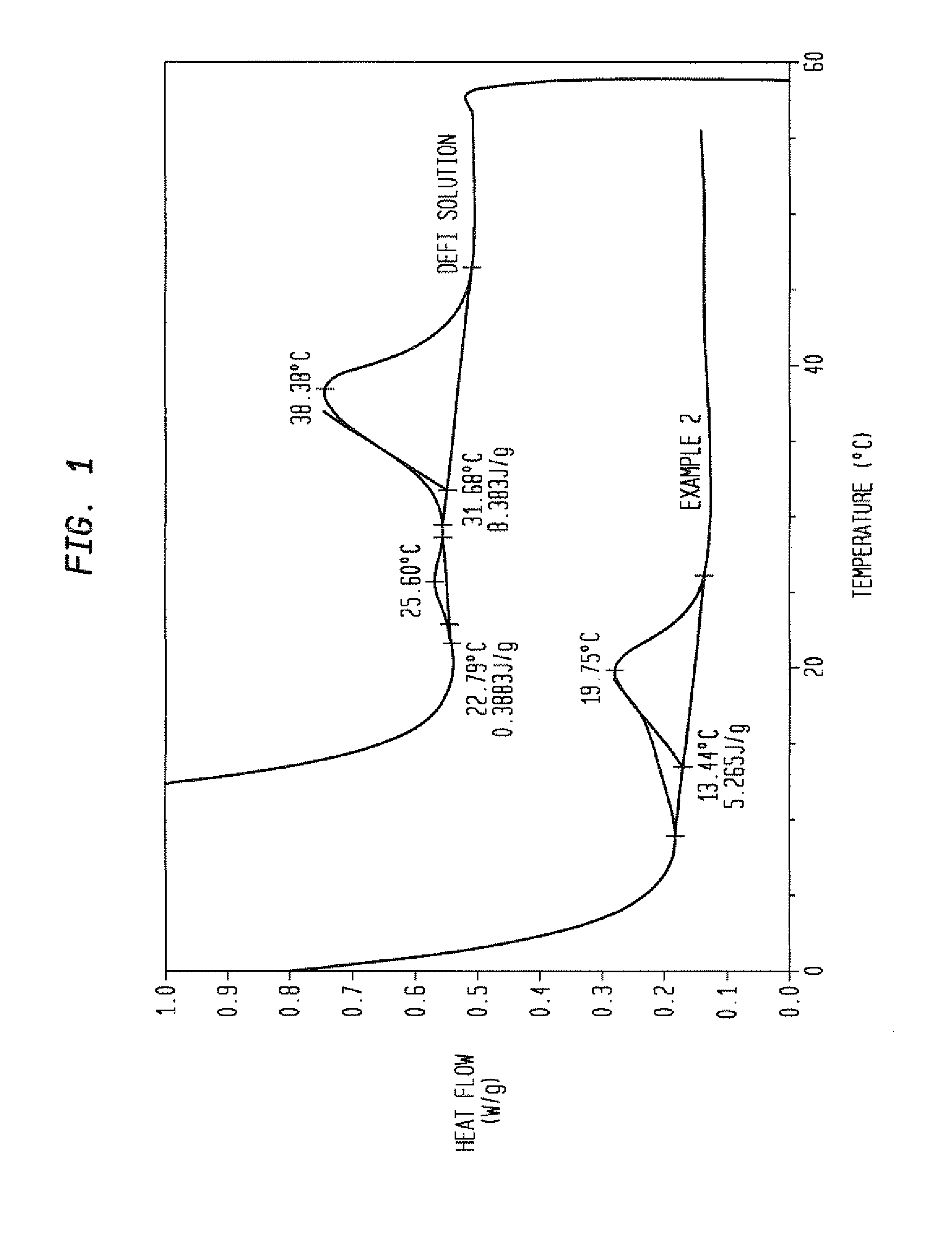
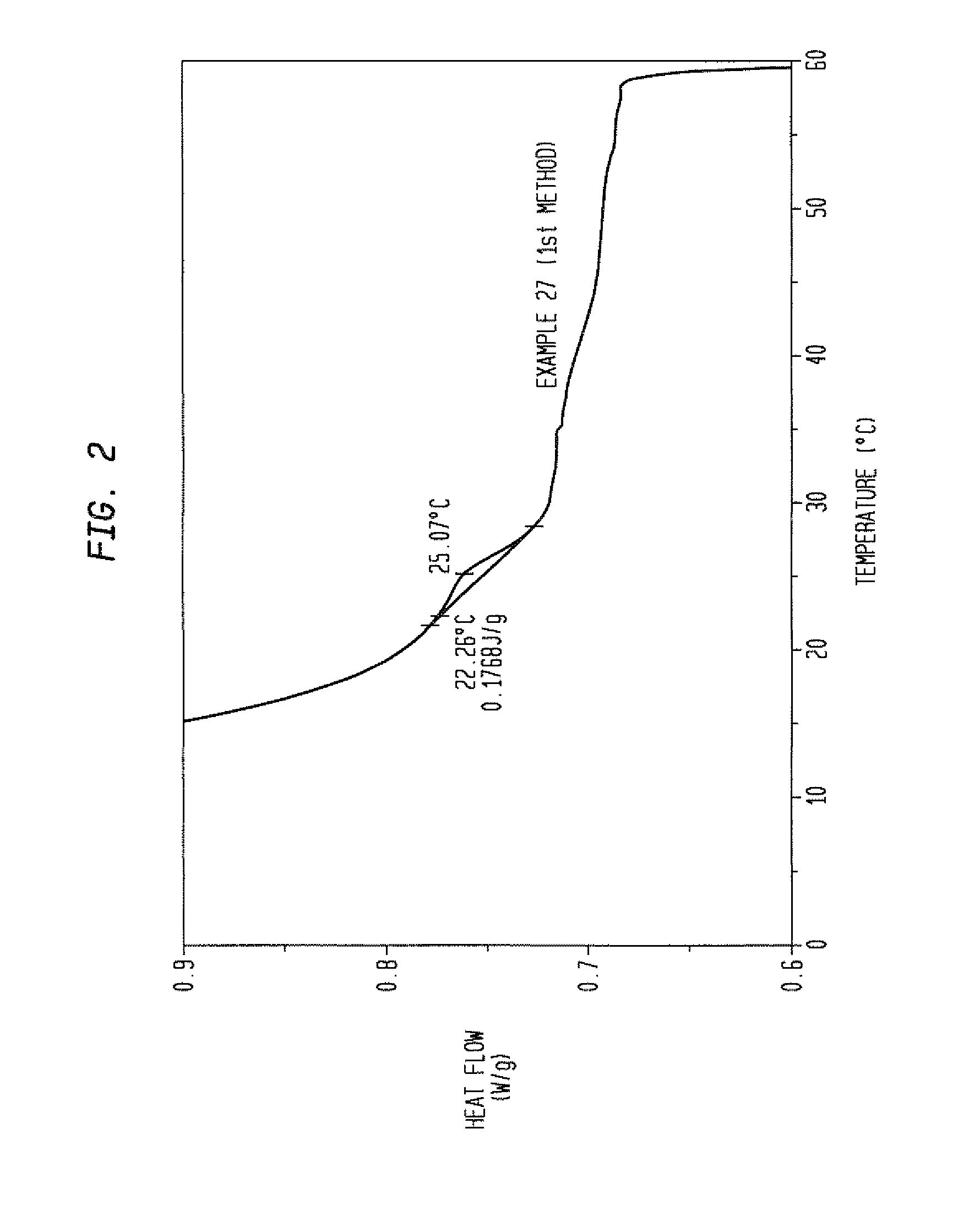
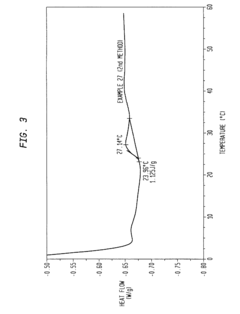
How to Address Stability Challenges of Alkyl Compounds?
JUL 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkyl Compound Stability Challenges and Objectives
Alkyl compounds play a crucial role in various industries, including pharmaceuticals, petrochemicals, and materials science. However, their inherent instability poses significant challenges for researchers and manufacturers alike. The stability issues of alkyl compounds stem from their reactive nature, primarily due to the presence of carbon-carbon and carbon-hydrogen bonds that are susceptible to various degradation mechanisms.
