How Thermoelectric Waste Recovery Enhances Pharmaceutical Processes
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Thermoelectric Waste Heat Recovery Background and Objectives
Thermoelectric waste heat recovery technology has evolved significantly over the past several decades, transforming from a niche scientific concept to a practical energy conservation solution. The fundamental principle, known as the Seebeck effect, was discovered in 1821, but commercial applications only gained momentum in the late 20th century with advances in semiconductor materials. This technology enables direct conversion of temperature differentials into electrical energy without moving parts, offering unique advantages for industrial settings where waste heat is abundant.
In pharmaceutical manufacturing, energy consumption represents a substantial operational cost, with thermal processes accounting for approximately 40-60% of total energy usage. Notably, 20-50% of this energy is typically lost as waste heat through various processes including sterilization, drying, distillation, and reactor operations. This presents a significant opportunity for energy recovery and efficiency improvement.
The pharmaceutical industry faces increasing pressure to reduce carbon footprints and operational costs while maintaining strict quality standards. Thermoelectric waste heat recovery aligns perfectly with these objectives by offering a pathway to energy recapture without compromising production processes. Recent regulatory developments, including the FDA's Quality by Design initiative and various international sustainability frameworks, further incentivize the adoption of energy-efficient technologies.
Current objectives for thermoelectric waste heat recovery in pharmaceutical applications focus on several key areas. First, improving conversion efficiency beyond the current industry average of 5-8% to make implementation economically viable across more processes. Second, developing materials and designs that can withstand the stringent cleanliness requirements and aggressive cleaning protocols common in pharmaceutical manufacturing. Third, creating modular, scalable systems that can be retrofitted to existing equipment with minimal disruption to validated processes.
Another critical objective is the integration of these systems with process control and monitoring infrastructure to enable real-time optimization and predictive maintenance. This integration supports both energy efficiency goals and the industry's move toward continuous manufacturing paradigms. Additionally, there is significant interest in developing systems capable of operating effectively at lower temperature differentials (below 100°C), which would dramatically expand the range of applicable processes.
The long-term vision for this technology includes closed-loop energy systems within pharmaceutical facilities, where waste heat from one process powers auxiliary systems or supplements energy needs in other processes. This approach could potentially reduce facility-wide energy consumption by 15-25%, representing both significant cost savings and environmental benefits.
In pharmaceutical manufacturing, energy consumption represents a substantial operational cost, with thermal processes accounting for approximately 40-60% of total energy usage. Notably, 20-50% of this energy is typically lost as waste heat through various processes including sterilization, drying, distillation, and reactor operations. This presents a significant opportunity for energy recovery and efficiency improvement.
The pharmaceutical industry faces increasing pressure to reduce carbon footprints and operational costs while maintaining strict quality standards. Thermoelectric waste heat recovery aligns perfectly with these objectives by offering a pathway to energy recapture without compromising production processes. Recent regulatory developments, including the FDA's Quality by Design initiative and various international sustainability frameworks, further incentivize the adoption of energy-efficient technologies.
Current objectives for thermoelectric waste heat recovery in pharmaceutical applications focus on several key areas. First, improving conversion efficiency beyond the current industry average of 5-8% to make implementation economically viable across more processes. Second, developing materials and designs that can withstand the stringent cleanliness requirements and aggressive cleaning protocols common in pharmaceutical manufacturing. Third, creating modular, scalable systems that can be retrofitted to existing equipment with minimal disruption to validated processes.
Another critical objective is the integration of these systems with process control and monitoring infrastructure to enable real-time optimization and predictive maintenance. This integration supports both energy efficiency goals and the industry's move toward continuous manufacturing paradigms. Additionally, there is significant interest in developing systems capable of operating effectively at lower temperature differentials (below 100°C), which would dramatically expand the range of applicable processes.
The long-term vision for this technology includes closed-loop energy systems within pharmaceutical facilities, where waste heat from one process powers auxiliary systems or supplements energy needs in other processes. This approach could potentially reduce facility-wide energy consumption by 15-25%, representing both significant cost savings and environmental benefits.
Pharmaceutical Industry Demand for Energy Efficiency Solutions
The pharmaceutical industry faces mounting pressure to enhance energy efficiency across its operations due to rising energy costs, stringent environmental regulations, and corporate sustainability commitments. Manufacturing pharmaceuticals is notably energy-intensive, with processes requiring precise temperature control, sterilization, and extensive heating and cooling cycles that consume substantial energy resources. Industry data indicates that energy costs typically represent 5-10% of total operational expenses in pharmaceutical manufacturing facilities, creating a significant financial incentive for efficiency improvements.
Environmental regulations worldwide are increasingly targeting industrial energy consumption and associated emissions. The European Union's Emissions Trading System, the United States EPA's greenhouse gas reporting requirements, and similar frameworks in Asia have established clear compliance thresholds that pharmaceutical companies must meet. Additionally, many major pharmaceutical corporations have voluntarily committed to ambitious carbon reduction targets, with several pledging carbon neutrality by 2030-2040, necessitating immediate action on energy efficiency.
Investors and stakeholders are also driving demand for energy-efficient solutions through ESG (Environmental, Social, and Governance) investment criteria. Major pharmaceutical companies report that sustainability performance increasingly influences capital access and valuation, creating market-based incentives for energy optimization beyond regulatory compliance.
