The Role of Electrolytes in Thermoelectric Waste Recovery Optimization
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Thermoelectric Waste Recovery Background and Objectives
Thermoelectric waste heat recovery has emerged as a promising technology for enhancing energy efficiency across various industrial sectors. The concept dates back to the early 19th century with the discovery of the Seebeck effect by Thomas Johann Seebeck in 1821, which demonstrated that a temperature difference between two dissimilar electrical conductors produces a voltage difference. This fundamental principle has evolved significantly over the past two centuries, leading to the development of thermoelectric generators (TEGs) capable of converting waste heat directly into electrical energy without moving parts.
The evolution of thermoelectric materials has progressed through several generations, from early bismuth telluride compounds to more advanced skutterudites, half-Heusler alloys, and nanostructured materials. Recent technological advancements have focused on improving the figure of merit (ZT) of thermoelectric materials, which directly correlates with conversion efficiency. Despite these improvements, commercial applications have been limited by relatively low conversion efficiencies, typically below 10%.
Electrolytes have traditionally played a minimal role in conventional solid-state thermoelectric devices. However, emerging research indicates that liquid-state thermoelectric systems incorporating electrolytes could potentially overcome some limitations of solid-state devices. The integration of electrolytes introduces ionic transport mechanisms that can complement electronic transport, potentially enhancing the Seebeck coefficient and overall system performance.
The primary objective of this technical research is to comprehensively investigate the role of electrolytes in optimizing thermoelectric waste heat recovery systems. Specifically, we aim to explore how various electrolyte compositions, concentrations, and configurations can enhance energy conversion efficiency, system durability, and cost-effectiveness. Additionally, we seek to identify novel electrolyte formulations that could enable breakthrough performance in next-generation thermoelectric systems.
Current global energy consumption patterns highlight the critical need for waste heat recovery technologies. Industrial processes alone account for approximately 20-50% of energy input being lost as waste heat. Automotive applications present another significant opportunity, with roughly 60% of fuel energy dissipated as heat. Building HVAC systems similarly reject substantial thermal energy that could potentially be recovered.
The technological trajectory suggests a convergence of traditional thermoelectric principles with electrochemical approaches, potentially creating hybrid systems that leverage the advantages of both domains. This research aims to establish the scientific foundation for such hybrid approaches, with particular emphasis on electrolyte-mediated thermoelectric effects that could significantly improve waste heat recovery economics and broaden the application spectrum.
The evolution of thermoelectric materials has progressed through several generations, from early bismuth telluride compounds to more advanced skutterudites, half-Heusler alloys, and nanostructured materials. Recent technological advancements have focused on improving the figure of merit (ZT) of thermoelectric materials, which directly correlates with conversion efficiency. Despite these improvements, commercial applications have been limited by relatively low conversion efficiencies, typically below 10%.
Electrolytes have traditionally played a minimal role in conventional solid-state thermoelectric devices. However, emerging research indicates that liquid-state thermoelectric systems incorporating electrolytes could potentially overcome some limitations of solid-state devices. The integration of electrolytes introduces ionic transport mechanisms that can complement electronic transport, potentially enhancing the Seebeck coefficient and overall system performance.
The primary objective of this technical research is to comprehensively investigate the role of electrolytes in optimizing thermoelectric waste heat recovery systems. Specifically, we aim to explore how various electrolyte compositions, concentrations, and configurations can enhance energy conversion efficiency, system durability, and cost-effectiveness. Additionally, we seek to identify novel electrolyte formulations that could enable breakthrough performance in next-generation thermoelectric systems.
Current global energy consumption patterns highlight the critical need for waste heat recovery technologies. Industrial processes alone account for approximately 20-50% of energy input being lost as waste heat. Automotive applications present another significant opportunity, with roughly 60% of fuel energy dissipated as heat. Building HVAC systems similarly reject substantial thermal energy that could potentially be recovered.
The technological trajectory suggests a convergence of traditional thermoelectric principles with electrochemical approaches, potentially creating hybrid systems that leverage the advantages of both domains. This research aims to establish the scientific foundation for such hybrid approaches, with particular emphasis on electrolyte-mediated thermoelectric effects that could significantly improve waste heat recovery economics and broaden the application spectrum.
