Regulatory Challenges in Metal Powders for Pharmaceutical Use
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal Powder Pharmaceutical Regulatory Background and Objectives
Metal powders have been increasingly utilized in pharmaceutical applications over the past several decades, evolving from simple tablet coatings to complex drug delivery systems. The regulatory landscape governing these materials has similarly evolved, shaped by significant historical events such as the thalidomide tragedy of the 1960s, which led to more stringent pharmaceutical regulations globally. This evolution reflects the growing understanding of how metal properties can impact drug safety, efficacy, and stability.
The pharmaceutical industry's interest in metal powders stems from their unique physical and chemical properties, including controlled dissolution rates, targeted delivery capabilities, and enhanced bioavailability. Historically, metals like iron, zinc, and magnesium were primarily used as nutritional supplements, but recent technological advancements have expanded their application to include active pharmaceutical ingredients (APIs), excipients, and specialized delivery systems.
Current regulatory frameworks for metal powders in pharmaceuticals vary significantly across regions, creating compliance challenges for global manufacturers. The FDA in the United States, the EMA in Europe, and the NMPA in China each maintain distinct requirements for metal powder characterization, impurity profiling, and manufacturing controls. This regulatory heterogeneity necessitates comprehensive understanding of multiple compliance pathways for successful product development and commercialization.
The technical objectives of this research include mapping the current global regulatory requirements specific to metal powders in pharmaceutical applications, identifying key compliance challenges, and developing strategic approaches to navigate these complex regulatory landscapes. Additionally, this analysis aims to anticipate future regulatory trends based on emerging scientific understanding of metal-based pharmaceuticals and evolving international harmonization efforts.
Particular attention will be given to regulatory considerations surrounding nano-sized metal powders, which present unique safety concerns due to their enhanced biological interactions and potential for unexpected toxicity profiles. The regulatory science in this area continues to develop, with agencies increasingly requiring specialized characterization and safety assessments for these materials.
This research will also examine how regulatory frameworks address the dual nature of many metal powders—beneficial at therapeutic doses but potentially toxic at higher concentrations—and how this impacts requirements for manufacturing controls, stability testing, and clinical development programs. Understanding these nuanced regulatory expectations is essential for pharmaceutical companies seeking to leverage the advantages of metal powders while ensuring patient safety and regulatory compliance.
The pharmaceutical industry's interest in metal powders stems from their unique physical and chemical properties, including controlled dissolution rates, targeted delivery capabilities, and enhanced bioavailability. Historically, metals like iron, zinc, and magnesium were primarily used as nutritional supplements, but recent technological advancements have expanded their application to include active pharmaceutical ingredients (APIs), excipients, and specialized delivery systems.
Current regulatory frameworks for metal powders in pharmaceuticals vary significantly across regions, creating compliance challenges for global manufacturers. The FDA in the United States, the EMA in Europe, and the NMPA in China each maintain distinct requirements for metal powder characterization, impurity profiling, and manufacturing controls. This regulatory heterogeneity necessitates comprehensive understanding of multiple compliance pathways for successful product development and commercialization.
The technical objectives of this research include mapping the current global regulatory requirements specific to metal powders in pharmaceutical applications, identifying key compliance challenges, and developing strategic approaches to navigate these complex regulatory landscapes. Additionally, this analysis aims to anticipate future regulatory trends based on emerging scientific understanding of metal-based pharmaceuticals and evolving international harmonization efforts.
Particular attention will be given to regulatory considerations surrounding nano-sized metal powders, which present unique safety concerns due to their enhanced biological interactions and potential for unexpected toxicity profiles. The regulatory science in this area continues to develop, with agencies increasingly requiring specialized characterization and safety assessments for these materials.
This research will also examine how regulatory frameworks address the dual nature of many metal powders—beneficial at therapeutic doses but potentially toxic at higher concentrations—and how this impacts requirements for manufacturing controls, stability testing, and clinical development programs. Understanding these nuanced regulatory expectations is essential for pharmaceutical companies seeking to leverage the advantages of metal powders while ensuring patient safety and regulatory compliance.
