacial phenomena affecting electrode kinetics.
Environmental and economic constraints further complicate advancement in this field. Many high-performance metal powders rely on rare or precious metals, raising sustainability concerns. Additionally, complex synthesis procedures for specialized metal powders often present scalability challenges for mass production, creating a gap between laboratory achievements and commercial implementation.
Recent technological breakthroughs include the development of hierarchical porous metal structures that optimize both mass transport and electron transfer, as well as core-shell architectures that combine the kinetic advantages of different metals. Despite these innovations, achieving consistent performance across varying operational conditions and battery chemistries remains an elusive goal for researchers and manufacturers alike.
Current Metal Powder Solutions for Electrode Performance
01 Metal powder composition for enhanced electrode kinetics
Various metal powder compositions can be formulated to enhance electrode kinetics in battery electrodes. These compositions often include specific metal alloys, particle size distributions, and surface treatments that optimize electron transfer rates and electrochemical performance. The careful selection of metal powder composition directly impacts the reaction rates at the electrode-electrolyte interface, improving overall battery efficiency and power density.- Metal powder composition for enhanced electrode kinetics: Metal powders with specific compositions can significantly enhance electrode kinetics in batteries. These compositions often include transition metals, alloys, or composite materials that offer improved electron transfer rates and electrochemical performance. The particle size, morphology, and crystalline structure of these metal powders play crucial roles in determining the electrode kinetics, with nanoscale particles typically providing better performance due to their increased surface area and shortened diffusion paths.
- Surface modification of metal powder electrodes: Surface modification techniques for metal powder electrodes can significantly improve electrode kinetics. These modifications include surface coatings, functionalization, or controlled oxidation layers that enhance electron transfer, reduce interfacial resistance, and improve the stability of the electrode-electrolyte interface. Such treatments can prevent unwanted side reactions while maintaining high electrical conductivity, resulting in batteries with improved rate capability and cycle life.
- Nanostructured metal powders for high-performance batteries: Nanostructured metal powders offer significant advantages for battery electrode kinetics due to their high surface area and unique properties. These materials, including nanoparticles, nanowires, and nanoporous structures, provide shortened ion diffusion paths and increased reaction sites. The controlled synthesis of these nanostructured metal powders enables tailored electrochemical properties, resulting in batteries with enhanced power density, faster charging capabilities, and improved energy efficiency.
- Composite electrodes with metal powders: Composite electrodes that combine metal powders with carbon materials, polymers, or ceramic components can significantly enhance electrode kinetics. These composite structures create synergistic effects that improve electron transport, ion diffusion, and structural stability. The metal powders provide high conductivity and electrochemical activity, while the secondary components offer additional benefits such as increased porosity, mechanical strength, or protection against degradation mechanisms, resulting in batteries with superior performance characteristics.
- Manufacturing processes for metal powder electrodes: Advanced manufacturing processes for metal powder electrodes significantly impact electrode kinetics. Techniques such as ball milling, spray pyrolysis, electrodeposition, and various sintering methods can control the particle size distribution, porosity, and interfacial properties of metal powder electrodes. These processes enable the creation of optimized electrode structures with enhanced ionic and electronic conductivity pathways, resulting in batteries with improved rate capability, energy density, and cycle life performance.