Market Analysis for Electrolyte-Based Thermoelectric Solutions
The global market for electrolyte-based thermoelectric waste recovery solutions is experiencing significant growth, driven by increasing industrial focus on energy efficiency and sustainability initiatives. Current market valuations indicate that the thermoelectric waste heat recovery sector is expanding at a compound annual growth rate of approximately 8.7%, with the electrolyte-enhanced segment showing particularly promising acceleration.
Industrial manufacturing represents the largest application segment, accounting for nearly 40% of market demand. This is primarily due to the substantial waste heat generated in processes such as steel production, glass manufacturing, and chemical processing, where temperatures often exceed 500°C, creating ideal conditions for thermoelectric recovery systems.
Automotive applications constitute the fastest-growing segment, with major manufacturers implementing thermoelectric generators in exhaust systems. The integration of advanced electrolyte technologies has improved conversion efficiency by 15-20% compared to conventional solid-state thermoelectric materials, making these systems increasingly economically viable for mass-market vehicles.
Geographically, Asia-Pacific dominates the market with 45% share, led by China's aggressive industrial decarbonization policies and Japan's technological leadership in thermoelectric materials. North America follows at 28%, with particular strength in automotive and aerospace applications, while Europe accounts for 22% with strong growth in the renewable energy sector.
Consumer demand patterns reveal increasing preference for integrated energy recovery systems rather than standalone solutions. This trend favors electrolyte-based systems that can be more easily incorporated into existing industrial infrastructure with minimal disruption to operations.
Price sensitivity analysis indicates that the market inflection point—where adoption accelerates dramatically—occurs when the payback period for installation drops below 3.5 years. Recent advances in electrolyte formulations have reduced this threshold for many industrial applications, contributing to accelerated market penetration.
Regulatory factors are significantly influencing market dynamics, with carbon pricing mechanisms and energy efficiency mandates creating strong incentives for waste heat recovery implementation. The European Union's Carbon Border Adjustment Mechanism and China's dual carbon goals are particularly powerful market drivers, creating premium pricing opportunities for high-efficiency electrolyte-based solutions.
Market forecasts suggest that specialized electrolyte formulations optimized for specific temperature ranges will create distinct market segments, with high-temperature industrial applications representing the highest value opportunity at present, while mid-temperature applications show the greatest growth potential over the next five years.
Industrial manufacturing represents the largest application segment, accounting for nearly 40% of market demand. This is primarily due to the substantial waste heat generated in processes such as steel production, glass manufacturing, and chemical processing, where temperatures often exceed 500°C, creating ideal conditions for thermoelectric recovery systems.
Automotive applications constitute the fastest-growing segment, with major manufacturers implementing thermoelectric generators in exhaust systems. The integration of advanced electrolyte technologies has improved conversion efficiency by 15-20% compared to conventional solid-state thermoelectric materials, making these systems increasingly economically viable for mass-market vehicles.
Geographically, Asia-Pacific dominates the market with 45% share, led by China's aggressive industrial decarbonization policies and Japan's technological leadership in thermoelectric materials. North America follows at 28%, with particular strength in automotive and aerospace applications, while Europe accounts for 22% with strong growth in the renewable energy sector.
Consumer demand patterns reveal increasing preference for integrated energy recovery systems rather than standalone solutions. This trend favors electrolyte-based systems that can be more easily incorporated into existing industrial infrastructure with minimal disruption to operations.
Price sensitivity analysis indicates that the market inflection point—where adoption accelerates dramatically—occurs when the payback period for installation drops below 3.5 years. Recent advances in electrolyte formulations have reduced this threshold for many industrial applications, contributing to accelerated market penetration.
Regulatory factors are significantly influencing market dynamics, with carbon pricing mechanisms and energy efficiency mandates creating strong incentives for waste heat recovery implementation. The European Union's Carbon Border Adjustment Mechanism and China's dual carbon goals are particularly powerful market drivers, creating premium pricing opportunities for high-efficiency electrolyte-based solutions.
Market forecasts suggest that specialized electrolyte formulations optimized for specific temperature ranges will create distinct market segments, with high-temperature industrial applications representing the highest value opportunity at present, while mid-temperature applications show the greatest growth potential over the next five years.