The industry's unique production requirements present specific energy efficiency challenges. Cleanroom operations demand continuous air handling and filtration systems that operate 24/7, consuming approximately 50-60% of a typical pharmaceutical facility's energy. Sterilization processes require high-temperature steam generation, while sensitive biological products necessitate precise refrigeration and freezing capabilities, often at ultra-low temperatures (-80°C or lower).
Market analysis reveals growing interest in technologies that can recover waste heat from these energy-intensive processes. Surveys of pharmaceutical manufacturing executives indicate that solutions offering energy recovery without compromising production reliability or product quality are highly valued, with payback periods of 2-3 years considered acceptable for capital investments in energy efficiency.
The COVID-19 pandemic has further accelerated this trend, as manufacturing capacity expansion has highlighted energy infrastructure limitations and costs. Pharmaceutical companies expanding vaccine production capabilities have reported energy supply constraints as a significant challenge, creating additional motivation to implement energy-efficient technologies and waste heat recovery systems.
As pharmaceutical manufacturing continues to evolve toward continuous processing and advanced manufacturing techniques, the industry increasingly seeks integrated energy solutions that can adapt to these new production paradigms while maximizing efficiency and minimizing environmental impact.
Environmental regulations worldwide are increasingly targeting industrial energy consumption and associated emissions. The European Union's Emissions Trading System, the United States EPA's greenhouse gas reporting requirements, and similar frameworks in Asia have established clear compliance thresholds that pharmaceutical companies must meet. Additionally, many major pharmaceutical corporations have voluntarily committed to ambitious carbon reduction targets, with several pledging carbon neutrality by 2030-2040, necessitating immediate action on energy efficiency.
Investors and stakeholders are also driving demand for energy-efficient solutions through ESG (Environmental, Social, and Governance) investment criteria. Major pharmaceutical companies report that sustainability performance increasingly influences capital access and valuation, creating market-based incentives for energy optimization beyond regulatory compliance.
The industry's unique production requirements present specific energy efficiency challenges. Cleanroom operations demand continuous air handling and filtration systems that operate 24/7, consuming approximately 50-60% of a typical pharmaceutical facility's energy. Sterilization processes require high-temperature steam generation, while sensitive biological products necessitate precise refrigeration and freezing capabilities, often at ultra-low temperatures (-80°C or lower).
Market analysis reveals growing interest in technologies that can recover waste heat from these energy-intensive processes. Surveys of pharmaceutical manufacturing executives indicate that solutions offering energy recovery without compromising production reliability or product quality are highly valued, with payback periods of 2-3 years considered acceptable for capital investments in energy efficiency.
The COVID-19 pandemic has further accelerated this trend, as manufacturing capacity expansion has highlighted energy infrastructure limitations and costs. Pharmaceutical companies expanding vaccine production capabilities have reported energy supply constraints as a significant challenge, creating additional motivation to implement energy-efficient technologies and waste heat recovery systems.
As pharmaceutical manufacturing continues to evolve toward continuous processing and advanced manufacturing techniques, the industry increasingly seeks integrated energy solutions that can adapt to these new production paradigms while maximizing efficiency and minimizing environmental impact.
Current Thermoelectric Technology Status and Barriers
Thermoelectric waste heat recovery technology in pharmaceutical processes currently faces several significant technical and economic barriers despite its promising potential. The global market for thermoelectric generators reached approximately $460 million in 2022, with a compound annual growth rate of 8.3%, indicating growing interest in this technology across industries including pharmaceuticals.
The current state-of-the-art thermoelectric materials achieve a figure of merit (ZT) between 1.0 and 2.0, which translates to conversion efficiencies of only 5-8% in real-world applications. This relatively low efficiency remains one of the primary technical limitations, particularly when compared to other energy recovery technologies that can achieve 15-20% efficiency. The pharmaceutical industry, with its precise temperature control requirements, finds these efficiency limitations especially challenging.
Material constraints present another significant barrier. Contemporary commercial thermoelectric devices primarily rely on bismuth telluride (Bi₂Te₃) for low to moderate temperature applications (up to 250°C) and lead telluride (PbTe) for higher temperatures. However, these materials contain rare or toxic elements, raising sustainability and regulatory concerns particularly relevant in pharmaceutical manufacturing environments where material safety is paramount.
Scalability issues further complicate implementation in pharmaceutical facilities. Current thermoelectric modules are typically small (10-50 cm²), requiring complex arrays for larger waste heat sources. This creates integration challenges with existing pharmaceutical equipment, which often features specialized designs for regulatory compliance and contamination prevention.
Cost remains a substantial barrier, with thermoelectric systems averaging $5,000-$10,000 per kilowatt of generating capacity. This high capital expenditure results in lengthy return-on-investment periods of 5-8 years in pharmaceutical applications, deterring widespread adoption despite the industry's focus on sustainability initiatives.
Geographically, thermoelectric technology development shows distinct patterns. North America and Europe lead in research and patent filings (approximately 45% and 30% respectively), while Asia, particularly China and Japan, dominates manufacturing capacity (over 60% of global production). This distribution creates supply chain vulnerabilities for pharmaceutical manufacturers seeking to implement these technologies.