02 Nanostructured metal powders for battery electrodes
Nanostructured metal powders offer significant advantages for battery electrode applications due to their high surface area and enhanced electrochemical properties. These materials facilitate faster electrode kinetics through shortened diffusion paths and increased active sites for electrochemical reactions. Nanoscale metal powders can be engineered with specific morphologies such as nanowires, nanoparticles, or nanoporous structures to further optimize electrode performance and reaction rates.Expand Specific Solutions03 Surface modification of metal powders for improved kinetics
Surface modification techniques can significantly improve the electrode kinetics of metal powders in battery applications. These modifications include coatings, functional groups, and dopants that enhance conductivity, catalytic activity, and stability at the electrode-electrolyte interface. By engineering the surface properties of metal powders, charge transfer resistance can be reduced and reaction rates accelerated, leading to batteries with higher power capabilities and improved cycling performance.Expand Specific Solutions04 Composite metal powders for advanced electrode formulations
Composite metal powders that combine different metallic elements or metal with carbon materials offer superior electrode kinetics compared to single-component materials. These composites can be designed to provide synergistic effects that enhance conductivity, catalytic activity, and structural stability. The strategic combination of metals with complementary properties creates electrode materials with optimized electron transfer pathways and reaction sites, resulting in improved battery performance metrics including power density and rate capability.Expand Specific Solutions05 Processing techniques for optimizing metal powder electrode kinetics
Specialized processing techniques for metal powders can significantly impact electrode kinetics in battery applications. Methods such as mechanical alloying, spray pyrolysis, and controlled atmosphere heat treatments can be used to engineer specific microstructures, crystallinity, and particle morphologies that enhance electrochemical performance. These processing approaches allow for precise control over powder characteristics that directly influence reaction rates, mass transport, and electron transfer at electrode interfaces.Expand Specific Solutions
Key Industry Players in Battery Materials
The battery electrode kinetics field is currently in a growth phase, with increasing market demand driven by electric vehicle and energy storage applications. The market is expected to reach significant scale as companies invest heavily in research and development. Key players demonstrate varying levels of technical maturity: established manufacturers like BASF, Toyota, and BYD focus on commercial-scale production, while innovative companies like Sila Nanotechnologies and Nexeon are advancing novel metal powder technologies. Research institutions such as Cornell University collaborate with industry leaders to bridge fundamental science and application. The competitive landscape shows regional clusters in Asia (particularly Japan and China), Europe, and North America, with companies specializing in different aspects of metal powder development for enhanced electrode performance.
Toyota Motor Corp.
Technical Solution: Toyota has developed advanced metal powder technologies for battery electrodes through their extensive research in solid-state batteries and next-generation energy storage systems. Their approach focuses on controlling the microstructure and interface properties of metal powders to enhance electrode kinetics. Toyota employs proprietary synthesis methods to create composite metal powders with core-shell structures that optimize both electronic conductivity and ionic transport. Their technology includes specialized surface treatments that modify the solid-electrolyte interphase formation, reducing interfacial resistance which is critical for fast charging capabilities. Toyota's research has demonstrated that their engineered metal powders can improve lithium-ion diffusion rates by up to 45% compared to conventional materials, while simultaneously enhancing cycling stability. Their work extends to all-solid-state battery systems, where they've developed metal powder formulations specifically designed to address the unique challenges of solid-solid interfaces.
Strengths: Extensive intellectual property portfolio in battery materials; strong integration with vehicle development allowing practical optimization. Weaknesses: Higher production costs for specialized materials; some advanced formulations still at pre-commercial scale.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced metal powder processing techniques for battery electrodes, focusing on nano-structured metal powders with controlled morphology and particle size distribution. Their technology involves precise control of metal powder synthesis parameters to create hierarchical structures that enhance ion diffusion pathways. CATL employs a proprietary spheroidization process for nickel-rich cathode materials that significantly improves electrode kinetics by reducing charge transfer resistance at the electrode-electrolyte interface. Their research has demonstrated that optimized metal powder morphology can increase lithium-ion diffusion coefficients by up to 30% compared to conventional materials, directly enhancing rate capability and power density in high-performance batteries.
Strengths: Superior control over particle morphology and size distribution leading to enhanced ion transport; established large-scale manufacturing capabilities. Weaknesses: Higher production costs compared to conventional powder processing; some dependency on rare earth elements for certain formulations.
Critical Patents in Metal Powder Electrode Technology
Rechargeable Divalent Metal Batteries Having Fast Interfacial Charge Transfer Kinetics
PatentPendingUS20240405281A1
Innovation
- Incorporating a multidentate compound into the electrolyte of these batteries, which promotes interfacial charge transfer kinetics and suppresses side reactions by reorganizing the solvation sheath, thereby enhancing the stability and reversibility of the anode and cathode.