The evolution of alkyl compound research has been driven by the need to overcome these stability challenges. Early studies focused on understanding the fundamental mechanisms of degradation, such as oxidation, hydrolysis, and thermal decomposition. As the field progressed, researchers began developing strategies to enhance the stability of these compounds, including the use of antioxidants, stabilizers, and novel synthetic approaches.
Current technological objectives in addressing alkyl compound stability revolve around several key areas. Firstly, there is a push towards developing more robust alkyl structures that inherently resist degradation. This involves exploring novel molecular designs and incorporating stabilizing functional groups. Secondly, researchers aim to create advanced stabilization techniques that can be applied to existing alkyl compounds without altering their desirable properties.
Another critical objective is the development of predictive models and analytical tools to assess and forecast the stability of alkyl compounds under various conditions. This includes leveraging computational chemistry and machine learning algorithms to simulate degradation processes and identify potential stability-enhancing modifications.
Furthermore, there is a growing emphasis on sustainable solutions to alkyl compound stability. This involves exploring bio-based alternatives and environmentally friendly stabilization methods that align with green chemistry principles. Researchers are also investigating ways to improve the recyclability and end-of-life management of alkyl compounds to address long-term stability concerns.
The pursuit of these objectives is driven by the increasing demand for high-performance and long-lasting alkyl-based products across various sectors. In the pharmaceutical industry, for instance, improved stability of alkyl compounds can lead to extended shelf lives of drugs and more effective drug delivery systems. In the petrochemical sector, enhanced stability can result in more efficient fuel additives and lubricants, contributing to improved engine performance and reduced environmental impact.
As we delve deeper into the challenges and objectives surrounding alkyl compound stability, it becomes clear that this field is at the intersection of chemistry, materials science, and engineering. The multidisciplinary nature of the problem necessitates collaborative efforts and innovative approaches to achieve significant breakthroughs in addressing the stability challenges of alkyl compounds.
The evolution of alkyl compound research has been driven by the need to overcome these stability challenges. Early studies focused on understanding the fundamental mechanisms of degradation, such as oxidation, hydrolysis, and thermal decomposition. As the field progressed, researchers began developing strategies to enhance the stability of these compounds, including the use of antioxidants, stabilizers, and novel synthetic approaches.
Current technological objectives in addressing alkyl compound stability revolve around several key areas. Firstly, there is a push towards developing more robust alkyl structures that inherently resist degradation. This involves exploring novel molecular designs and incorporating stabilizing functional groups. Secondly, researchers aim to create advanced stabilization techniques that can be applied to existing alkyl compounds without altering their desirable properties.
Another critical objective is the development of predictive models and analytical tools to assess and forecast the stability of alkyl compounds under various conditions. This includes leveraging computational chemistry and machine learning algorithms to simulate degradation processes and identify potential stability-enhancing modifications.
Furthermore, there is a growing emphasis on sustainable solutions to alkyl compound stability. This involves exploring bio-based alternatives and environmentally friendly stabilization methods that align with green chemistry principles. Researchers are also investigating ways to improve the recyclability and end-of-life management of alkyl compounds to address long-term stability concerns.
The pursuit of these objectives is driven by the increasing demand for high-performance and long-lasting alkyl-based products across various sectors. In the pharmaceutical industry, for instance, improved stability of alkyl compounds can lead to extended shelf lives of drugs and more effective drug delivery systems. In the petrochemical sector, enhanced stability can result in more efficient fuel additives and lubricants, contributing to improved engine performance and reduced environmental impact.
As we delve deeper into the challenges and objectives surrounding alkyl compound stability, it becomes clear that this field is at the intersection of chemistry, materials science, and engineering. The multidisciplinary nature of the problem necessitates collaborative efforts and innovative approaches to achieve significant breakthroughs in addressing the stability challenges of alkyl compounds.
Market Demand for Stable Alkyl Compounds
The market demand for stable alkyl compounds has been steadily increasing across various industries due to their versatile applications and unique properties. In the pharmaceutical sector, stable alkyl compounds play a crucial role in drug development and formulation, particularly in the synthesis of active pharmaceutical ingredients (APIs) and excipients. The growing emphasis on drug stability and shelf life has further amplified the need for these compounds, driving research and development efforts to enhance their stability profiles.
In the agrochemical industry, stable alkyl compounds are essential components in the production of pesticides, herbicides, and fertilizers. As global agricultural demands continue to rise, the market for these compounds is expected to expand significantly. The push for more environmentally friendly and sustainable agricultural practices has also led to increased interest in developing stable alkyl compounds with improved biodegradability and reduced ecological impact.