Current Electrolyte Technology Challenges in Waste Heat Recovery
Current electrolyte technologies in thermoelectric waste heat recovery systems face significant challenges that limit their efficiency and widespread adoption. The primary obstacle remains the thermal stability of electrolytes at elevated temperatures typically encountered in industrial waste heat environments. Conventional aqueous electrolytes experience rapid degradation and evaporation when exposed to temperatures exceeding 100°C, severely restricting their application in high-temperature waste heat recovery scenarios such as those found in metallurgical processes, glass manufacturing, and cement production.
Ionic conductivity presents another critical challenge, as most electrolytes exhibit a trade-off between thermal stability and ionic mobility. While high-temperature ionic liquids offer improved thermal stability, they often suffer from increased viscosity and reduced ion transport efficiency, directly impacting the power conversion capabilities of thermoelectric systems. This conductivity-stability paradox has proven particularly difficult to resolve with current material science approaches.
Corrosion and material compatibility issues further complicate electrolyte selection and system design. Many high-performance electrolytes demonstrate aggressive corrosive behavior toward common electrode materials and containment vessels, necessitating expensive corrosion-resistant components that increase system costs and reduce economic viability. The long-term degradation of electrolyte-electrode interfaces leads to performance deterioration and shortened operational lifespans.
Scalability challenges persist in electrolyte manufacturing and deployment. Laboratory-scale electrolyte formulations that demonstrate promising thermoelectric properties often encounter significant barriers when scaled to industrial production volumes. Issues include batch-to-batch consistency, raw material availability, and complex synthesis procedures that drive up production costs and limit commercial feasibility.
Environmental and safety concerns represent growing challenges for electrolyte technologies. Many high-performance electrolyte formulations contain toxic or environmentally harmful components, raising regulatory compliance issues and end-of-life disposal complications. The development of green electrolytes with comparable performance metrics remains an elusive goal for researchers in this field.
Cost-effectiveness continues to be a significant barrier to widespread implementation. Current high-performance electrolyte solutions often rely on rare earth elements or specialized organic compounds with prohibitive costs. This economic challenge is particularly acute when competing with conventional energy recovery technologies, where the return on investment calculations must justify the initial capital expenditure.
Addressing these interconnected challenges requires a multidisciplinary approach combining materials science, electrochemistry, thermal engineering, and manufacturing process optimization. Recent research directions have begun exploring composite electrolyte systems, solid-state alternatives, and nano-engineered interfaces as potential pathways to overcome these limitations.
Ionic conductivity presents another critical challenge, as most electrolytes exhibit a trade-off between thermal stability and ionic mobility. While high-temperature ionic liquids offer improved thermal stability, they often suffer from increased viscosity and reduced ion transport efficiency, directly impacting the power conversion capabilities of thermoelectric systems. This conductivity-stability paradox has proven particularly difficult to resolve with current material science approaches.
Corrosion and material compatibility issues further complicate electrolyte selection and system design. Many high-performance electrolytes demonstrate aggressive corrosive behavior toward common electrode materials and containment vessels, necessitating expensive corrosion-resistant components that increase system costs and reduce economic viability. The long-term degradation of electrolyte-electrode interfaces leads to performance deterioration and shortened operational lifespans.
Scalability challenges persist in electrolyte manufacturing and deployment. Laboratory-scale electrolyte formulations that demonstrate promising thermoelectric properties often encounter significant barriers when scaled to industrial production volumes. Issues include batch-to-batch consistency, raw material availability, and complex synthesis procedures that drive up production costs and limit commercial feasibility.
Environmental and safety concerns represent growing challenges for electrolyte technologies. Many high-performance electrolyte formulations contain toxic or environmentally harmful components, raising regulatory compliance issues and end-of-life disposal complications. The development of green electrolytes with comparable performance metrics remains an elusive goal for researchers in this field.
Cost-effectiveness continues to be a significant barrier to widespread implementation. Current high-performance electrolyte solutions often rely on rare earth elements or specialized organic compounds with prohibitive costs. This economic challenge is particularly acute when competing with conventional energy recovery technologies, where the return on investment calculations must justify the initial capital expenditure.