Regulatory frameworks present additional challenges specific to pharmaceutical applications. The FDA and similar international bodies maintain strict requirements for equipment used in drug manufacturing, necessitating extensive validation processes for new technologies like thermoelectric waste heat recovery systems. This regulatory burden extends development timelines and increases implementation costs.
The current state-of-the-art thermoelectric materials achieve a figure of merit (ZT) between 1.0 and 2.0, which translates to conversion efficiencies of only 5-8% in real-world applications. This relatively low efficiency remains one of the primary technical limitations, particularly when compared to other energy recovery technologies that can achieve 15-20% efficiency. The pharmaceutical industry, with its precise temperature control requirements, finds these efficiency limitations especially challenging.
Material constraints present another significant barrier. Contemporary commercial thermoelectric devices primarily rely on bismuth telluride (Bi₂Te₃) for low to moderate temperature applications (up to 250°C) and lead telluride (PbTe) for higher temperatures. However, these materials contain rare or toxic elements, raising sustainability and regulatory concerns particularly relevant in pharmaceutical manufacturing environments where material safety is paramount.
Scalability issues further complicate implementation in pharmaceutical facilities. Current thermoelectric modules are typically small (10-50 cm²), requiring complex arrays for larger waste heat sources. This creates integration challenges with existing pharmaceutical equipment, which often features specialized designs for regulatory compliance and contamination prevention.
Cost remains a substantial barrier, with thermoelectric systems averaging $5,000-$10,000 per kilowatt of generating capacity. This high capital expenditure results in lengthy return-on-investment periods of 5-8 years in pharmaceutical applications, deterring widespread adoption despite the industry's focus on sustainability initiatives.
Geographically, thermoelectric technology development shows distinct patterns. North America and Europe lead in research and patent filings (approximately 45% and 30% respectively), while Asia, particularly China and Japan, dominates manufacturing capacity (over 60% of global production). This distribution creates supply chain vulnerabilities for pharmaceutical manufacturers seeking to implement these technologies.
Regulatory frameworks present additional challenges specific to pharmaceutical applications. The FDA and similar international bodies maintain strict requirements for equipment used in drug manufacturing, necessitating extensive validation processes for new technologies like thermoelectric waste heat recovery systems. This regulatory burden extends development timelines and increases implementation costs.
Existing Thermoelectric Integration Methods for Pharma Processes
01 Thermoelectric material optimization for waste heat recovery
Advanced thermoelectric materials can significantly enhance waste heat recovery efficiency. These materials are specifically engineered to improve the conversion of thermal energy into electrical energy through the Seebeck effect. Optimization techniques include doping, nanostructuring, and creating composite materials to increase the figure of merit (ZT) value, which directly correlates with conversion efficiency. These enhanced materials can operate effectively across various temperature ranges, making them suitable for diverse industrial waste heat recovery applications.- Thermoelectric material optimization for waste heat recovery: Advanced thermoelectric materials can be engineered to improve conversion efficiency in waste heat recovery systems. These materials exhibit enhanced Seebeck coefficients and reduced thermal conductivity, allowing for better conversion of temperature differentials into electrical energy. Optimization techniques include nanostructuring, doping, and creating composite materials that can operate effectively across various temperature ranges typically found in industrial waste heat streams.
- System design and integration for industrial waste heat recovery: Specialized system designs can maximize the capture and conversion of waste heat in industrial settings. These systems incorporate optimized heat exchangers, thermal management components, and electrical power conditioning circuits. The integration approach considers factors such as heat source characteristics, temperature gradients, and operational conditions to ensure maximum energy recovery. Advanced designs may include cascaded modules to capture heat across different temperature ranges or modular configurations for adaptability to various industrial processes.
- Automotive exhaust heat recovery systems: Thermoelectric generators specifically designed for automotive applications can recover waste heat from exhaust systems. These systems are engineered to withstand high temperatures, vibration, and thermal cycling while efficiently converting exhaust heat into usable electrical power. The recovered energy can supplement the vehicle's electrical system, reducing alternator load and improving fuel efficiency. Design considerations include compact packaging, durability under harsh conditions, and integration with existing vehicle systems.
- Heat exchanger design optimization for thermoelectric systems: Specialized heat exchanger designs can significantly improve the performance of thermoelectric waste heat recovery systems. These heat exchangers maximize thermal transfer to thermoelectric modules while minimizing pressure drops in fluid systems. Advanced designs incorporate features such as turbulators, extended surfaces, and optimized flow paths to enhance heat transfer coefficients. Materials selection and manufacturing techniques focus on balancing thermal conductivity, mechanical strength, and cost-effectiveness for industrial applications.
- Control systems and power management for thermoelectric generators: Sophisticated control and power management systems can optimize the operation of thermoelectric waste heat recovery installations. These systems dynamically adjust operating parameters based on heat source conditions and load requirements to maximize energy harvest. Advanced power conditioning circuits ensure efficient conversion of the variable DC output from thermoelectric modules to usable power for the grid or local consumption. Monitoring capabilities provide real-time performance data and predictive maintenance information to ensure sustained optimal operation.
