How to Address Hypochlorous Acid's Stability in Transport?
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HOCl Stability Challenges
Hypochlorous acid (HOCl) presents significant stability challenges during transport, primarily due to its inherent chemical properties and reactivity. The molecule's instability stems from its tendency to decompose into chlorine gas and water, especially when exposed to light, heat, or certain impurities. This decomposition not only reduces the effectiveness of HOCl solutions but also poses safety risks during storage and transportation.
One of the primary factors affecting HOCl stability is pH level. The acid is most stable at a pH range of 3.5 to 5.5, with optimal stability around pH 4. Outside this range, the molecule rapidly decomposes, losing its antimicrobial efficacy. Temperature also plays a crucial role in HOCl stability. Higher temperatures accelerate the decomposition process, necessitating careful temperature control during transport and storage.
Light exposure is another significant challenge for HOCl stability. Ultraviolet (UV) light catalyzes the breakdown of HOCl, leading to a rapid loss of active chlorine content. This photosensitivity necessitates the use of opaque or UV-resistant packaging materials to protect the solution during transport and storage.
The presence of organic matter or metal ions can also compromise HOCl stability. These contaminants can react with HOCl, leading to its rapid degradation. This issue is particularly problematic in industrial settings where the purity of water used for HOCl production may vary.
The concentration of HOCl in solution is another critical factor affecting its stability. Higher concentrations tend to be less stable, as they are more prone to self-decomposition. This creates a challenge in balancing the need for potent solutions with the requirement for stability during transport.
Packaging materials used for HOCl transport must be carefully selected to avoid reactions with the acid. Many common materials, including certain plastics and metals, can react with HOCl, leading to both degradation of the solution and potential damage to the container. This necessitates the use of specialized, chemically resistant packaging materials.
Addressing these stability challenges requires a multifaceted approach. Strategies may include precise pH control, temperature-regulated transport conditions, use of UV-resistant and chemically inert packaging, and the addition of stabilizing agents. Some researchers have explored the use of buffer systems to maintain optimal pH levels, while others have investigated novel packaging technologies that can actively regulate the internal environment of HOCl containers.
One of the primary factors affecting HOCl stability is pH level. The acid is most stable at a pH range of 3.5 to 5.5, with optimal stability around pH 4. Outside this range, the molecule rapidly decomposes, losing its antimicrobial efficacy. Temperature also plays a crucial role in HOCl stability. Higher temperatures accelerate the decomposition process, necessitating careful temperature control during transport and storage.
Light exposure is another significant challenge for HOCl stability. Ultraviolet (UV) light catalyzes the breakdown of HOCl, leading to a rapid loss of active chlorine content. This photosensitivity necessitates the use of opaque or UV-resistant packaging materials to protect the solution during transport and storage.
The presence of organic matter or metal ions can also compromise HOCl stability. These contaminants can react with HOCl, leading to its rapid degradation. This issue is particularly problematic in industrial settings where the purity of water used for HOCl production may vary.
The concentration of HOCl in solution is another critical factor affecting its stability. Higher concentrations tend to be less stable, as they are more prone to self-decomposition. This creates a challenge in balancing the need for potent solutions with the requirement for stability during transport.
Packaging materials used for HOCl transport must be carefully selected to avoid reactions with the acid. Many common materials, including certain plastics and metals, can react with HOCl, leading to both degradation of the solution and potential damage to the container. This necessitates the use of specialized, chemically resistant packaging materials.
Addressing these stability challenges requires a multifaceted approach. Strategies may include precise pH control, temperature-regulated transport conditions, use of UV-resistant and chemically inert packaging, and the addition of stabilizing agents. Some researchers have explored the use of buffer systems to maintain optimal pH levels, while others have investigated novel packaging technologies that can actively regulate the internal environment of HOCl containers.
Market Demand Analysis
The market demand for hypochlorous acid (HOCl) has been steadily increasing across various industries due to its powerful disinfectant properties and eco-friendly nature. The global hypochlorous acid market size was valued at USD 1.42 billion in 2020 and is projected to grow at a compound annual growth rate (CAGR) of 7.8% from 2021 to 2028. This growth is primarily driven by the rising awareness of hygiene and sanitation, especially in the wake of the COVID-19 pandemic.
The healthcare sector remains the largest consumer of hypochlorous acid, accounting for over 35% of the market share. Hospitals, clinics, and other healthcare facilities use HOCl for surface disinfection, wound care, and sterilization of medical equipment. The increasing focus on preventing healthcare-associated infections is expected to further boost demand in this sector.