Market Analysis of Metal Powders in Pharmaceutical Applications
The global market for metal powders in pharmaceutical applications has witnessed significant growth in recent years, driven by advancements in drug delivery systems and manufacturing technologies. The market size for pharmaceutical-grade metal powders reached approximately $1.2 billion in 2022, with projections indicating a compound annual growth rate of 6.8% through 2028. This growth trajectory is primarily fueled by increasing demand for specialized drug delivery systems and the expanding application scope of metal powders in pharmaceutical formulations.
North America currently dominates the market with a 38% share, followed by Europe at 32% and Asia-Pacific at 24%. The remaining 6% is distributed across other regions. The Asia-Pacific region, particularly China and India, is expected to exhibit the highest growth rate due to expanding pharmaceutical manufacturing capabilities and increasing healthcare investments.
Within the pharmaceutical sector, metal powders find applications across various segments. Injectable drug delivery systems account for approximately 35% of the market, followed by oral drug formulations (28%), topical applications (18%), implantable devices (12%), and others (7%). The injectable segment's dominance is attributed to the growing prevalence of chronic diseases requiring precise drug delivery mechanisms and the increasing adoption of self-administration devices.
From a demand perspective, pharmaceutical companies are increasingly seeking metal powders with enhanced properties such as controlled particle size distribution, high purity levels, and improved biocompatibility. This trend is driven by stringent regulatory requirements and the need for improved drug efficacy and safety profiles. The demand for titanium, stainless steel, and platinum-group metals has shown particularly strong growth due to their excellent biocompatibility and corrosion resistance properties.
Market analysis indicates that pricing trends for pharmaceutical-grade metal powders have remained relatively stable despite fluctuations in raw material costs. This stability is primarily due to long-term supply contracts between manufacturers and pharmaceutical companies. However, premium pricing is observed for specialized metal powders with unique properties or those meeting specific regulatory standards.
The competitive landscape features both specialized metal powder manufacturers and diversified materials companies. Key market players include established materials science companies expanding their pharmaceutical portfolios and specialized manufacturers focusing exclusively on high-purity metal powders for medical and pharmaceutical applications. Strategic partnerships between metal powder suppliers and pharmaceutical companies are becoming increasingly common, driving innovation and ensuring compliance with evolving regulatory standards.
North America currently dominates the market with a 38% share, followed by Europe at 32% and Asia-Pacific at 24%. The remaining 6% is distributed across other regions. The Asia-Pacific region, particularly China and India, is expected to exhibit the highest growth rate due to expanding pharmaceutical manufacturing capabilities and increasing healthcare investments.
Within the pharmaceutical sector, metal powders find applications across various segments. Injectable drug delivery systems account for approximately 35% of the market, followed by oral drug formulations (28%), topical applications (18%), implantable devices (12%), and others (7%). The injectable segment's dominance is attributed to the growing prevalence of chronic diseases requiring precise drug delivery mechanisms and the increasing adoption of self-administration devices.
From a demand perspective, pharmaceutical companies are increasingly seeking metal powders with enhanced properties such as controlled particle size distribution, high purity levels, and improved biocompatibility. This trend is driven by stringent regulatory requirements and the need for improved drug efficacy and safety profiles. The demand for titanium, stainless steel, and platinum-group metals has shown particularly strong growth due to their excellent biocompatibility and corrosion resistance properties.
Market analysis indicates that pricing trends for pharmaceutical-grade metal powders have remained relatively stable despite fluctuations in raw material costs. This stability is primarily due to long-term supply contracts between manufacturers and pharmaceutical companies. However, premium pricing is observed for specialized metal powders with unique properties or those meeting specific regulatory standards.
The competitive landscape features both specialized metal powder manufacturers and diversified materials companies. Key market players include established materials science companies expanding their pharmaceutical portfolios and specialized manufacturers focusing exclusively on high-purity metal powders for medical and pharmaceutical applications. Strategic partnerships between metal powder suppliers and pharmaceutical companies are becoming increasingly common, driving innovation and ensuring compliance with evolving regulatory standards.