02 System design and integration for thermoelectric generators
Innovative system designs and integration methods can maximize the efficiency of thermoelectric waste heat recovery. These designs focus on optimizing heat transfer between the heat source and thermoelectric modules, improving thermal management, and reducing thermal resistance. Advanced heat exchanger designs, module arrangements, and integration techniques ensure maximum temperature differential across the thermoelectric elements. Proper system integration with existing industrial processes or vehicle exhaust systems allows for efficient capture and conversion of waste heat that would otherwise be lost.Expand Specific Solutions03 Heat exchanger and thermal interface improvements
Enhanced heat exchanger designs and thermal interface materials play a crucial role in thermoelectric waste heat recovery. These improvements focus on maximizing heat transfer to and from thermoelectric modules while minimizing thermal losses. Advanced heat sink designs, phase-change materials, and specialized thermal interface compounds reduce thermal resistance at critical junctions. Innovations in this area include micro-channel heat exchangers, porous media for increased surface area, and composite materials that provide both electrical insulation and thermal conductivity, resulting in higher overall system efficiency.Expand Specific Solutions04 Control systems and power management for thermoelectric recovery
Sophisticated control systems and power management technologies optimize the performance of thermoelectric waste heat recovery systems under varying conditions. These systems include maximum power point tracking algorithms, dynamic load matching circuits, and intelligent thermal management controls. Advanced power conditioning electronics convert the variable DC output from thermoelectric generators into usable power for specific applications. These control systems can adapt to fluctuating heat sources and changing environmental conditions, ensuring optimal energy harvesting across different operational scenarios.Expand Specific Solutions05 Application-specific thermoelectric waste heat recovery solutions
Specialized thermoelectric waste heat recovery solutions designed for specific applications maximize energy recapture in targeted industries. These include systems for automotive exhaust heat recovery, industrial furnace waste heat utilization, and power plant efficiency enhancement. Application-specific designs consider unique operational parameters such as temperature ranges, space constraints, and integration requirements. Customized solutions may incorporate hybrid approaches combining thermoelectric generation with other waste heat recovery technologies to achieve optimal efficiency for particular use cases.Expand Specific Solutions
Leading Companies in Pharmaceutical Thermoelectric Applications
Thermoelectric waste recovery in pharmaceutical processes is emerging as a promising technology in the early growth stage, with an estimated market size of $150-200 million and projected annual growth of 12-15%. The technology is approaching commercial maturity, with varying implementation levels across key players. Toyota Motor Corp. and LG Electronics demonstrate advanced applications in industrial settings, while pharmaceutical companies like Abbott Laboratories and Anhui Double-Crane Pharmaceutical are in early adoption phases. Academic institutions including Nanjing Tech University and Xi'an Jiaotong University are driving fundamental research, while specialized firms such as Shanghai Future High-tech and Shuangliang Eco-Energy Systems are developing tailored solutions for pharmaceutical applications, indicating a competitive landscape with diverse expertise levels.
Anhui Double-Crane Pharmaceutical Co., Ltd.
Technical Solution: Anhui Double-Crane Pharmaceutical has developed an innovative thermoelectric waste heat recovery system specifically optimized for their fermentation and sterilization processes. Their proprietary solution captures waste heat from steam sterilization units and fermentation tanks, where temperatures typically range from 100-130°C. The system employs custom-designed thermoelectric modules with bismuth-antimony-telluride compounds that deliver enhanced performance in this specific temperature range. Double-Crane's implementation features a closed-loop heat transfer system that maintains strict separation between process equipment and power generation components, ensuring compliance with pharmaceutical GMP requirements. Their solution incorporates specialized heat exchangers with pharmaceutical-grade stainless steel surfaces that prevent contamination while maximizing heat transfer efficiency. The recovered electrical energy is primarily utilized to power auxiliary systems such as clean room HVAC controls and water purification systems, creating a partially self-sustaining manufacturing environment.
Strengths: Purpose-built for pharmaceutical applications with full GMP compliance; seamless integration with existing production lines; demonstrated energy cost reduction of approximately 15-20% in implemented facilities. Weaknesses: Limited scalability beyond specific temperature ranges found in fermentation processes; requires periodic recalibration to maintain optimal performance; higher maintenance requirements compared to passive heat recovery systems.
Abbott Laboratories
Technical Solution: Abbott Laboratories has developed an integrated thermoelectric waste heat recovery system specifically designed for pharmaceutical manufacturing processes. Their solution captures waste heat from various production stages including fermentation, distillation, and drying operations. The system employs advanced bismuth telluride-based thermoelectric generators (TEGs) with optimized n-type and p-type semiconductor materials to achieve conversion efficiencies of 5-7% in temperature differentials common in pharmaceutical operations (80-200°C). Abbott's implementation includes a modular design that can be retrofitted to existing equipment without significant process modifications, featuring heat exchangers with specialized coatings to prevent pharmaceutical contamination concerns. Their system incorporates real-time monitoring capabilities that integrate with manufacturing execution systems (MES) to provide continuous optimization of energy recovery based on production conditions.
Strengths: Seamless integration with existing pharmaceutical GMP environments; contamination-resistant materials compatible with strict regulatory requirements; modular approach allowing scalable implementation. Weaknesses: Higher initial capital costs compared to conventional heat exchangers; requires specialized maintenance expertise; efficiency drops significantly at lower temperature differentials.