The food and beverage industry is another significant market for hypochlorous acid. With stringent food safety regulations and growing consumer awareness about foodborne illnesses, there is a rising demand for effective and safe disinfectants in food processing and packaging. HOCl's ability to eliminate pathogens without leaving harmful residues makes it an attractive option for this industry.
Water treatment is an emerging application area for hypochlorous acid. As concerns about water quality and scarcity grow, the demand for efficient water treatment solutions is increasing. HOCl's effectiveness in treating both potable and wastewater is driving its adoption in municipal water treatment plants and industrial facilities.
The agriculture sector is also showing increased interest in hypochlorous acid. Its use in crop protection, soil treatment, and livestock care is gaining traction due to its non-toxic nature and ability to control plant pathogens without harming beneficial microorganisms.
However, the market demand for hypochlorous acid is significantly impacted by its stability issues during transport and storage. The short shelf life of HOCl solutions, typically ranging from a few days to a few weeks, poses a major challenge for manufacturers and end-users. This instability limits the product's distribution range and increases the need for on-site generation systems, which can be costly for smaller businesses.
To address this challenge, there is a growing demand for stabilized hypochlorous acid formulations and improved packaging solutions that can extend the product's shelf life. Manufacturers are investing in research and development to create more stable HOCl products that can withstand transportation and longer storage periods without losing efficacy.
The market is also seeing increased demand for concentrated HOCl formulations that can be diluted at the point of use, reducing transportation costs and improving stability during transit. Additionally, there is a rising interest in dry hypochlorous acid precursors that can be activated with water when needed, offering a more stable alternative to liquid formulations.
The healthcare sector remains the largest consumer of hypochlorous acid, accounting for over 35% of the market share. Hospitals, clinics, and other healthcare facilities use HOCl for surface disinfection, wound care, and sterilization of medical equipment. The increasing focus on preventing healthcare-associated infections is expected to further boost demand in this sector.
The food and beverage industry is another significant market for hypochlorous acid. With stringent food safety regulations and growing consumer awareness about foodborne illnesses, there is a rising demand for effective and safe disinfectants in food processing and packaging. HOCl's ability to eliminate pathogens without leaving harmful residues makes it an attractive option for this industry.
Water treatment is an emerging application area for hypochlorous acid. As concerns about water quality and scarcity grow, the demand for efficient water treatment solutions is increasing. HOCl's effectiveness in treating both potable and wastewater is driving its adoption in municipal water treatment plants and industrial facilities.
The agriculture sector is also showing increased interest in hypochlorous acid. Its use in crop protection, soil treatment, and livestock care is gaining traction due to its non-toxic nature and ability to control plant pathogens without harming beneficial microorganisms.
However, the market demand for hypochlorous acid is significantly impacted by its stability issues during transport and storage. The short shelf life of HOCl solutions, typically ranging from a few days to a few weeks, poses a major challenge for manufacturers and end-users. This instability limits the product's distribution range and increases the need for on-site generation systems, which can be costly for smaller businesses.
To address this challenge, there is a growing demand for stabilized hypochlorous acid formulations and improved packaging solutions that can extend the product's shelf life. Manufacturers are investing in research and development to create more stable HOCl products that can withstand transportation and longer storage periods without losing efficacy.
The market is also seeing increased demand for concentrated HOCl formulations that can be diluted at the point of use, reducing transportation costs and improving stability during transit. Additionally, there is a rising interest in dry hypochlorous acid precursors that can be activated with water when needed, offering a more stable alternative to liquid formulations.
Current Limitations
Hypochlorous acid (HOCl) presents significant challenges in terms of stability during transport, which limits its widespread application despite its potent antimicrobial properties. The primary limitation stems from its inherent chemical instability, as HOCl readily decomposes into chlorine gas and water, especially when exposed to light, heat, or certain metals.
One of the major hurdles in transporting HOCl is its sensitivity to temperature fluctuations. Even slight increases in temperature can accelerate the decomposition process, reducing the concentration and effectiveness of the solution. This temperature sensitivity necessitates stringent control measures during transportation, which can be both costly and logistically complex.
The pH of HOCl solutions also plays a crucial role in their stability. The optimal pH range for HOCl stability is narrow, typically between 3.5 and 5.5. Deviations from this range can lead to rapid degradation of the active compound. Maintaining this precise pH balance during transport, especially over long distances or extended periods, poses a significant challenge.