Current Regulatory Landscape and Technical Barriers
The regulatory landscape for metal powders in pharmaceutical applications is characterized by a complex web of international and regional frameworks. The U.S. Food and Drug Administration (FDA) maintains stringent requirements through 21 CFR parts 210 and 211, which govern good manufacturing practices for pharmaceutical ingredients. Similarly, the European Medicines Agency (EMA) enforces regulations through EudraLex Volume 4, with specific attention to metallic impurities in medicinal products. These regulatory bodies have established increasingly strict limits on elemental impurities, as outlined in ICH Q3D guidelines.
A significant technical barrier in this domain is the analytical methodology for detecting and quantifying metal contaminants. Current pharmacopoeial methods often lack the sensitivity required for the increasingly stringent regulatory limits. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has emerged as the gold standard, but implementation challenges persist due to high equipment costs and specialized expertise requirements.
Material characterization presents another substantial challenge. Metal powders used in pharmaceuticals must meet precise specifications for particle size distribution, surface area, and morphology. However, standardized testing protocols specifically designed for pharmaceutical-grade metal powders remain underdeveloped, creating inconsistencies in quality assessment across the industry.
Cross-contamination control during manufacturing represents a critical regulatory concern. Metal powders, particularly those with nano-scale dimensions, pose unique containment challenges due to their high mobility and potential for airborne dispersion. Current Good Manufacturing Practice (cGMP) guidelines require dedicated facilities and specialized handling procedures, which significantly increase production costs and complexity.
Stability testing requirements constitute another regulatory hurdle. Metal powders can undergo oxidation, agglomeration, or other physical changes during storage, potentially affecting their pharmaceutical performance. Regulatory expectations for demonstrating long-term stability remain ambiguous, particularly for novel metal-based formulations.
International regulatory harmonization remains incomplete despite ICH efforts. Significant discrepancies exist between regions regarding acceptable limits for certain metals, required testing methodologies, and documentation standards. These inconsistencies create substantial compliance challenges for global pharmaceutical manufacturers utilizing metal powders in their formulations.
The emergence of advanced manufacturing technologies, such as 3D printing of metal-containing pharmaceuticals, has outpaced regulatory frameworks. Current regulations were not designed with these novel production methods in mind, creating uncertainty regarding quality control requirements and validation approaches for these innovative manufacturing processes.
A significant technical barrier in this domain is the analytical methodology for detecting and quantifying metal contaminants. Current pharmacopoeial methods often lack the sensitivity required for the increasingly stringent regulatory limits. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) has emerged as the gold standard, but implementation challenges persist due to high equipment costs and specialized expertise requirements.
Material characterization presents another substantial challenge. Metal powders used in pharmaceuticals must meet precise specifications for particle size distribution, surface area, and morphology. However, standardized testing protocols specifically designed for pharmaceutical-grade metal powders remain underdeveloped, creating inconsistencies in quality assessment across the industry.
Cross-contamination control during manufacturing represents a critical regulatory concern. Metal powders, particularly those with nano-scale dimensions, pose unique containment challenges due to their high mobility and potential for airborne dispersion. Current Good Manufacturing Practice (cGMP) guidelines require dedicated facilities and specialized handling procedures, which significantly increase production costs and complexity.
Stability testing requirements constitute another regulatory hurdle. Metal powders can undergo oxidation, agglomeration, or other physical changes during storage, potentially affecting their pharmaceutical performance. Regulatory expectations for demonstrating long-term stability remain ambiguous, particularly for novel metal-based formulations.
International regulatory harmonization remains incomplete despite ICH efforts. Significant discrepancies exist between regions regarding acceptable limits for certain metals, required testing methodologies, and documentation standards. These inconsistencies create substantial compliance challenges for global pharmaceutical manufacturers utilizing metal powders in their formulations.