Key Patents and Innovations in Pharmaceutical Heat Recovery
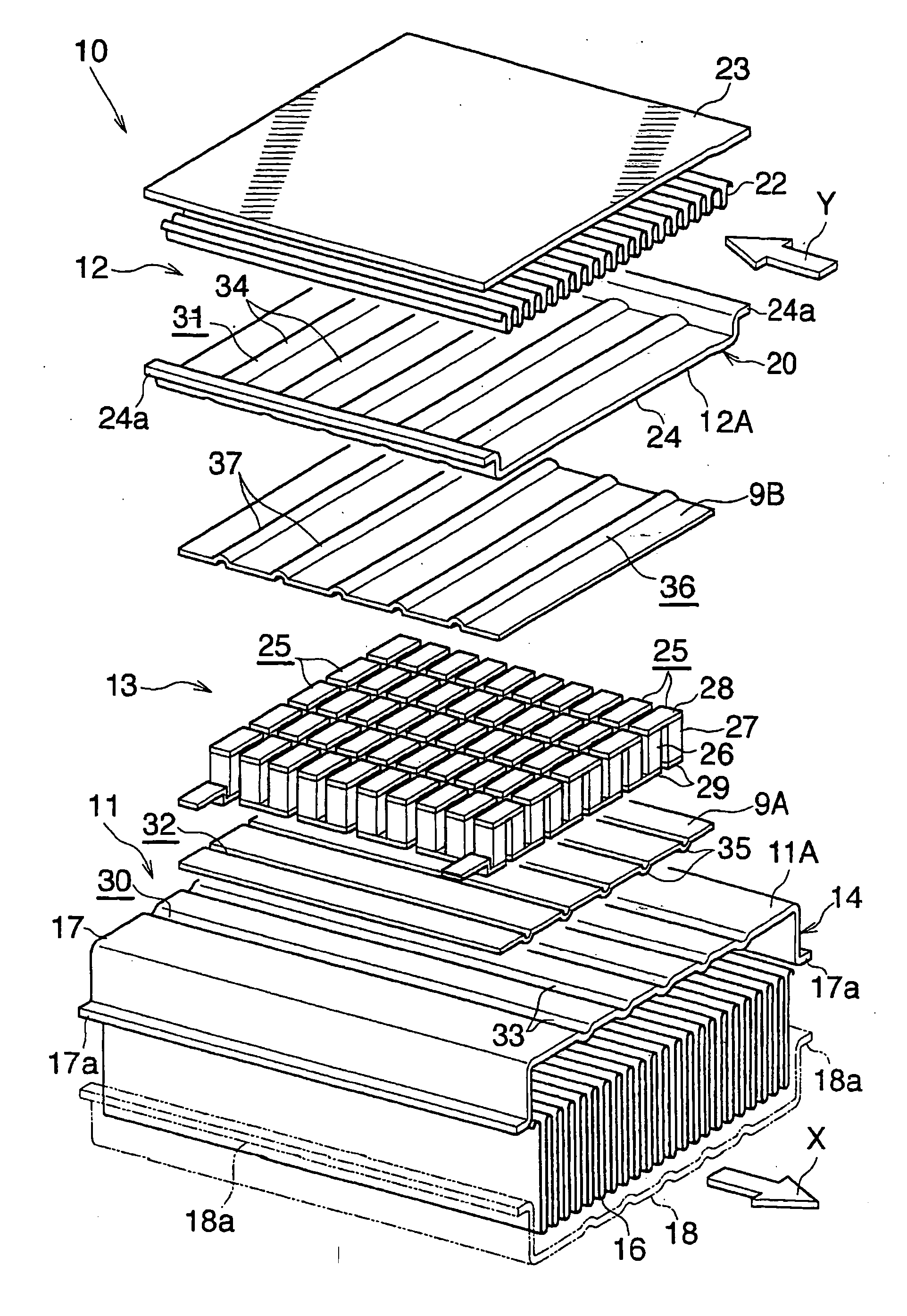
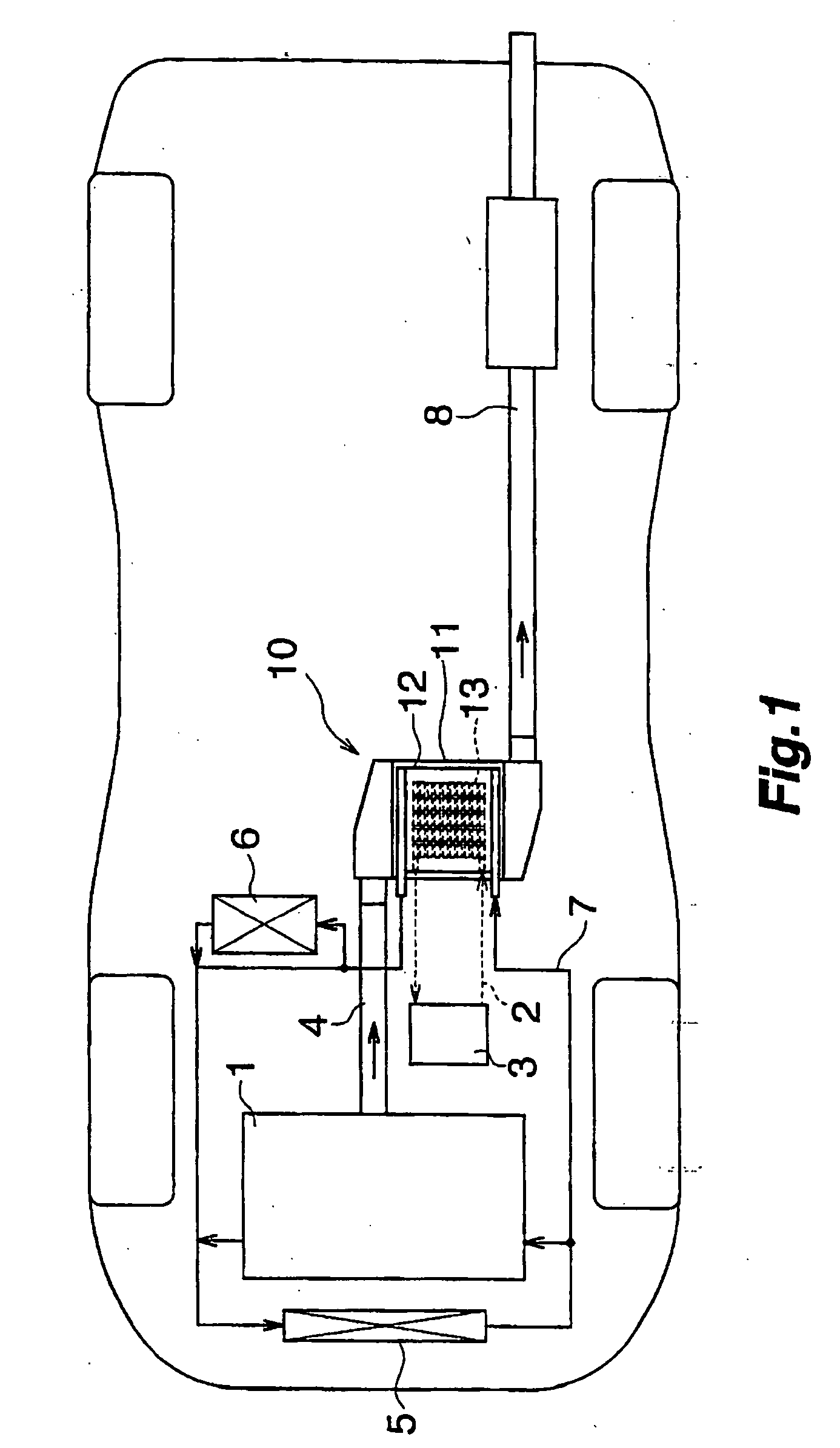
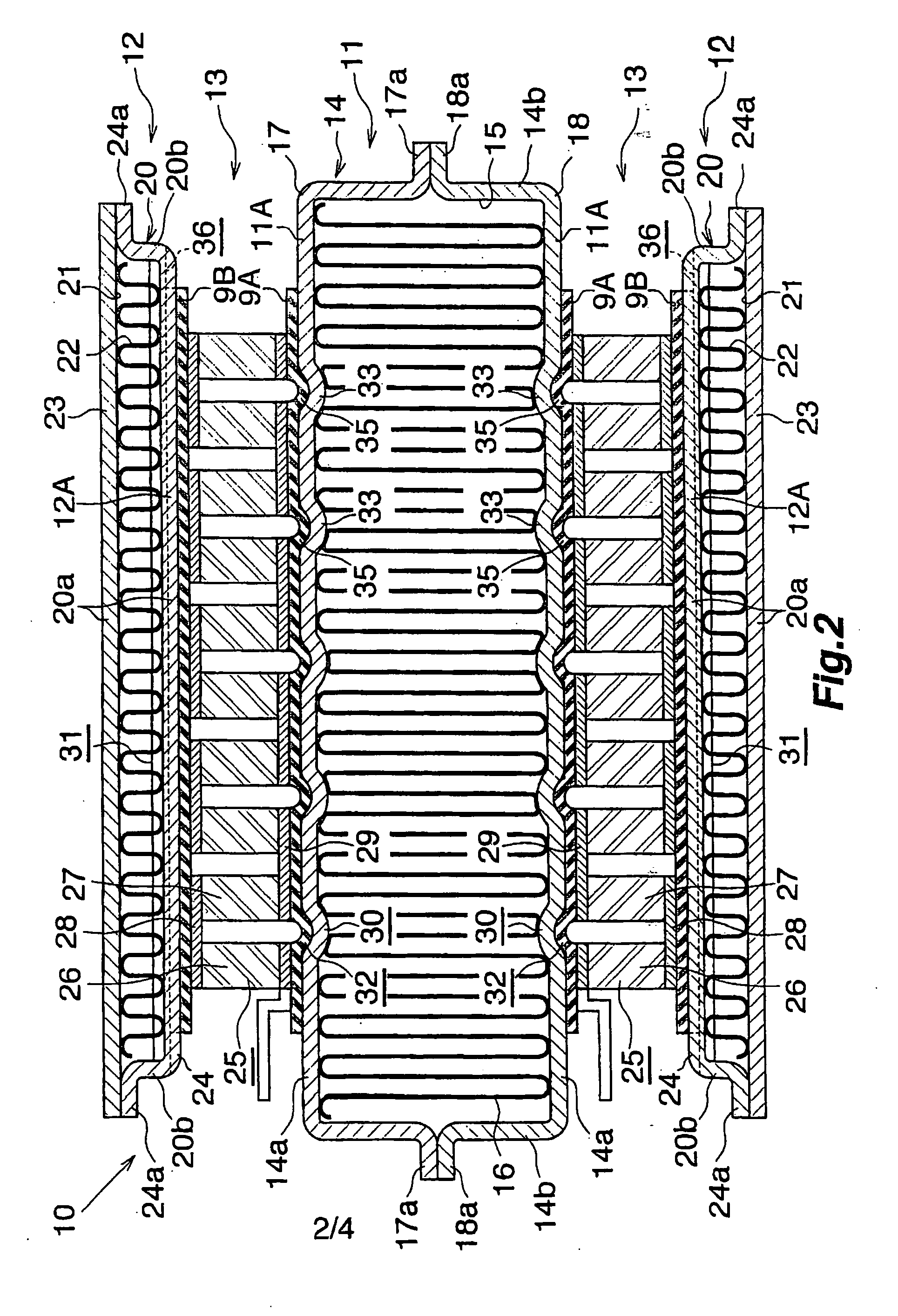
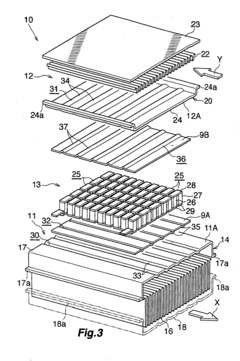
Waste heat recovery system and thermoelectric conversion system
PatentInactiveUS20060157102A1
Innovation
- A waste heat recovery system utilizing a thermoelectric conversion unit with sintered crystals of specific structures, connected in series with high-temperature and low-temperature heat exchangers, and thermal-stress relaxation features to enhance heat transfer and reduce thermal stress, allowing for efficient conversion of waste heat to electricity.
Waste heat recovery system and thermoelectric conversion unit
PatentWO2006075571A8
Innovation
- A waste heat recovery system incorporating a thermoelectric conversion unit with a sintered body of crystals less than 200 µm in size, utilizing a high-temperature side and low-temperature side heat exchanger configuration, and featuring thermal stress relief mechanisms to improve heat transfer and reliability, allowing for efficient conversion of waste heat into electricity and utilization for heating, defrosting, and engine temperature control.
ROI Analysis of Thermoelectric Implementation
Implementing thermoelectric waste heat recovery systems in pharmaceutical manufacturing processes represents a significant capital investment that requires thorough financial analysis. The return on investment (ROI) for these systems typically ranges from 2-5 years, depending on facility size, energy costs, and process characteristics.