The polymer and plastics industry represents another major market for stable alkyl compounds. These compounds are widely used as stabilizers, plasticizers, and additives in various polymer formulations. With the growing demand for high-performance materials in automotive, construction, and consumer goods sectors, the need for stable alkyl compounds that can enhance the durability and longevity of polymeric products has surged.
In the energy sector, particularly in the oil and gas industry, stable alkyl compounds find applications in lubricants, fuel additives, and drilling fluids. The increasing exploration and production activities in challenging environments, such as deep-sea drilling and hydraulic fracturing, have created a demand for more stable and efficient alkyl-based products that can withstand extreme conditions.
The personal care and cosmetics industry also contributes significantly to the market demand for stable alkyl compounds. These compounds are used in a wide range of products, including skincare formulations, hair care products, and fragrances. The trend towards natural and organic cosmetics has spurred research into developing stable alkyl compounds derived from renewable sources, further expanding market opportunities.
As environmental regulations become more stringent globally, there is a growing demand for stable alkyl compounds that meet sustainability criteria. This has led to increased investment in green chemistry initiatives and the development of bio-based alkyl compounds. The shift towards circular economy models in various industries has also created new market opportunities for recyclable and biodegradable alkyl-based products.
In the agrochemical industry, stable alkyl compounds are essential components in the production of pesticides, herbicides, and fertilizers. As global agricultural demands continue to rise, the market for these compounds is expected to expand significantly. The push for more environmentally friendly and sustainable agricultural practices has also led to increased interest in developing stable alkyl compounds with improved biodegradability and reduced ecological impact.
The polymer and plastics industry represents another major market for stable alkyl compounds. These compounds are widely used as stabilizers, plasticizers, and additives in various polymer formulations. With the growing demand for high-performance materials in automotive, construction, and consumer goods sectors, the need for stable alkyl compounds that can enhance the durability and longevity of polymeric products has surged.
In the energy sector, particularly in the oil and gas industry, stable alkyl compounds find applications in lubricants, fuel additives, and drilling fluids. The increasing exploration and production activities in challenging environments, such as deep-sea drilling and hydraulic fracturing, have created a demand for more stable and efficient alkyl-based products that can withstand extreme conditions.
The personal care and cosmetics industry also contributes significantly to the market demand for stable alkyl compounds. These compounds are used in a wide range of products, including skincare formulations, hair care products, and fragrances. The trend towards natural and organic cosmetics has spurred research into developing stable alkyl compounds derived from renewable sources, further expanding market opportunities.
As environmental regulations become more stringent globally, there is a growing demand for stable alkyl compounds that meet sustainability criteria. This has led to increased investment in green chemistry initiatives and the development of bio-based alkyl compounds. The shift towards circular economy models in various industries has also created new market opportunities for recyclable and biodegradable alkyl-based products.
Current State and Limitations of Alkyl Compound Stabilization
The current state of alkyl compound stabilization presents both significant advancements and persistent challenges. Alkyl compounds, characterized by their carbon-hydrogen bonds, are widely used in various industries, including pharmaceuticals, petrochemicals, and materials science. However, their inherent instability poses a major obstacle to their widespread application and long-term storage.
Recent developments in stabilization techniques have primarily focused on three main approaches: antioxidant additives, physical encapsulation, and chemical modification. Antioxidant additives, such as butylated hydroxytoluene (BHT) and propyl gallate, have shown promising results in preventing oxidative degradation of alkyl compounds. These additives work by scavenging free radicals and interrupting the chain reaction of oxidation.
Physical encapsulation methods, including microencapsulation and nanoencapsulation, have emerged as effective strategies for protecting alkyl compounds from environmental factors. These techniques involve surrounding the compound with a protective barrier, which can significantly extend shelf life and improve stability under various conditions.
Chemical modification of alkyl compounds has also gained traction as a stabilization approach. This method involves altering the molecular structure to enhance stability without compromising the desired properties. For instance, the introduction of electron-withdrawing groups or the incorporation of sterically hindering substituents has shown potential in improving the overall stability of certain alkyl compounds.