Light exposure is another critical factor affecting HOCl stability. Ultraviolet (UV) light, in particular, can catalyze the breakdown of HOCl, necessitating opaque or UV-resistant packaging. However, such packaging solutions often come with increased costs and may not be fully effective in all transport scenarios.
The presence of organic matter or contaminants can also accelerate the decomposition of HOCl. This is particularly problematic in scenarios where the transport containers or pipelines are not thoroughly cleaned or when the HOCl solution comes into contact with incompatible materials during handling and transfer processes.
The reactivity of HOCl with certain metals presents another limitation. Materials such as copper, brass, or certain grades of stainless steel can catalyze the decomposition of HOCl. This restricts the choice of transport containers and pipelines, often necessitating the use of more expensive, specialized materials that are resistant to HOCl's corrosive properties.
Time is a critical factor in HOCl stability. Even under optimal conditions, HOCl solutions gradually lose their potency over time. This time-dependent degradation imposes limitations on transport duration and storage periods, affecting supply chain logistics and inventory management strategies.
Lastly, the concentration of HOCl solutions impacts their stability during transport. Higher concentrations, while more potent, tend to be less stable and more prone to rapid decomposition. This creates a trade-off between potency and stability, often requiring compromises in formulation that may limit the effectiveness of the final product at the point of use.
One of the major hurdles in transporting HOCl is its sensitivity to temperature fluctuations. Even slight increases in temperature can accelerate the decomposition process, reducing the concentration and effectiveness of the solution. This temperature sensitivity necessitates stringent control measures during transportation, which can be both costly and logistically complex.
The pH of HOCl solutions also plays a crucial role in their stability. The optimal pH range for HOCl stability is narrow, typically between 3.5 and 5.5. Deviations from this range can lead to rapid degradation of the active compound. Maintaining this precise pH balance during transport, especially over long distances or extended periods, poses a significant challenge.
Light exposure is another critical factor affecting HOCl stability. Ultraviolet (UV) light, in particular, can catalyze the breakdown of HOCl, necessitating opaque or UV-resistant packaging. However, such packaging solutions often come with increased costs and may not be fully effective in all transport scenarios.
The presence of organic matter or contaminants can also accelerate the decomposition of HOCl. This is particularly problematic in scenarios where the transport containers or pipelines are not thoroughly cleaned or when the HOCl solution comes into contact with incompatible materials during handling and transfer processes.
The reactivity of HOCl with certain metals presents another limitation. Materials such as copper, brass, or certain grades of stainless steel can catalyze the decomposition of HOCl. This restricts the choice of transport containers and pipelines, often necessitating the use of more expensive, specialized materials that are resistant to HOCl's corrosive properties.
Time is a critical factor in HOCl stability. Even under optimal conditions, HOCl solutions gradually lose their potency over time. This time-dependent degradation imposes limitations on transport duration and storage periods, affecting supply chain logistics and inventory management strategies.
Lastly, the concentration of HOCl solutions impacts their stability during transport. Higher concentrations, while more potent, tend to be less stable and more prone to rapid decomposition. This creates a trade-off between potency and stability, often requiring compromises in formulation that may limit the effectiveness of the final product at the point of use.
Existing Stabilization Methods
01 pH control for stability
Maintaining an optimal pH range is crucial for the stability of hypochlorous acid. Adjusting and controlling the pH level can significantly enhance the shelf life and effectiveness of hypochlorous acid solutions. This can be achieved through the use of buffer systems or pH adjusting agents.- pH control for stability: Maintaining an optimal pH range is crucial for the stability of hypochlorous acid. Adjusting and controlling the pH level can significantly enhance the shelf life and effectiveness of hypochlorous acid solutions. This can be achieved through the use of buffer systems or pH adjusting agents.
- Stabilizing additives: Incorporating specific additives can improve the stability of hypochlorous acid. These may include antioxidants, chelating agents, or other stabilizing compounds that prevent degradation and maintain the acid's potency over time. The choice of additives depends on the intended application and storage conditions.
- Packaging and storage solutions: Proper packaging and storage conditions play a vital role in maintaining hypochlorous acid stability. This includes using light-resistant containers, controlling temperature and humidity during storage, and minimizing exposure to air and contaminants. Innovative packaging designs can help preserve the acid's efficacy for extended periods.