Initial capital expenditure for thermoelectric implementation includes equipment costs ($150-300 per kW capacity), installation expenses (typically 30-40% of equipment costs), and system integration costs. For a medium-sized pharmaceutical facility, total implementation costs generally range from $500,000 to $2 million.
Energy savings constitute the primary financial benefit, with most pharmaceutical facilities able to recover 10-15% of waste heat energy. For a facility consuming 10 million kWh annually at $0.12/kWh, this translates to potential savings of $120,000-180,000 per year. Additional cost reductions come from decreased cooling requirements, as waste heat removal reduces the load on cooling systems.
Maintenance costs remain relatively low for thermoelectric systems, averaging 2-3% of capital costs annually, which is significantly lower than traditional heat recovery systems. This advantage stems from the solid-state nature of thermoelectric devices, which have no moving parts and minimal maintenance requirements.
Government incentives substantially improve ROI calculations. Many regions offer energy efficiency tax credits (10-30% of implementation costs), accelerated depreciation benefits, and utility company rebates that can cover up to 50% of project costs in some jurisdictions. These incentives can reduce payback periods by 1-2 years.
Sensitivity analysis reveals that ROI is most affected by energy prices, with each $0.01/kWh increase improving payback periods by approximately 8%. Production volume also significantly impacts returns, as facilities operating at higher capacities generate more waste heat available for recovery.