The emergence of advanced manufacturing technologies, such as 3D printing of metal-containing pharmaceuticals, has outpaced regulatory frameworks. Current regulations were not designed with these novel production methods in mind, creating uncertainty regarding quality control requirements and validation approaches for these innovative manufacturing processes.
Compliance Strategies for Metal Powder Formulations
01 Production methods for metal powders
Various methods are employed for producing metal powders, including atomization, reduction, and electrolytic processes. These techniques control particle size, shape, and purity, which are critical for downstream applications. The production methods can be optimized to yield powders with specific characteristics such as spherical morphology, narrow size distribution, or enhanced surface properties.- Production methods for metal powders: Various methods are employed for producing metal powders, including atomization, reduction, and electrolytic processes. These techniques allow for control over particle size, shape, and purity of the resulting powders. The production method significantly influences the properties of the metal powders, making them suitable for specific applications in industries such as additive manufacturing, powder metallurgy, and electronics.
- Metal powder compositions and alloys: Metal powder compositions can include various alloys and mixtures designed for specific applications. These compositions may combine different metals to achieve desired properties such as improved strength, corrosion resistance, or thermal conductivity. The precise formulation of metal powder alloys is critical for applications in industries ranging from aerospace to medical devices, where performance requirements are stringent.
- Applications in additive manufacturing and 3D printing: Metal powders are extensively used in additive manufacturing processes, including selective laser melting, electron beam melting, and direct metal laser sintering. These powders must possess specific characteristics such as flowability, particle size distribution, and spherical morphology to ensure successful printing operations. The quality of the metal powder directly impacts the mechanical properties, surface finish, and dimensional accuracy of the final printed components.
- Surface treatment and coating of metal powders: Surface treatments and coatings can be applied to metal powders to enhance their properties or provide additional functionality. These treatments may include oxidation prevention coatings, surface activation for improved bonding, or functionalization for specific chemical reactions. Coated metal powders find applications in catalysts, electronic components, and composite materials where the surface properties are as important as the bulk material characteristics.
- Processing and handling techniques for metal powders: Specialized techniques for processing and handling metal powders are essential due to their reactive nature and safety considerations. These include methods for compaction, sintering, and consolidation to transform powders into solid components. Additionally, proper handling procedures are necessary to prevent oxidation, contamination, or potential hazards such as dust explosions. Advanced processing techniques can improve powder characteristics and the properties of final products.
02 Metal powder applications in additive manufacturing
Metal powders are extensively used in additive manufacturing processes such as selective laser melting, electron beam melting, and direct metal laser sintering. These powders require specific characteristics including flowability, particle size distribution, and chemical composition to ensure successful 3D printing of complex metal components with desired mechanical properties and minimal defects.Expand Specific Solutions03 Metal powder compositions and alloys
Specialized metal powder compositions and alloys are developed for specific applications, including high-temperature resistant alloys, corrosion-resistant compositions, and materials with enhanced magnetic or electrical properties. These compositions often involve precise blending of multiple metallic elements and sometimes non-metallic additives to achieve the desired performance characteristics.Expand Specific Solutions04 Surface treatment and coating of metal powders
Metal powders can be surface-treated or coated to enhance their properties such as oxidation resistance, flowability, or compatibility with binding materials. Surface modification techniques include chemical treatments, polymer coating, and oxide layer formation. These treatments can significantly improve the performance of metal powders in various applications including powder metallurgy, thermal spraying, and composite materials.Expand Specific Solutions05 Metal powder processing and sintering techniques
Processing techniques for metal powders include compaction, sintering, and heat treatment methods that transform loose powder into solid components. Advanced sintering approaches such as spark plasma sintering, hot isostatic pressing, and microwave sintering enable the production of high-density parts with minimal porosity. These techniques control the microstructure development and final properties of the sintered components.Expand Specific Solutions
Key Stakeholders in Pharmaceutical Metal Powder Industry
The regulatory landscape for metal powders in pharmaceutical applications is evolving within a maturing industry that faces complex compliance challenges. The market is experiencing moderate growth, estimated at $1.2-1.5 billion annually, driven by advanced drug delivery systems and specialized formulations. Technical maturity varies significantly across applications, with established players like Höganäs AB and Cabot Corp. leading in traditional metal powder production, while pharmaceutical specialists such as AstraZeneca and Palatin Technologies focus on novel therapeutic applications. Regulatory frameworks remain fragmented globally, creating compliance challenges particularly for cross-border operations. Companies like Umicore and TANIOBIS GmbH are investing in developing pharmaceutical-grade metal powders that meet stringent purity and safety requirements, while government agencies continue refining standards for this specialized intersection of metallurgy and pharmaceutical sciences.