- Production methods for enhanced stability: Developing improved production methods can lead to more stable hypochlorous acid formulations. This may involve optimizing electrolysis parameters, using high-purity precursors, or implementing novel synthesis techniques that result in a more stable end product with fewer impurities and longer shelf life.
- Combination with other compounds: Combining hypochlorous acid with compatible compounds can enhance its stability and functionality. This may include creating synergistic formulations with other antimicrobial agents, incorporating it into stable emulsions, or developing novel delivery systems that protect the acid from degradation while maintaining its effectiveness.
02 Stabilizing additives
Incorporating specific additives can improve the stability of hypochlorous acid. These may include antioxidants, chelating agents, or other stabilizing compounds that prevent degradation and maintain the active concentration of hypochlorous acid over time.Expand Specific Solutions03 Packaging and storage solutions
Proper packaging and storage conditions play a vital role in maintaining hypochlorous acid stability. This includes using light-resistant containers, controlling storage temperature, and minimizing exposure to air and contaminants. Innovative packaging designs can help preserve the integrity of the solution.Expand Specific Solutions04 Electrochemical production methods
Advanced electrochemical techniques for producing hypochlorous acid can lead to more stable formulations. These methods focus on generating hypochlorous acid with minimal by-products and impurities, resulting in a purer and more stable product.Expand Specific Solutions05 Formulation with compatible ingredients
Developing formulations that include ingredients compatible with hypochlorous acid can enhance its stability. This involves selecting components that do not react with or degrade hypochlorous acid, and may even provide synergistic stabilizing effects.Expand Specific Solutions
Key Industry Players
The market for addressing hypochlorous acid's stability in transport is in a growth phase, driven by increasing demand for safe and effective disinfectants. The global market size is expanding, with applications across healthcare, water treatment, and consumer products. Technologically, the field is advancing rapidly, with companies like WIAB WATER INNOVATION AB, Parasol Medical LLC, and Industrie De Nora SpA leading innovation. These firms are developing proprietary formulations and delivery systems to enhance hypochlorous acid stability. Other key players such as Zep, Inc. and Aquaox, Inc. are focusing on commercialization and market penetration, indicating a maturing industry with diverse technological approaches and growing competition.
Industrie De Nora SpA
Technical Solution: De Nora has developed an innovative on-site generation system for hypochlorous acid called GISELLE®. This system addresses the stability issue by producing hypochlorous acid on-demand, eliminating the need for long-term storage and transport. The GISELLE® technology uses a patented electrolytic cell to generate a stable hypochlorous acid solution with a concentration of 500-700 mg/L and a pH of 5-6.5[3]. The system incorporates advanced control mechanisms to maintain optimal production parameters, ensuring consistent quality and stability of the generated hypochlorous acid. De Nora's approach also includes the use of high-purity salt and water, along with precise control of the electrolysis process, to minimize by-product formation and enhance stability[4].
Strengths: On-site generation eliminates transport stability issues, consistent quality production, and minimal by-product formation. Weaknesses: Requires installation and maintenance of on-site equipment, potentially higher initial investment costs.
Aquaox, Inc.
Technical Solution: Aquaox has developed a patented Electrochemical Activation (ECA) technology for producing stable hypochlorous acid solutions. Their approach focuses on creating a highly pure and stable form of hypochlorous acid through a controlled electrolysis process. The Aquaox system generates hypochlorous acid with a pH range of 6.2 to 6.5 and an oxidation-reduction potential (ORP) of +800 to +1000 mV, which contributes to its stability[5]. The company has also implemented advanced packaging solutions, including opaque containers and air-tight seals, to protect the hypochlorous acid from light and air exposure during transport. Additionally, Aquaox has developed a proprietary stabilization process that involves the careful balancing of minerals and electrolytes in the solution to enhance its shelf life[6].
Strengths: Highly pure and stable hypochlorous acid production, advanced packaging solutions, and proprietary stabilization process. Weaknesses: May require specialized equipment for production, potentially higher costs compared to traditional disinfectants.