Long-term financial modeling demonstrates that thermoelectric systems maintain efficiency with minimal degradation over 15+ year lifespans, providing continued returns well beyond the initial payback period. When factoring in projected energy price increases of 3-5% annually, the lifetime value of these systems increases substantially.
Risk assessment indicates that the primary financial risks include longer-than-expected implementation timelines affecting cash flow projections and potential process disruptions during installation. However, modular implementation approaches can mitigate these risks by allowing phased deployment and validation.
Initial capital expenditure for thermoelectric implementation includes equipment costs ($150-300 per kW capacity), installation expenses (typically 30-40% of equipment costs), and system integration costs. For a medium-sized pharmaceutical facility, total implementation costs generally range from $500,000 to $2 million.
Energy savings constitute the primary financial benefit, with most pharmaceutical facilities able to recover 10-15% of waste heat energy. For a facility consuming 10 million kWh annually at $0.12/kWh, this translates to potential savings of $120,000-180,000 per year. Additional cost reductions come from decreased cooling requirements, as waste heat removal reduces the load on cooling systems.
Maintenance costs remain relatively low for thermoelectric systems, averaging 2-3% of capital costs annually, which is significantly lower than traditional heat recovery systems. This advantage stems from the solid-state nature of thermoelectric devices, which have no moving parts and minimal maintenance requirements.