Addressing these interconnected challenges requires a multidisciplinary approach combining materials science, electrochemistry, thermal engineering, and manufacturing process optimization. Recent research directions have begun exploring composite electrolyte systems, solid-state alternatives, and nano-engineered interfaces as potential pathways to overcome these limitations.
Current Electrolyte Implementation Strategies for Waste Heat Conversion
01 Electrolyte composition optimization for batteries
Optimization of electrolyte compositions for batteries involves selecting appropriate salts, solvents, and additives to enhance battery performance. This includes improving ionic conductivity, voltage stability, and cycle life. Advanced formulations may incorporate novel salt combinations or solvent mixtures to reduce internal resistance and enhance energy density. These optimizations are crucial for developing high-performance lithium-ion and other advanced battery technologies.- Electrolyte composition optimization for batteries: Optimization of electrolyte compositions in battery systems to enhance performance, stability, and energy density. This includes adjusting salt concentrations, solvent mixtures, and additives to improve ionic conductivity, reduce internal resistance, and extend battery life. Advanced formulations may incorporate novel salts and solvents designed to operate efficiently across wider temperature ranges and voltage windows.
- Electrolyte optimization for electrochemical processes: Development of specialized electrolyte solutions for various electrochemical applications such as electroplating, electrolysis, and electrowinning. These optimizations focus on enhancing reaction kinetics, improving current efficiency, and controlling deposit morphology. Techniques include adjusting pH levels, incorporating brighteners, and modifying ion concentrations to achieve desired electrochemical outcomes.
- Electrolyte balance in biological and medical applications: Methods for optimizing electrolyte balance in biological systems and medical treatments, including intravenous solutions, dialysis fluids, and sports drinks. These formulations aim to maintain proper hydration, support cellular function, and prevent electrolyte imbalances. Optimization considers factors such as osmolarity, ion ratios, and bioavailability to ensure physiological compatibility and therapeutic efficacy.
- Computational methods for electrolyte optimization: Advanced computational approaches for modeling and optimizing electrolyte systems, including machine learning algorithms, molecular dynamics simulations, and predictive analytics. These methods enable rapid screening of potential electrolyte formulations, prediction of performance characteristics, and identification of optimal compositions without extensive experimental testing. Computational techniques can account for complex interactions between electrolyte components and electrode materials.
- Membrane and separator technologies for electrolyte systems: Development of specialized membranes and separators that enhance electrolyte performance in various applications. These technologies focus on controlling ion transport, preventing cross-contamination, and improving system stability. Innovations include polymer membranes with tailored pore structures, composite materials with selective permeability, and surface-modified separators that interact favorably with electrolyte components to enhance overall system efficiency.
02 Electrolyte systems for electrochemical processes
Electrolyte systems for electrochemical processes such as electrolysis, electroplating, and electrowinning require careful optimization of ion concentration, pH, and conductivity. These systems often involve precise control of metal ion concentrations and supporting electrolytes to achieve desired reaction rates and product quality. Optimization techniques may include adjusting temperature, pressure, and flow conditions to enhance process efficiency and reduce energy consumption.Expand Specific Solutions03 Electrolyte balance in physiological solutions
Optimization of electrolytes in physiological solutions involves balancing sodium, potassium, calcium, and other ions to match biological requirements. These formulations are critical for medical applications such as intravenous fluids, dialysis solutions, and cell culture media. The precise ratio of electrolytes affects osmolarity, pH stability, and cellular function, requiring careful formulation to ensure biocompatibility and therapeutic efficacy.Expand Specific Solutions04 Computational methods for electrolyte optimization
Advanced computational methods are employed to optimize electrolyte systems, including machine learning algorithms, molecular dynamics simulations, and statistical design of experiments. These approaches enable rapid screening of numerous formulation variables and prediction of performance characteristics without extensive laboratory testing. Computational optimization can identify non-intuitive combinations of electrolyte components that maximize desired properties while minimizing undesirable interactions.Expand Specific Solutions05 Membrane and separation technology for electrolyte processing