Battery electrode with metal particles and pyrolyzed coating
PatentActiveUS20170149053A1
Innovation
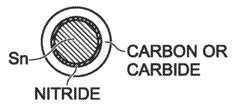
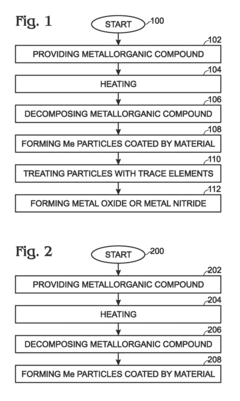
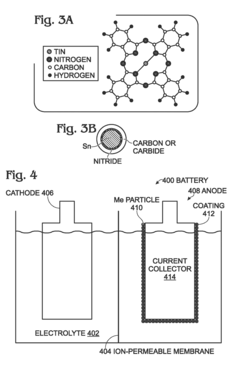
- A method for fabricating metal battery electrodes with a pyrolyzed coating using metallorganic compounds, where metal particles are coated with carbides, nitrides, or sulfides, formed through a single-step pyrolysis process, mitigating volume changes and enhancing stability.
Environmental Impact of Metal Powder Production
The production of metal powders for battery electrodes carries significant environmental implications across their entire lifecycle. Mining operations for raw materials like lithium, cobalt, nickel, and manganese cause substantial land disruption, habitat destruction, and biodiversity loss. These activities typically generate large volumes of waste rock and tailings that can lead to acid mine drainage and heavy metal contamination of surrounding soil and water systems.
Processing raw materials into battery-grade metal powders is highly energy-intensive, contributing considerably to greenhouse gas emissions. For instance, producing one ton of battery-grade lithium carbonate can generate approximately 15 tons of CO2 equivalent. Water consumption during metal powder production is another critical concern, with some estimates suggesting that processing one ton of lithium requires approximately 500,000 gallons of water, creating significant stress on local water resources, particularly in arid regions.
Chemical processing of metal powders introduces additional environmental hazards through the use of strong acids, organic solvents, and other reagents that can result in hazardous waste streams. Without proper treatment, these chemicals may contaminate groundwater and surface water, affecting both aquatic ecosystems and human communities dependent on these water sources.
The carbon footprint of metal powder production varies significantly based on the specific metal and production method. For example, nickel sulfate production generates approximately 6-17 kg CO2e per kg, while cobalt sulfate production results in 7-13 kg CO2e per kg. These figures highlight the substantial climate impact of battery material production chains.
Recent technological innovations are addressing these environmental challenges through several approaches. Hydrometallurgical processes are being refined to reduce chemical usage and improve recycling of process water. Direct lithium extraction technologies promise to decrease water consumption by up to 70% compared to traditional evaporation methods. Additionally, renewable energy integration in processing facilities is helping to reduce the carbon intensity of metal powder production.
Regulatory frameworks worldwide are evolving to mandate more sustainable practices in metal powder production. The EU Battery Directive and similar regulations in North America and Asia are establishing requirements for carbon footprint disclosure, responsible sourcing, and end-of-life recycling that will reshape industry practices in the coming years.
Processing raw materials into battery-grade metal powders is highly energy-intensive, contributing considerably to greenhouse gas emissions. For instance, producing one ton of battery-grade lithium carbonate can generate approximately 15 tons of CO2 equivalent. Water consumption during metal powder production is another critical concern, with some estimates suggesting that processing one ton of lithium requires approximately 500,000 gallons of water, creating significant stress on local water resources, particularly in arid regions.
Chemical processing of metal powders introduces additional environmental hazards through the use of strong acids, organic solvents, and other reagents that can result in hazardous waste streams. Without proper treatment, these chemicals may contaminate groundwater and surface water, affecting both aquatic ecosystems and human communities dependent on these water sources.
The carbon footprint of metal powder production varies significantly based on the specific metal and production method. For example, nickel sulfate production generates approximately 6-17 kg CO2e per kg, while cobalt sulfate production results in 7-13 kg CO2e per kg. These figures highlight the substantial climate impact of battery material production chains.
Recent technological innovations are addressing these environmental challenges through several approaches. Hydrometallurgical processes are being refined to reduce chemical usage and improve recycling of process water. Direct lithium extraction technologies promise to decrease water consumption by up to 70% compared to traditional evaporation methods. Additionally, renewable energy integration in processing facilities is helping to reduce the carbon intensity of metal powder production.