Despite these advancements, several limitations persist in the field of alkyl compound stabilization. One major challenge is the trade-off between stability and reactivity. Stabilization techniques often reduce the compound's reactivity, which can be detrimental in applications where high reactivity is desired. Finding the right balance between stability and functionality remains a key area of research.
Another significant limitation is the lack of universal stabilization solutions. Different alkyl compounds exhibit varying degrees of instability and respond differently to stabilization methods. This necessitates a tailored approach for each compound or class of compounds, making large-scale implementation challenging and costly.
Environmental concerns also pose limitations to current stabilization methods. Some stabilizers, particularly certain antioxidants, have raised health and environmental safety concerns. This has led to increased regulatory scrutiny and a push towards developing more environmentally friendly stabilization techniques.
The stability of alkyl compounds under extreme conditions, such as high temperatures or pressures, continues to be a major challenge. Many current stabilization methods are ineffective under these conditions, limiting the use of alkyl compounds in harsh industrial environments or specialized applications.
Recent developments in stabilization techniques have primarily focused on three main approaches: antioxidant additives, physical encapsulation, and chemical modification. Antioxidant additives, such as butylated hydroxytoluene (BHT) and propyl gallate, have shown promising results in preventing oxidative degradation of alkyl compounds. These additives work by scavenging free radicals and interrupting the chain reaction of oxidation.
Physical encapsulation methods, including microencapsulation and nanoencapsulation, have emerged as effective strategies for protecting alkyl compounds from environmental factors. These techniques involve surrounding the compound with a protective barrier, which can significantly extend shelf life and improve stability under various conditions.
Chemical modification of alkyl compounds has also gained traction as a stabilization approach. This method involves altering the molecular structure to enhance stability without compromising the desired properties. For instance, the introduction of electron-withdrawing groups or the incorporation of sterically hindering substituents has shown potential in improving the overall stability of certain alkyl compounds.
Despite these advancements, several limitations persist in the field of alkyl compound stabilization. One major challenge is the trade-off between stability and reactivity. Stabilization techniques often reduce the compound's reactivity, which can be detrimental in applications where high reactivity is desired. Finding the right balance between stability and functionality remains a key area of research.
Another significant limitation is the lack of universal stabilization solutions. Different alkyl compounds exhibit varying degrees of instability and respond differently to stabilization methods. This necessitates a tailored approach for each compound or class of compounds, making large-scale implementation challenging and costly.
Environmental concerns also pose limitations to current stabilization methods. Some stabilizers, particularly certain antioxidants, have raised health and environmental safety concerns. This has led to increased regulatory scrutiny and a push towards developing more environmentally friendly stabilization techniques.
The stability of alkyl compounds under extreme conditions, such as high temperatures or pressures, continues to be a major challenge. Many current stabilization methods are ineffective under these conditions, limiting the use of alkyl compounds in harsh industrial environments or specialized applications.
Existing Solutions for Enhancing Alkyl Compound Stability
01 Thermal stability of alkyl compounds
The thermal stability of alkyl compounds is an important factor in their industrial applications. Various methods are employed to enhance the thermal stability of these compounds, including the use of additives and structural modifications. Improved thermal stability can lead to better performance in high-temperature environments and extended product lifetimes.- Thermal stability of alkyl compounds: The thermal stability of alkyl compounds is an important factor in their industrial applications. Various methods are employed to enhance the thermal stability of these compounds, including the use of additives and structural modifications. Improved thermal stability can lead to better performance in high-temperature environments and extended product lifetimes.
- Chemical stability of alkyl compounds: Chemical stability of alkyl compounds is crucial for their use in various applications. Factors affecting chemical stability include molecular structure, presence of functional groups, and environmental conditions. Techniques to improve chemical stability may involve the use of stabilizers, antioxidants, or chemical modifications to the alkyl chain.
- Stabilization of alkyl compounds in formulations: Incorporating alkyl compounds into various formulations while maintaining their stability is a key challenge. Formulation techniques, such as encapsulation, emulsification, or the use of compatible solvents, can help improve the stability of alkyl compounds in complex mixtures. This is particularly important in industries such as pharmaceuticals, cosmetics, and agrochemicals.
- Environmental stability of alkyl compounds: The stability of alkyl compounds under various environmental conditions, such as exposure to light, moisture, or air, is an important consideration for their practical applications. Strategies to enhance environmental stability may include the use of UV stabilizers, moisture barriers, or oxygen scavengers. Improving environmental stability can lead to increased shelf life and better performance in diverse applications.