Höganäs AB
Technical Solution: Höganäs AB has developed comprehensive technical solutions addressing regulatory challenges in metal powders for pharmaceutical applications. Their approach includes specialized water atomization processes that produce high-purity metal powders with controlled particle size distribution specifically designed to meet pharmaceutical regulatory standards. The company has implemented advanced quality management systems compliant with ISO 13485 for medical device applications, which they've extended to pharmaceutical metal powder production. Höganäs has pioneered analytical methods for detecting trace contaminants at parts-per-billion levels, crucial for meeting USP <232> and ICH Q3D guidelines on elemental impurities. Their metal powders undergo rigorous stability testing to ensure consistent performance throughout shelf life, addressing FDA concerns about material degradation. Additionally, they've developed specialized packaging systems that maintain powder integrity and prevent contamination during storage and transport, a key regulatory requirement for pharmaceutical-grade materials.
Strengths: Industry-leading expertise in high-purity metal powder production with decades of experience in powder metallurgy. Established quality management systems specifically adapted for pharmaceutical applications. Weaknesses: Their primary focus on industrial applications may limit specialized pharmaceutical regulatory knowledge compared to dedicated pharmaceutical material suppliers.
Tekna Plasma Systems, Inc.
Technical Solution: Tekna Plasma Systems has developed an innovative plasma induction technology specifically designed to address regulatory challenges in pharmaceutical-grade metal powders. Their proprietary plasma atomization process creates spherical metal powders with exceptional purity levels (>99.9%) and precise particle size control, critical for meeting stringent pharmaceutical regulations. The company has implemented a comprehensive regulatory compliance framework that includes full material traceability, batch-to-batch consistency validation, and detailed documentation systems aligned with GMP requirements. Tekna's technology enables the production of titanium, tantalum, and other biocompatible metal powders with minimal oxygen content and controlled surface chemistry, addressing key regulatory concerns about material biocompatibility and patient safety. Their advanced in-process monitoring systems provide real-time quality control data that supports regulatory submissions and helps pharmaceutical manufacturers demonstrate compliance with FDA and EMA requirements for starting materials. Tekna has also developed specialized handling protocols to prevent cross-contamination and maintain powder sterility throughout the production process.
Strengths: Cutting-edge plasma technology enables production of ultra-high purity spherical powders with exceptional consistency. Their specialized focus on advanced materials provides deep expertise in challenging metal powder applications. Weaknesses: Relatively smaller scale compared to traditional metal powder producers may limit production capacity for large pharmaceutical applications.
Critical Regulatory Documentation and Testing Requirements
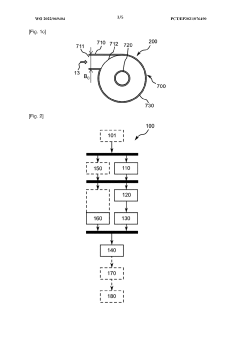
Metallic powders for use as electrode material in multilayer ceramic capacitors and method of manufacturing and of using same
PatentWO2019148277A1
Innovation
- The development of metal-based spherical particles doped with a doping agent to increase sintering temperature, with a controlled particle size distribution of 20 nm to 350 nm and reduced carbon content, produced through a process involving vaporization and cooling of metal-based particles with a doping agent, ensuring less than 1000 ppm of carbon content and minimal coarse particle presence.