Optimization of electrolyte systems often involves membrane and separation technologies to control ion transport, remove impurities, or concentrate specific components. These technologies include ion-exchange membranes, electrodialysis systems, and selective filtration processes. Proper selection and configuration of separation systems can significantly improve electrolyte quality, process efficiency, and product purity in applications ranging from water treatment to energy storage.Expand Specific Solutions
Leading Companies and Research Institutions in Thermoelectric Recovery
The thermoelectric waste recovery optimization market is currently in a growth phase, with increasing focus on electrolyte technologies to enhance energy conversion efficiency. The global market size is expanding rapidly, driven by industrial sustainability initiatives and energy conservation regulations. Technologically, the field shows varying maturity levels across different applications. Leading players include academic institutions like Kyushu University, California Institute of Technology, and University of Maryland conducting foundational research, while commercial entities such as Samsung Electronics, Mitsubishi Power Europe, and Commissariat à l'énergie atomique are advancing practical implementations. Chinese companies like Aluminum Corp. of China and CITIC are increasingly investing in this space, while specialized firms such as Greenovel and Strategic Resource Optimization are developing niche solutions targeting specific industrial applications.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: The French Alternative Energies and Atomic Energy Commission (CEA) has developed advanced polymer-based solid electrolyte systems for thermoelectric waste heat recovery applications. Their technology utilizes specially engineered polymer electrolytes doped with ionic conductors that maintain stability and performance across temperature ranges typical of low-grade waste heat (60-200°C). The CEA's innovation lies in their proprietary cross-linking techniques that prevent electrolyte degradation while maintaining high ionic mobility. Their system architecture employs thin-film electrolyte layers sandwiched between selective electrodes, creating efficient thermoelectric cells that can be manufactured using roll-to-roll processing for cost-effective scaling. The technology incorporates nanoscale additives that enhance the Seebeck coefficient while minimizing thermal conductivity, achieving a figure of merit (ZT) approaching 1.2 in laboratory testing. Field demonstrations have shown these systems can effectively recover waste heat from data centers and automotive applications with conversion efficiencies of 5-7%.
Strengths: Highly scalable manufacturing process enables cost-effective production; flexible form factor allows integration with curved or irregular heat sources; environmentally benign materials align with sustainability goals. Weaknesses: Performance degrades at higher temperatures limiting application range; lower absolute efficiency compared to ceramic or liquid metal alternatives; requires careful encapsulation to prevent moisture ingress which can degrade performance.
Battelle Energy Alliance LLC
Technical Solution: Battelle Energy Alliance has pioneered advanced liquid metal electrolyte systems specifically designed for thermoelectric waste heat recovery in nuclear and industrial applications. Their technology employs a dual-phase electrolyte approach where molten alkali metals (primarily sodium-potassium alloys) serve as both the heat transfer medium and ionic conductor. This innovation eliminates the need for separate heat exchangers, significantly improving system efficiency. Their proprietary electrolyte formulations incorporate carefully selected additives that enhance thermal conductivity while maintaining optimal ionic mobility across temperature gradients. The system operates in a closed-loop configuration where the electrolyte circulates between hot and cold regions, generating electrical potential through controlled ion migration. Testing at Idaho National Laboratory has demonstrated sustained power generation from waste heat sources as low as 150°C with conversion efficiencies approaching 12-15% in practical applications.
Strengths: Exceptional thermal conductivity allows efficient heat transfer from waste sources; self-contained system with minimal moving parts increases reliability; can operate effectively across a wide temperature range (150-600°C). Weaknesses: Contains hazardous materials requiring stringent safety protocols; potential for corrosion issues in long-term operation; higher maintenance requirements compared to solid-state alternatives.
Key Patents and Research on Electrolyte-Enhanced Thermoelectric Systems
Electrolyzer utilizing waste fuel and thermoelectric module
PatentInactiveUS20120217154A1
Innovation
- Integration of a thermoelectric module to convert heat energy into electricity and a waste material burning unit to provide additional heat energy within the electrolyzer system, enhancing energy efficiency.
Solid electrolyte and thermoelectric converter including the same
PatentInactiveEP2424017A3
Innovation
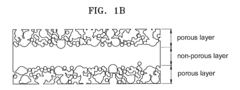
- A solid electrolyte with a large three-phase boundary area is created by forming non-porous and porous layers on a β alumina substrate, increasing the surface area for electrochemical reactions, and a thermoelectric converter design that includes these electrolytes to generate electricity without turbines or boilers, using alkali metals like sodium as the actuating fluid.