- Structural modifications for improved alkyl compound stability: Modifying the molecular structure of alkyl compounds can significantly impact their stability. Techniques such as introducing branching, altering chain length, or incorporating specific functional groups can enhance the overall stability of these compounds. These structural modifications can lead to improved resistance to degradation and better performance in various applications.
02 Chemical stability of alkyl compounds
Chemical stability of alkyl compounds is crucial for their use in various applications. Factors affecting chemical stability include molecular structure, presence of functional groups, and environmental conditions. Techniques to improve chemical stability may involve the use of stabilizers, antioxidants, or chemical modifications to the alkyl chain.Expand Specific Solutions03 Stabilization of alkyl compounds in formulations
Incorporating alkyl compounds into various formulations while maintaining their stability is a key challenge. This may involve the use of specific solvents, emulsifiers, or encapsulation techniques to protect the alkyl compounds from degradation. The choice of stabilization method depends on the nature of the formulation and the intended application.Expand Specific Solutions04 Environmental stability of alkyl compounds
The stability of alkyl compounds under various environmental conditions, such as exposure to light, moisture, or air, is an important consideration for their use and storage. Strategies to improve environmental stability may include packaging innovations, addition of UV stabilizers, or chemical modifications to enhance resistance to oxidation or hydrolysis.Expand Specific Solutions05 Long-term storage stability of alkyl compounds
Ensuring the long-term storage stability of alkyl compounds is essential for their commercial viability. This may involve the use of specialized storage conditions, such as controlled temperature and humidity, as well as the addition of stabilizers or preservatives. Understanding the degradation mechanisms and developing appropriate mitigation strategies is crucial for maintaining product quality over extended periods.Expand Specific Solutions
Key Players in Alkyl Compound Research and Production
The stability challenges of alkyl compounds present a complex competitive landscape in the chemical industry. The market is in a mature stage, with established players like BASF, Sinopec, and Arkema dominating. However, ongoing research and development efforts by companies such as Wacker Chemie and Rohm & Haas indicate potential for innovation and market growth. The global market size for alkyl compounds is substantial, driven by diverse applications in industries ranging from petrochemicals to pharmaceuticals. Technologically, while the basic chemistry is well-understood, companies like Henkel and Sumitomo Chemical are focusing on enhancing stability and performance, suggesting that there is still room for technological advancement and differentiation in this field.
Arkema France SA
Technical Solution: Arkema has focused on developing innovative solutions for alkyl compound stabilization, particularly in the field of polymer additives. Their approach includes the use of proprietary thiodipropionate antioxidants and phosphite processing stabilizers[4]. Arkema's research has led to the development of the Luperox® line of organic peroxides, which can be used to control the molecular weight and improve the stability of certain alkyl-based polymers[5]. They have also introduced novel UV stabilizers and heat stabilizers designed specifically for long-chain alkyl compounds used in high-performance plastics and elastomers[6].
Strengths: Specialized solutions for high-performance materials, strong focus on sustainable additives. Weaknesses: Limited coverage in some traditional alkyl compound applications, potentially higher costs for specialized solutions.
BASF Corp.
Technical Solution: BASF has developed innovative stabilization techniques for alkyl compounds, focusing on antioxidants and light stabilizers. Their approach involves the use of hindered amine light stabilizers (HALS) and phenolic antioxidants to prevent degradation of alkyl compounds[1]. They have also introduced novel synergistic stabilizer blends that combine different types of stabilizers to address multiple degradation pathways simultaneously[2]. BASF's research has led to the development of high-performance stabilizers like Tinuvin® and Irganox® series, which offer long-term thermal and light stability to alkyl compounds in various applications, including automotive, construction, and packaging industries[3].
Strengths: Comprehensive stabilizer portfolio, synergistic blends for enhanced performance, global research capabilities. Weaknesses: Higher cost compared to generic stabilizers, potential over-engineering for simple applications.
Core Innovations in Alkyl Compound Stabilization Methods
Method of providing stability for liquid cleansing compositions comprising broad selection fatty acyl isethionate surfactants
PatentInactiveUS20080153727A1
Innovation
- Converting part or all of the fatty acyl isethionate surfactant crystals to surfactant liquid crystals by using a specific combination of liquid crystal modifiers, such as fatty acids and alkanolamide, to create a stable liquid crystal phase that maintains consistent viscosity regardless of free fatty acid content or chain length, ensuring stability at various temperatures.