Device and method for producing metal powders
PatentWO2022069404A1
Innovation
- A process involving melting and spraying of materials using an electric arc, followed by cooling with a carrier gas to form spherical particles, and an enrichment step with an active substance to control chemical composition, while using a gas/particle separation system to prevent aggregation and adhesion.
International Harmonization of Metal Powder Standards
The global pharmaceutical industry faces significant challenges due to the lack of harmonized standards for metal powders used in pharmaceutical applications. Currently, major regulatory bodies such as the FDA, EMA, and NMPA maintain different specifications and testing requirements, creating compliance complexities for manufacturers operating across multiple markets. This regulatory fragmentation increases production costs, delays market entry, and potentially compromises product quality consistency.
Efforts toward international harmonization have gained momentum through initiatives like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The ICH Q3D guideline on elemental impurities represents a significant step forward, establishing consistent limits for metal contaminants across regions. However, specific standards for metal powders as active ingredients or excipients remain inconsistently defined across jurisdictions.
The Pharmacopoeial Discussion Group (PDG), comprising representatives from the European, Japanese, and United States pharmacopoeias, has been working to align monographs for metal-containing materials. Their progress, while notable, has been slowed by technical disagreements regarding appropriate analytical methodologies and acceptance criteria. The International Organization for Standardization (ISO) has also developed relevant standards, particularly ISO 13320 for particle size analysis, though these are not uniformly adopted across pharmaceutical regulatory frameworks.
Regional differences in metal powder standards create particular challenges for emerging markets. Countries developing pharmaceutical manufacturing capabilities must navigate conflicting requirements when designing quality systems, often implementing redundant testing protocols to satisfy multiple regulatory authorities. This creates inefficiencies that ultimately impact medication affordability and accessibility.
Industry consortia like the Metal Powders Industries Federation (MPIF) have proposed standardized testing protocols specifically for pharmaceutical applications, but regulatory adoption remains inconsistent. The development of reference materials certified across multiple jurisdictions represents a promising approach to practical harmonization, allowing manufacturers to validate analytical methods against universally recognized standards.
Future harmonization efforts will likely focus on risk-based approaches that maintain safety while allowing reasonable flexibility in manufacturing processes. Digital tracking systems using blockchain technology are being explored to enhance supply chain transparency and facilitate regulatory convergence. Success will require continued collaboration between industry stakeholders, regulatory authorities, and international standards organizations to develop technically sound, globally applicable standards for pharmaceutical metal powders.
Efforts toward international harmonization have gained momentum through initiatives like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The ICH Q3D guideline on elemental impurities represents a significant step forward, establishing consistent limits for metal contaminants across regions. However, specific standards for metal powders as active ingredients or excipients remain inconsistently defined across jurisdictions.
The Pharmacopoeial Discussion Group (PDG), comprising representatives from the European, Japanese, and United States pharmacopoeias, has been working to align monographs for metal-containing materials. Their progress, while notable, has been slowed by technical disagreements regarding appropriate analytical methodologies and acceptance criteria. The International Organization for Standardization (ISO) has also developed relevant standards, particularly ISO 13320 for particle size analysis, though these are not uniformly adopted across pharmaceutical regulatory frameworks.
Regional differences in metal powder standards create particular challenges for emerging markets. Countries developing pharmaceutical manufacturing capabilities must navigate conflicting requirements when designing quality systems, often implementing redundant testing protocols to satisfy multiple regulatory authorities. This creates inefficiencies that ultimately impact medication affordability and accessibility.
Industry consortia like the Metal Powders Industries Federation (MPIF) have proposed standardized testing protocols specifically for pharmaceutical applications, but regulatory adoption remains inconsistent. The development of reference materials certified across multiple jurisdictions represents a promising approach to practical harmonization, allowing manufacturers to validate analytical methods against universally recognized standards.
Future harmonization efforts will likely focus on risk-based approaches that maintain safety while allowing reasonable flexibility in manufacturing processes. Digital tracking systems using blockchain technology are being explored to enhance supply chain transparency and facilitate regulatory convergence. Success will require continued collaboration between industry stakeholders, regulatory authorities, and international standards organizations to develop technically sound, globally applicable standards for pharmaceutical metal powders.