Regulatory frameworks worldwide are evolving to mandate more sustainable practices in metal powder production. The EU Battery Directive and similar regulations in North America and Asia are establishing requirements for carbon footprint disclosure, responsible sourcing, and end-of-life recycling that will reshape industry practices in the coming years.
Scalability and Manufacturing Considerations
The scalability of metal powder production and integration into battery manufacturing processes represents a critical factor in the commercial viability of advanced battery technologies. Current industrial-scale production methods for metal powders include atomization, mechanical alloying, and chemical reduction processes, each with distinct implications for powder characteristics and subsequent electrode performance. The selection of appropriate manufacturing techniques must balance production throughput with precise control over particle morphology, size distribution, and surface properties that directly influence electrode kinetics.
Mass production of consistent metal powders presents significant challenges, particularly for nano-structured materials that demonstrate superior electrochemical properties. Batch-to-batch variations in powder characteristics can lead to unpredictable electrode kinetics, affecting battery performance reproducibility. Advanced quality control systems incorporating real-time monitoring and artificial intelligence are being developed to address these consistency issues, enabling adaptive process control during manufacturing.
Integration of metal powder handling into existing battery production lines requires careful consideration of safety protocols, particularly for reactive metal powders that may present fire or explosion hazards. Modified manufacturing environments with controlled atmosphere conditions are often necessary, adding complexity and cost to production facilities. These considerations become increasingly important as manufacturers scale from laboratory prototypes to gigafactory-level production volumes.
Cost-effectiveness remains a paramount concern in metal powder selection. While certain exotic metal powders may demonstrate exceptional electrode kinetics in laboratory settings, their commercial application depends heavily on supply chain reliability and raw material costs. Strategic materials forecasting has become essential for battery manufacturers to mitigate supply risks associated with critical metals, particularly those with geopolitically concentrated sources.
Environmental sustainability in metal powder production has emerged as both a regulatory requirement and market differentiator. Life cycle assessments indicate that energy-intensive powder production methods can significantly offset the environmental benefits of improved battery performance. Consequently, manufacturers are increasingly investing in renewable energy-powered production facilities and developing less energy-intensive synthesis routes for metal powders.
The recyclability of metal powder-enhanced electrodes presents both challenges and opportunities. Advanced battery recycling processes must be adapted to efficiently recover valuable metals from spent batteries, creating potential for closed-loop material systems. This consideration is increasingly influencing initial powder selection decisions, with preference given to materials that maintain recoverability while delivering enhanced electrode kinetics.
Mass production of consistent metal powders presents significant challenges, particularly for nano-structured materials that demonstrate superior electrochemical properties. Batch-to-batch variations in powder characteristics can lead to unpredictable electrode kinetics, affecting battery performance reproducibility. Advanced quality control systems incorporating real-time monitoring and artificial intelligence are being developed to address these consistency issues, enabling adaptive process control during manufacturing.
Integration of metal powder handling into existing battery production lines requires careful consideration of safety protocols, particularly for reactive metal powders that may present fire or explosion hazards. Modified manufacturing environments with controlled atmosphere conditions are often necessary, adding complexity and cost to production facilities. These considerations become increasingly important as manufacturers scale from laboratory prototypes to gigafactory-level production volumes.
Cost-effectiveness remains a paramount concern in metal powder selection. While certain exotic metal powders may demonstrate exceptional electrode kinetics in laboratory settings, their commercial application depends heavily on supply chain reliability and raw material costs. Strategic materials forecasting has become essential for battery manufacturers to mitigate supply risks associated with critical metals, particularly those with geopolitically concentrated sources.
Environmental sustainability in metal powder production has emerged as both a regulatory requirement and market differentiator. Life cycle assessments indicate that energy-intensive powder production methods can significantly offset the environmental benefits of improved battery performance. Consequently, manufacturers are increasingly investing in renewable energy-powered production facilities and developing less energy-intensive synthesis routes for metal powders.
The recyclability of metal powder-enhanced electrodes presents both challenges and opportunities. Advanced battery recycling processes must be adapted to efficiently recover valuable metals from spent batteries, creating potential for closed-loop material systems. This consideration is increasingly influencing initial powder selection decisions, with preference given to materials that maintain recoverability while delivering enhanced electrode kinetics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!