Environmental Impact of Alkyl Compound Stabilization
The stabilization of alkyl compounds presents significant environmental challenges that require careful consideration. These compounds, widely used in various industries, can have detrimental effects on ecosystems and human health if not properly managed. The environmental impact of alkyl compound stabilization primarily stems from the chemicals and processes used to enhance their stability.
One of the main concerns is the potential release of stabilizing agents into the environment. Many traditional stabilizers, such as antioxidants and UV absorbers, can persist in soil and water systems, leading to long-term contamination. These substances may bioaccumulate in organisms, causing disruptions in food chains and ecosystems. Furthermore, some stabilizers can react with other environmental pollutants, forming more toxic compounds and exacerbating the overall environmental impact.
The production and disposal of stabilized alkyl compounds also contribute to environmental issues. Manufacturing processes often involve energy-intensive operations and the use of hazardous materials, resulting in increased carbon emissions and potential chemical spills. When these compounds reach the end of their lifecycle, improper disposal can lead to soil and water pollution, particularly if the stabilizers prevent natural degradation processes.
Efforts to address these environmental concerns have led to the development of more eco-friendly stabilization methods. Green chemistry approaches focus on using biodegradable stabilizers and minimizing the use of harmful substances. For instance, natural antioxidants derived from plant extracts are being explored as alternatives to synthetic stabilizers. These bio-based solutions offer the potential for reduced environmental persistence and toxicity.
Another promising avenue is the development of self-stabilizing alkyl compounds, which could eliminate the need for additional stabilizing agents altogether. This approach involves modifying the molecular structure of the compounds to enhance their inherent stability, potentially reducing the environmental footprint associated with stabilization processes.
The environmental impact of alkyl compound stabilization also extends to air quality. Volatile organic compounds (VOCs) emitted during the production and use of stabilized alkyl products can contribute to smog formation and air pollution. Regulatory bodies worldwide are implementing stricter emission controls, driving the industry towards cleaner stabilization technologies and improved manufacturing practices.
In conclusion, addressing the environmental impact of alkyl compound stabilization requires a multifaceted approach. This includes developing greener stabilization methods, improving production processes, and implementing more effective waste management strategies. As research in this field progresses, it is crucial to balance the need for stable alkyl compounds with the imperative of environmental protection, ensuring sustainable practices in their production and use.
One of the main concerns is the potential release of stabilizing agents into the environment. Many traditional stabilizers, such as antioxidants and UV absorbers, can persist in soil and water systems, leading to long-term contamination. These substances may bioaccumulate in organisms, causing disruptions in food chains and ecosystems. Furthermore, some stabilizers can react with other environmental pollutants, forming more toxic compounds and exacerbating the overall environmental impact.
The production and disposal of stabilized alkyl compounds also contribute to environmental issues. Manufacturing processes often involve energy-intensive operations and the use of hazardous materials, resulting in increased carbon emissions and potential chemical spills. When these compounds reach the end of their lifecycle, improper disposal can lead to soil and water pollution, particularly if the stabilizers prevent natural degradation processes.
Efforts to address these environmental concerns have led to the development of more eco-friendly stabilization methods. Green chemistry approaches focus on using biodegradable stabilizers and minimizing the use of harmful substances. For instance, natural antioxidants derived from plant extracts are being explored as alternatives to synthetic stabilizers. These bio-based solutions offer the potential for reduced environmental persistence and toxicity.
Another promising avenue is the development of self-stabilizing alkyl compounds, which could eliminate the need for additional stabilizing agents altogether. This approach involves modifying the molecular structure of the compounds to enhance their inherent stability, potentially reducing the environmental footprint associated with stabilization processes.
The environmental impact of alkyl compound stabilization also extends to air quality. Volatile organic compounds (VOCs) emitted during the production and use of stabilized alkyl products can contribute to smog formation and air pollution. Regulatory bodies worldwide are implementing stricter emission controls, driving the industry towards cleaner stabilization technologies and improved manufacturing practices.
In conclusion, addressing the environmental impact of alkyl compound stabilization requires a multifaceted approach. This includes developing greener stabilization methods, improving production processes, and implementing more effective waste management strategies. As research in this field progresses, it is crucial to balance the need for stable alkyl compounds with the imperative of environmental protection, ensuring sustainable practices in their production and use.