Core Stabilization Patents
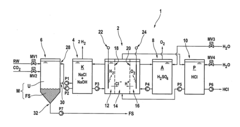
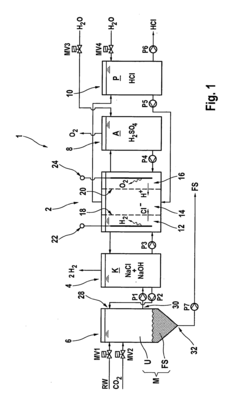
Method for producing hydrogen chloride or an aqueous solution thereof using untreated salt water, thus produced product, use of the product and electrodialysis system
PatentActiveUS20130272952A1
Innovation
- Producing hydrogen chloride or an aqueous hydrochloric acid solution on-site using untreated saline water through an electrodialysis method, which involves furnishing a chloride-ion-containing electrolyte, subjecting it to cathodic reduction, processing the catholyte with untreated saline water to increase chloride ions and decrease hydroxide ions, and reusing the catholyte as the electrolyte, thereby avoiding costly disposal and additional chemical use.
Regulatory Considerations
The regulatory landscape surrounding hypochlorous acid (HOCl) transport and stability is complex and multifaceted, requiring careful consideration by manufacturers and distributors. In the United States, the Environmental Protection Agency (EPA) regulates HOCl as a pesticide under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). This classification necessitates strict adherence to labeling requirements, registration processes, and safety standards for production, transport, and use.
The Food and Drug Administration (FDA) also plays a role in regulating HOCl, particularly when it is used in food-contact applications or medical devices. Manufacturers must ensure compliance with FDA guidelines on good manufacturing practices (GMP) and provide evidence of safety and efficacy for intended uses.
Internationally, the transport of HOCl is subject to various regulations, including the United Nations Recommendations on the Transport of Dangerous Goods. These guidelines classify HOCl solutions based on concentration and pH levels, impacting packaging, labeling, and shipping requirements. The International Maritime Dangerous Goods (IMDG) Code and the International Air Transport Association (IATA) Dangerous Goods Regulations further govern the maritime and air transport of HOCl, respectively.
Stability concerns during transport have led to the development of specific regulatory guidelines. For instance, the Department of Transportation (DOT) in the United States has established requirements for the packaging and labeling of HOCl solutions to ensure safe handling and prevent degradation during transit. These regulations often mandate the use of specific container materials, such as high-density polyethylene (HDPE), and may require temperature-controlled shipping conditions.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation impacts the production, import, and use of HOCl. Manufacturers and importers must register HOCl with the European Chemicals Agency (ECHA) and provide comprehensive safety data, including stability information under various transport conditions.
Regulatory bodies also emphasize the importance of proper documentation and traceability throughout the supply chain. This includes maintaining detailed records of production batches, stability testing results, and transport conditions to ensure compliance and facilitate rapid response in case of any safety concerns or product recalls.
As the use of HOCl expands into new applications, regulatory frameworks continue to evolve. Manufacturers and distributors must stay informed about changes in regulations across different jurisdictions and adapt their practices accordingly. This may involve investing in research and development to improve stability formulations, exploring innovative packaging solutions, and collaborating with regulatory agencies to establish new standards that address the unique challenges of HOCl transport and storage.
The Food and Drug Administration (FDA) also plays a role in regulating HOCl, particularly when it is used in food-contact applications or medical devices. Manufacturers must ensure compliance with FDA guidelines on good manufacturing practices (GMP) and provide evidence of safety and efficacy for intended uses.
Internationally, the transport of HOCl is subject to various regulations, including the United Nations Recommendations on the Transport of Dangerous Goods. These guidelines classify HOCl solutions based on concentration and pH levels, impacting packaging, labeling, and shipping requirements. The International Maritime Dangerous Goods (IMDG) Code and the International Air Transport Association (IATA) Dangerous Goods Regulations further govern the maritime and air transport of HOCl, respectively.
Stability concerns during transport have led to the development of specific regulatory guidelines. For instance, the Department of Transportation (DOT) in the United States has established requirements for the packaging and labeling of HOCl solutions to ensure safe handling and prevent degradation during transit. These regulations often mandate the use of specific container materials, such as high-density polyethylene (HDPE), and may require temperature-controlled shipping conditions.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation impacts the production, import, and use of HOCl. Manufacturers and importers must register HOCl with the European Chemicals Agency (ECHA) and provide comprehensive safety data, including stability information under various transport conditions.
Regulatory bodies also emphasize the importance of proper documentation and traceability throughout the supply chain. This includes maintaining detailed records of production batches, stability testing results, and transport conditions to ensure compliance and facilitate rapid response in case of any safety concerns or product recalls.