Risk Assessment Frameworks for Metal Powder Safety
Risk assessment frameworks for metal powder safety in pharmaceutical applications have evolved significantly in response to increasing regulatory scrutiny. These frameworks typically incorporate a multi-tiered approach that evaluates hazards, exposure pathways, and risk characterization specific to metal powders used in drug formulations and manufacturing processes.
The ICH Q3D Elemental Impurities guideline represents the cornerstone of modern risk assessment for pharmaceutical metal powders, establishing permitted daily exposure (PDE) limits for 24 elemental impurities across various administration routes. This framework employs a risk-based approach that considers toxicological data, bioavailability factors, and exposure scenarios to determine acceptable limits.
Complementary to ICH guidelines, the Quality Risk Management (QRM) principles outlined in ICH Q9 provide structured methodologies for evaluating metal powder risks. These include Failure Mode Effects Analysis (FMEA), Hazard Analysis and Critical Control Points (HACCP), and Preliminary Hazard Analysis (PHA), which systematically identify potential failure points in metal powder handling processes.
For occupational safety considerations, frameworks developed by organizations such as NIOSH and OSHA establish exposure limits and control measures for workers handling metal powders. These frameworks incorporate particle size distribution analysis, since finer particles present greater inhalation risks and potentially enhanced bioavailability when incorporated into pharmaceutical products.
Environmental risk assessment frameworks for metal powders have also gained prominence, particularly in evaluating manufacturing waste streams and potential ecological impacts. These frameworks typically employ a tiered approach beginning with screening-level assessments followed by more detailed evaluations when warranted by initial findings.
Recent innovations in risk assessment include computational toxicology models that predict metal bioavailability and toxicity based on physicochemical properties. These in silico approaches complement traditional toxicological testing and can significantly reduce the need for animal studies while providing more comprehensive safety profiles for novel metal-containing formulations.
Harmonization efforts between different regulatory jurisdictions have led to more consistent risk assessment frameworks globally, though regional variations persist in implementation requirements and acceptance criteria. This convergence facilitates international pharmaceutical development while maintaining appropriate safety standards for metal powder applications.
The ICH Q3D Elemental Impurities guideline represents the cornerstone of modern risk assessment for pharmaceutical metal powders, establishing permitted daily exposure (PDE) limits for 24 elemental impurities across various administration routes. This framework employs a risk-based approach that considers toxicological data, bioavailability factors, and exposure scenarios to determine acceptable limits.
Complementary to ICH guidelines, the Quality Risk Management (QRM) principles outlined in ICH Q9 provide structured methodologies for evaluating metal powder risks. These include Failure Mode Effects Analysis (FMEA), Hazard Analysis and Critical Control Points (HACCP), and Preliminary Hazard Analysis (PHA), which systematically identify potential failure points in metal powder handling processes.
For occupational safety considerations, frameworks developed by organizations such as NIOSH and OSHA establish exposure limits and control measures for workers handling metal powders. These frameworks incorporate particle size distribution analysis, since finer particles present greater inhalation risks and potentially enhanced bioavailability when incorporated into pharmaceutical products.
Environmental risk assessment frameworks for metal powders have also gained prominence, particularly in evaluating manufacturing waste streams and potential ecological impacts. These frameworks typically employ a tiered approach beginning with screening-level assessments followed by more detailed evaluations when warranted by initial findings.
Recent innovations in risk assessment include computational toxicology models that predict metal bioavailability and toxicity based on physicochemical properties. These in silico approaches complement traditional toxicological testing and can significantly reduce the need for animal studies while providing more comprehensive safety profiles for novel metal-containing formulations.
Harmonization efforts between different regulatory jurisdictions have led to more consistent risk assessment frameworks globally, though regional variations persist in implementation requirements and acceptance criteria. This convergence facilitates international pharmaceutical development while maintaining appropriate safety standards for metal powder applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!