Safety Regulations for Alkyl Compound Handling and Storage
The handling and storage of alkyl compounds present significant safety challenges due to their inherent instability and reactivity. To address these concerns, stringent safety regulations have been established across various industries and jurisdictions. These regulations typically encompass several key areas, including storage conditions, handling procedures, personal protective equipment (PPE), and emergency response protocols.
Storage regulations for alkyl compounds often mandate the use of specialized containment systems designed to minimize exposure to air, moisture, and heat. These may include inert gas blanketing systems, temperature-controlled storage units, and corrosion-resistant containers. Facilities are required to maintain proper ventilation and implement strict inventory management practices to prevent the accumulation of potentially hazardous quantities.
Handling procedures for alkyl compounds are subject to rigorous guidelines that emphasize the importance of proper training and supervision. Regulations often stipulate that only qualified personnel should be permitted to work with these substances, and that all operations involving alkyl compounds must be conducted in designated areas with appropriate safety equipment. This may include the use of fume hoods, glove boxes, or other containment systems designed to minimize the risk of exposure.
Personal protective equipment requirements for working with alkyl compounds are typically extensive. Regulations often mandate the use of chemical-resistant gloves, protective eyewear, and respiratory protection appropriate to the specific compounds being handled. In some cases, full-body protective suits may be required, particularly when dealing with highly reactive or pyrophoric alkyl compounds.
Emergency response protocols form a critical component of safety regulations for alkyl compound handling and storage. Facilities are required to develop and maintain comprehensive emergency plans that address potential scenarios such as spills, fires, or explosions. These plans must include procedures for evacuation, containment, and decontamination, as well as protocols for notifying emergency services and relevant authorities.
Regulatory bodies also impose strict requirements for labeling, documentation, and record-keeping related to alkyl compound handling and storage. This includes maintaining up-to-date safety data sheets (SDS), implementing proper hazard communication systems, and conducting regular safety audits and inspections to ensure compliance with all applicable regulations.
As research continues to advance our understanding of the stability challenges associated with alkyl compounds, safety regulations are regularly reviewed and updated to reflect the latest scientific knowledge and best practices. This ongoing process of refinement helps to ensure that the handling and storage of these potentially hazardous substances remain as safe as possible, minimizing risks to personnel, facilities, and the environment.
Storage regulations for alkyl compounds often mandate the use of specialized containment systems designed to minimize exposure to air, moisture, and heat. These may include inert gas blanketing systems, temperature-controlled storage units, and corrosion-resistant containers. Facilities are required to maintain proper ventilation and implement strict inventory management practices to prevent the accumulation of potentially hazardous quantities.
Handling procedures for alkyl compounds are subject to rigorous guidelines that emphasize the importance of proper training and supervision. Regulations often stipulate that only qualified personnel should be permitted to work with these substances, and that all operations involving alkyl compounds must be conducted in designated areas with appropriate safety equipment. This may include the use of fume hoods, glove boxes, or other containment systems designed to minimize the risk of exposure.
Personal protective equipment requirements for working with alkyl compounds are typically extensive. Regulations often mandate the use of chemical-resistant gloves, protective eyewear, and respiratory protection appropriate to the specific compounds being handled. In some cases, full-body protective suits may be required, particularly when dealing with highly reactive or pyrophoric alkyl compounds.
Emergency response protocols form a critical component of safety regulations for alkyl compound handling and storage. Facilities are required to develop and maintain comprehensive emergency plans that address potential scenarios such as spills, fires, or explosions. These plans must include procedures for evacuation, containment, and decontamination, as well as protocols for notifying emergency services and relevant authorities.
Regulatory bodies also impose strict requirements for labeling, documentation, and record-keeping related to alkyl compound handling and storage. This includes maintaining up-to-date safety data sheets (SDS), implementing proper hazard communication systems, and conducting regular safety audits and inspections to ensure compliance with all applicable regulations.
As research continues to advance our understanding of the stability challenges associated with alkyl compounds, safety regulations are regularly reviewed and updated to reflect the latest scientific knowledge and best practices. This ongoing process of refinement helps to ensure that the handling and storage of these potentially hazardous substances remain as safe as possible, minimizing risks to personnel, facilities, and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!