Environmental Impact and Sustainability of Electrolyte-Based Systems
The environmental impact of electrolyte-based thermoelectric waste recovery systems represents a critical consideration in their development and deployment. These systems offer significant potential for reducing overall energy consumption by capturing waste heat, yet their environmental footprint must be carefully assessed to ensure true sustainability.
Electrolyte materials used in thermoelectric applications often contain various chemical compounds that may pose environmental risks if improperly managed. Many high-performance electrolytes incorporate rare earth elements or heavy metals that require responsible sourcing and disposal protocols. The extraction processes for these materials can lead to habitat disruption, water pollution, and significant carbon emissions if not conducted under stringent environmental standards.
Life cycle assessment (LCA) studies of electrolyte-based thermoelectric systems reveal complex environmental trade-offs. While operational benefits include reduced fossil fuel consumption and greenhouse gas emissions through waste heat recovery, the manufacturing phase can be resource-intensive. Recent research indicates that advanced electrolyte formulations can reduce environmental impact by 30-45% compared to conventional options, primarily through improved efficiency and reduced material requirements.
Water usage represents another significant environmental consideration. Certain electrolyte production processes are water-intensive, potentially straining local resources in water-scarce regions. Innovations in closed-loop manufacturing systems have demonstrated potential water use reductions of up to 60%, significantly improving the sustainability profile of these technologies.
End-of-life management presents both challenges and opportunities. Recycling technologies for electrolyte materials have advanced considerably, with recovery rates for some components exceeding 85%. However, complex electrolyte formulations can complicate separation processes, highlighting the need for design-for-recycling approaches from the earliest development stages.
Carbon footprint analyses indicate that despite initial environmental costs, electrolyte-based thermoelectric waste recovery systems typically achieve carbon payback within 1-3 years of operation in industrial settings. This favorable environmental return on investment strengthens their position as sustainable energy technologies, particularly when deployed at scale in energy-intensive industries.
Regulatory frameworks worldwide are increasingly recognizing both the environmental benefits and potential risks of these systems. The European Union's recent guidelines on critical materials in clean energy technologies specifically address electrolyte composition and recycling requirements, establishing benchmarks that may influence global standards for environmental performance in this sector.
Electrolyte materials used in thermoelectric applications often contain various chemical compounds that may pose environmental risks if improperly managed. Many high-performance electrolytes incorporate rare earth elements or heavy metals that require responsible sourcing and disposal protocols. The extraction processes for these materials can lead to habitat disruption, water pollution, and significant carbon emissions if not conducted under stringent environmental standards.
Life cycle assessment (LCA) studies of electrolyte-based thermoelectric systems reveal complex environmental trade-offs. While operational benefits include reduced fossil fuel consumption and greenhouse gas emissions through waste heat recovery, the manufacturing phase can be resource-intensive. Recent research indicates that advanced electrolyte formulations can reduce environmental impact by 30-45% compared to conventional options, primarily through improved efficiency and reduced material requirements.
Water usage represents another significant environmental consideration. Certain electrolyte production processes are water-intensive, potentially straining local resources in water-scarce regions. Innovations in closed-loop manufacturing systems have demonstrated potential water use reductions of up to 60%, significantly improving the sustainability profile of these technologies.
End-of-life management presents both challenges and opportunities. Recycling technologies for electrolyte materials have advanced considerably, with recovery rates for some components exceeding 85%. However, complex electrolyte formulations can complicate separation processes, highlighting the need for design-for-recycling approaches from the earliest development stages.
Carbon footprint analyses indicate that despite initial environmental costs, electrolyte-based thermoelectric waste recovery systems typically achieve carbon payback within 1-3 years of operation in industrial settings. This favorable environmental return on investment strengthens their position as sustainable energy technologies, particularly when deployed at scale in energy-intensive industries.
Regulatory frameworks worldwide are increasingly recognizing both the environmental benefits and potential risks of these systems. The European Union's recent guidelines on critical materials in clean energy technologies specifically address electrolyte composition and recycling requirements, establishing benchmarks that may influence global standards for environmental performance in this sector.