Government incentives substantially improve ROI calculations. Many regions offer energy efficiency tax credits (10-30% of implementation costs), accelerated depreciation benefits, and utility company rebates that can cover up to 50% of project costs in some jurisdictions. These incentives can reduce payback periods by 1-2 years.
Sensitivity analysis reveals that ROI is most affected by energy prices, with each $0.01/kWh increase improving payback periods by approximately 8%. Production volume also significantly impacts returns, as facilities operating at higher capacities generate more waste heat available for recovery.
Long-term financial modeling demonstrates that thermoelectric systems maintain efficiency with minimal degradation over 15+ year lifespans, providing continued returns well beyond the initial payback period. When factoring in projected energy price increases of 3-5% annually, the lifetime value of these systems increases substantially.
Risk assessment indicates that the primary financial risks include longer-than-expected implementation timelines affecting cash flow projections and potential process disruptions during installation. However, modular implementation approaches can mitigate these risks by allowing phased deployment and validation.
Regulatory Compliance and GMP Considerations
The implementation of thermoelectric waste recovery systems in pharmaceutical manufacturing processes necessitates careful consideration of regulatory frameworks and Good Manufacturing Practice (GMP) requirements. Pharmaceutical operations are heavily regulated to ensure product quality, safety, and efficacy, making compliance a critical factor when introducing new technologies.
The FDA, EMA, and other global regulatory bodies have established specific guidelines for energy recovery systems that may impact pharmaceutical production environments. These regulations primarily focus on ensuring that waste heat recovery systems do not compromise product integrity or introduce contamination risks. Thermoelectric generators (TEGs) must be designed and installed in accordance with these requirements, particularly when implemented in critical processing areas.
GMP considerations for thermoelectric waste recovery systems include material compatibility, cleanability, and validation protocols. Materials used in TEG construction must be non-reactive, non-leaching, and compatible with cleaning agents commonly used in pharmaceutical facilities. Surfaces that may come into contact with pharmaceutical products or their components must meet stringent cleanability standards to prevent cross-contamination.
Documentation requirements represent another significant regulatory aspect. Pharmaceutical manufacturers must maintain comprehensive records of system design, installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) for thermoelectric waste recovery systems. These validation protocols must demonstrate that the systems operate consistently within specified parameters without adversely affecting product quality.
Risk assessment methodologies such as Failure Mode and Effects Analysis (FMEA) are essential when implementing thermoelectric waste recovery in pharmaceutical processes. These assessments help identify potential failure points and establish appropriate control measures to mitigate risks to product quality and patient safety.
Energy efficiency improvements through thermoelectric waste recovery can also support compliance with environmental regulations and sustainability initiatives. Many regulatory frameworks now include provisions for energy efficiency and carbon footprint reduction, making thermoelectric waste recovery systems potentially advantageous from a regulatory perspective.
Change control procedures must be rigorously applied when retrofitting existing pharmaceutical processes with thermoelectric waste recovery systems. These procedures ensure that modifications are properly evaluated, documented, and approved before implementation, maintaining the validated state of the manufacturing process.
Ongoing monitoring and periodic review of thermoelectric waste recovery systems are required to maintain regulatory compliance throughout the system lifecycle. This includes regular calibration of temperature sensors, performance verification of thermoelectric modules, and assessment of energy recovery efficiency against established parameters.
The FDA, EMA, and other global regulatory bodies have established specific guidelines for energy recovery systems that may impact pharmaceutical production environments. These regulations primarily focus on ensuring that waste heat recovery systems do not compromise product integrity or introduce contamination risks. Thermoelectric generators (TEGs) must be designed and installed in accordance with these requirements, particularly when implemented in critical processing areas.
GMP considerations for thermoelectric waste recovery systems include material compatibility, cleanability, and validation protocols. Materials used in TEG construction must be non-reactive, non-leaching, and compatible with cleaning agents commonly used in pharmaceutical facilities. Surfaces that may come into contact with pharmaceutical products or their components must meet stringent cleanability standards to prevent cross-contamination.
Documentation requirements represent another significant regulatory aspect. Pharmaceutical manufacturers must maintain comprehensive records of system design, installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) for thermoelectric waste recovery systems. These validation protocols must demonstrate that the systems operate consistently within specified parameters without adversely affecting product quality.
Risk assessment methodologies such as Failure Mode and Effects Analysis (FMEA) are essential when implementing thermoelectric waste recovery in pharmaceutical processes. These assessments help identify potential failure points and establish appropriate control measures to mitigate risks to product quality and patient safety.
Energy efficiency improvements through thermoelectric waste recovery can also support compliance with environmental regulations and sustainability initiatives. Many regulatory frameworks now include provisions for energy efficiency and carbon footprint reduction, making thermoelectric waste recovery systems potentially advantageous from a regulatory perspective.
Change control procedures must be rigorously applied when retrofitting existing pharmaceutical processes with thermoelectric waste recovery systems. These procedures ensure that modifications are properly evaluated, documented, and approved before implementation, maintaining the validated state of the manufacturing process.
Ongoing monitoring and periodic review of thermoelectric waste recovery systems are required to maintain regulatory compliance throughout the system lifecycle. This includes regular calibration of temperature sensors, performance verification of thermoelectric modules, and assessment of energy recovery efficiency against established parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!