As the use of HOCl expands into new applications, regulatory frameworks continue to evolve. Manufacturers and distributors must stay informed about changes in regulations across different jurisdictions and adapt their practices accordingly. This may involve investing in research and development to improve stability formulations, exploring innovative packaging solutions, and collaborating with regulatory agencies to establish new standards that address the unique challenges of HOCl transport and storage.
Environmental Impact
The environmental impact of addressing hypochlorous acid's stability in transport is a critical consideration that extends beyond the immediate technical challenges. Hypochlorous acid (HOCl) is known for its potent disinfectant properties, making it valuable in various applications, including water treatment, healthcare, and agriculture. However, its instability during transport poses not only logistical challenges but also potential environmental risks.
One of the primary environmental concerns is the potential for accidental release during transportation. If unstable HOCl solutions leak or spill, they can have immediate effects on local ecosystems. The high oxidative nature of HOCl can lead to the rapid oxidation of organic matter in soil and water bodies, potentially disrupting microbial communities and aquatic life. This impact could be particularly severe in sensitive ecological areas or water sources.
Furthermore, the degradation of HOCl can result in the formation of chlorine gas, which is highly toxic and can have far-reaching environmental consequences if released into the atmosphere. Even in small quantities, chlorine gas can cause respiratory issues in humans and animals, and damage vegetation. The potential for such releases necessitates stringent safety measures and environmental risk assessments in the development of stabilization techniques.
On the other hand, improving HOCl stability could have positive environmental implications. Enhanced stability would reduce the need for frequent production and transportation, potentially lowering the overall carbon footprint associated with its use. Additionally, more stable formulations could lead to reduced packaging requirements, minimizing waste and the environmental impact of packaging materials.
The development of stabilization methods may involve the use of additional chemicals or processes, which themselves must be evaluated for their environmental impact. Ideally, any additives or stabilization techniques should be environmentally benign and not introduce new pollutants or toxins into ecosystems upon eventual disposal or release.
In addressing HOCl stability, there is also an opportunity to explore more sustainable production methods. For instance, on-site generation of HOCl could be optimized, reducing the need for long-distance transportation altogether. This approach could significantly decrease the environmental risks associated with transport while also reducing energy consumption and emissions from transportation vehicles.
Ultimately, the environmental impact of stabilizing HOCl for transport must be carefully balanced against the benefits of its use. Comprehensive life cycle assessments should be conducted to evaluate the full environmental footprint of different stabilization strategies, from production to end-use and disposal. This holistic approach will ensure that efforts to improve HOCl stability do not inadvertently create new environmental challenges while solving existing ones.
One of the primary environmental concerns is the potential for accidental release during transportation. If unstable HOCl solutions leak or spill, they can have immediate effects on local ecosystems. The high oxidative nature of HOCl can lead to the rapid oxidation of organic matter in soil and water bodies, potentially disrupting microbial communities and aquatic life. This impact could be particularly severe in sensitive ecological areas or water sources.
Furthermore, the degradation of HOCl can result in the formation of chlorine gas, which is highly toxic and can have far-reaching environmental consequences if released into the atmosphere. Even in small quantities, chlorine gas can cause respiratory issues in humans and animals, and damage vegetation. The potential for such releases necessitates stringent safety measures and environmental risk assessments in the development of stabilization techniques.
On the other hand, improving HOCl stability could have positive environmental implications. Enhanced stability would reduce the need for frequent production and transportation, potentially lowering the overall carbon footprint associated with its use. Additionally, more stable formulations could lead to reduced packaging requirements, minimizing waste and the environmental impact of packaging materials.
The development of stabilization methods may involve the use of additional chemicals or processes, which themselves must be evaluated for their environmental impact. Ideally, any additives or stabilization techniques should be environmentally benign and not introduce new pollutants or toxins into ecosystems upon eventual disposal or release.
In addressing HOCl stability, there is also an opportunity to explore more sustainable production methods. For instance, on-site generation of HOCl could be optimized, reducing the need for long-distance transportation altogether. This approach could significantly decrease the environmental risks associated with transport while also reducing energy consumption and emissions from transportation vehicles.
Ultimately, the environmental impact of stabilizing HOCl for transport must be carefully balanced against the benefits of its use. Comprehensive life cycle assessments should be conducted to evaluate the full environmental footprint of different stabilization strategies, from production to end-use and disposal. This holistic approach will ensure that efforts to improve HOCl stability do not inadvertently create new environmental challenges while solving existing ones.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!