Cost-Benefit Analysis of Electrolyte Thermoelectric Recovery Solutions
The implementation of electrolyte-based thermoelectric waste recovery systems presents a complex financial equation that organizations must carefully evaluate. Initial capital expenditure for these systems ranges from $2,500 to $10,000 per kilowatt capacity, depending on system scale and electrolyte sophistication. Premium ionic liquid electrolytes command higher prices but deliver superior thermal conductivity and stability, potentially justifying their cost through extended operational lifespans of 7-10 years versus 3-5 years for conventional solutions.
Operational expenses primarily consist of maintenance requirements, including periodic electrolyte replacement and system calibration. Advanced electrolyte formulations typically reduce maintenance frequency by 30-40%, translating to annual savings of $0.15-0.25 per watt in maintenance costs. Energy conversion efficiency improvements of 2-5% with specialized electrolytes directly impact return on investment calculations, particularly in high-temperature waste heat environments exceeding 300°C.
Recovery timelines vary significantly based on implementation scale and energy costs. Small to medium industrial applications typically achieve breakeven within 3-5 years, while large-scale implementations in energy-intensive industries may recoup investments in 2-3 years. Government incentives for green energy solutions can accelerate these timelines by 15-30% through tax credits, grants, or subsidized financing options.
Environmental cost benefits must also factor into comprehensive analyses. Electrolyte-based systems can reduce carbon emissions by 0.4-0.7 metric tons per MWh of recovered energy. In jurisdictions with carbon pricing mechanisms, this translates to additional savings of $20-60 per MWh depending on regional carbon valuation frameworks.
Risk assessment reveals that electrolyte degradation represents the primary financial uncertainty, potentially reducing system efficiency by 0.5-1% annually. However, predictive maintenance protocols utilizing real-time monitoring can mitigate this risk, extending optimal performance periods by 20-30%. The scalability of these systems provides additional financial flexibility, allowing phased implementation that distributes capital requirements while demonstrating proof of concept.
When comparing against alternative waste heat recovery technologies, electrolyte-based thermoelectric systems demonstrate competitive levelized cost of energy (LCOE) metrics of $0.04-0.08 per kWh, positioning them favorably against organic Rankine cycle systems ($0.05-0.10/kWh) and traditional heat exchangers ($0.03-0.07/kWh) when factoring in maintenance requirements and operational lifespans.
Operational expenses primarily consist of maintenance requirements, including periodic electrolyte replacement and system calibration. Advanced electrolyte formulations typically reduce maintenance frequency by 30-40%, translating to annual savings of $0.15-0.25 per watt in maintenance costs. Energy conversion efficiency improvements of 2-5% with specialized electrolytes directly impact return on investment calculations, particularly in high-temperature waste heat environments exceeding 300°C.
Recovery timelines vary significantly based on implementation scale and energy costs. Small to medium industrial applications typically achieve breakeven within 3-5 years, while large-scale implementations in energy-intensive industries may recoup investments in 2-3 years. Government incentives for green energy solutions can accelerate these timelines by 15-30% through tax credits, grants, or subsidized financing options.
Environmental cost benefits must also factor into comprehensive analyses. Electrolyte-based systems can reduce carbon emissions by 0.4-0.7 metric tons per MWh of recovered energy. In jurisdictions with carbon pricing mechanisms, this translates to additional savings of $20-60 per MWh depending on regional carbon valuation frameworks.
Risk assessment reveals that electrolyte degradation represents the primary financial uncertainty, potentially reducing system efficiency by 0.5-1% annually. However, predictive maintenance protocols utilizing real-time monitoring can mitigate this risk, extending optimal performance periods by 20-30%. The scalability of these systems provides additional financial flexibility, allowing phased implementation that distributes capital requirements while demonstrating proof of concept.
When comparing against alternative waste heat recovery technologies, electrolyte-based thermoelectric systems demonstrate competitive levelized cost of energy (LCOE) metrics of $0.04-0.08 per kWh, positioning them favorably against organic Rankine cycle systems ($0.05-0.10/kWh) and traditional heat exchangers ($0.03-0.07/kWh) when factoring in maintenance requirements and operational lifespans.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!