How to Assess Tartaric Acid Stability in Heat Exposure
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid Thermal Stability Background & Objectives
Tartaric acid, a naturally occurring organic acid predominantly found in grapes and wine, has been a subject of scientific interest since its discovery in the 18th century. The stability of tartaric acid under thermal conditions has become increasingly important across multiple industries including food and beverage, pharmaceuticals, and chemical manufacturing. Historically, tartaric acid was primarily studied in the context of wine production, where its stability directly impacts product quality and shelf life.
The evolution of tartaric acid research has progressed from basic characterization of its physical properties to sophisticated analytical methods for assessing its thermal degradation pathways. Early studies in the 1950s and 1960s established fundamental understanding of tartaric acid's melting point (around 170°C) and initial decomposition behaviors. By the 1980s, researchers began employing more advanced techniques such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) to quantify thermal stability parameters.
Recent technological advancements have enabled more precise examination of tartaric acid's molecular behavior under various temperature conditions. Modern spectroscopic methods and computational modeling have revealed complex degradation mechanisms involving dehydration, decarboxylation, and potential formation of pyrotartaric acid and other byproducts when exposed to elevated temperatures.
The current technological trajectory points toward developing standardized protocols for assessing tartaric acid stability across different matrices and environmental conditions. Industry demands for more heat-resistant formulations and processing techniques have accelerated research in this domain, particularly for applications requiring thermal processing or storage in variable temperature environments.
This technical research report aims to establish comprehensive methodologies for evaluating tartaric acid stability under heat exposure conditions. Specifically, our objectives include: developing reproducible analytical protocols for quantifying thermal degradation rates; identifying key molecular markers indicative of stability compromise; establishing temperature thresholds for various application contexts; and proposing novel approaches to enhance thermal stability without compromising functional properties.
Additionally, we seek to correlate theoretical degradation models with practical application scenarios, bridging the gap between laboratory findings and industrial implementation. By synthesizing existing knowledge and generating new insights, this research endeavors to provide actionable guidance for industries reliant on tartaric acid's functional properties in thermally challenging environments.
The ultimate goal is to establish a scientific foundation that enables prediction of tartaric acid behavior under diverse thermal conditions, thereby facilitating innovation in product formulation, processing techniques, and quality control methodologies across multiple sectors.
The evolution of tartaric acid research has progressed from basic characterization of its physical properties to sophisticated analytical methods for assessing its thermal degradation pathways. Early studies in the 1950s and 1960s established fundamental understanding of tartaric acid's melting point (around 170°C) and initial decomposition behaviors. By the 1980s, researchers began employing more advanced techniques such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) to quantify thermal stability parameters.
Recent technological advancements have enabled more precise examination of tartaric acid's molecular behavior under various temperature conditions. Modern spectroscopic methods and computational modeling have revealed complex degradation mechanisms involving dehydration, decarboxylation, and potential formation of pyrotartaric acid and other byproducts when exposed to elevated temperatures.
The current technological trajectory points toward developing standardized protocols for assessing tartaric acid stability across different matrices and environmental conditions. Industry demands for more heat-resistant formulations and processing techniques have accelerated research in this domain, particularly for applications requiring thermal processing or storage in variable temperature environments.
This technical research report aims to establish comprehensive methodologies for evaluating tartaric acid stability under heat exposure conditions. Specifically, our objectives include: developing reproducible analytical protocols for quantifying thermal degradation rates; identifying key molecular markers indicative of stability compromise; establishing temperature thresholds for various application contexts; and proposing novel approaches to enhance thermal stability without compromising functional properties.
Additionally, we seek to correlate theoretical degradation models with practical application scenarios, bridging the gap between laboratory findings and industrial implementation. By synthesizing existing knowledge and generating new insights, this research endeavors to provide actionable guidance for industries reliant on tartaric acid's functional properties in thermally challenging environments.
The ultimate goal is to establish a scientific foundation that enables prediction of tartaric acid behavior under diverse thermal conditions, thereby facilitating innovation in product formulation, processing techniques, and quality control methodologies across multiple sectors.
Market Applications and Industry Demand Analysis
Tartaric acid stability assessment under heat exposure has significant market applications across multiple industries, with demand driven by quality control requirements and consumer expectations. The wine industry represents the largest market segment, where tartaric acid stability directly impacts product quality, shelf life, and consumer acceptance. With the global wine market valued at over 417 billion USD in 2023 and projected to grow at a CAGR of 5.5% through 2028, the demand for reliable tartaric acid stability testing methods continues to expand proportionally.
The food and beverage sector beyond wine also demonstrates substantial demand for tartaric acid stability assessment. As a key ingredient in confectionery, baked goods, and beverages, manufacturers require dependable methods to ensure product consistency under varying temperature conditions. The global food additives market, within which tartaric acid is a significant component, exceeded 83 billion USD in 2022, with heat-stable additives commanding premium pricing.
Pharmaceutical applications represent another growth area, where tartaric acid serves as an excipient in numerous formulations. The pharmaceutical excipients market reached approximately 10.3 billion USD in 2022, with stability testing protocols becoming increasingly stringent due to regulatory requirements. Manufacturers must demonstrate that tartaric acid-containing formulations maintain stability across various environmental conditions, including temperature fluctuations during transport and storage.
The cosmetics and personal care industry utilizes tartaric acid in formulations requiring pH adjustment and as a chelating agent. With this sector growing at 4.8% annually and valued at over 560 billion USD globally, stability testing has become essential for product development and quality assurance. Heat exposure during manufacturing, shipping, and consumer use necessitates reliable assessment methods.
Regional market analysis reveals varying demand patterns, with established wine regions in Europe showing consistent demand for advanced stability testing methods, while emerging wine markets in Asia-Pacific and South America demonstrate the fastest growth rates for such technologies. North America leads in pharmaceutical and food applications of tartaric acid stability assessment.
Industry stakeholders increasingly demand automated, rapid, and cost-effective methods for assessing tartaric acid stability under heat exposure. Traditional methods requiring specialized laboratory equipment and trained personnel are being supplemented or replaced by portable testing devices and predictive algorithms. This shift is driven by industry needs for faster decision-making, reduced testing costs, and integration with existing quality management systems.
The food and beverage sector beyond wine also demonstrates substantial demand for tartaric acid stability assessment. As a key ingredient in confectionery, baked goods, and beverages, manufacturers require dependable methods to ensure product consistency under varying temperature conditions. The global food additives market, within which tartaric acid is a significant component, exceeded 83 billion USD in 2022, with heat-stable additives commanding premium pricing.
Pharmaceutical applications represent another growth area, where tartaric acid serves as an excipient in numerous formulations. The pharmaceutical excipients market reached approximately 10.3 billion USD in 2022, with stability testing protocols becoming increasingly stringent due to regulatory requirements. Manufacturers must demonstrate that tartaric acid-containing formulations maintain stability across various environmental conditions, including temperature fluctuations during transport and storage.
The cosmetics and personal care industry utilizes tartaric acid in formulations requiring pH adjustment and as a chelating agent. With this sector growing at 4.8% annually and valued at over 560 billion USD globally, stability testing has become essential for product development and quality assurance. Heat exposure during manufacturing, shipping, and consumer use necessitates reliable assessment methods.
Regional market analysis reveals varying demand patterns, with established wine regions in Europe showing consistent demand for advanced stability testing methods, while emerging wine markets in Asia-Pacific and South America demonstrate the fastest growth rates for such technologies. North America leads in pharmaceutical and food applications of tartaric acid stability assessment.
Industry stakeholders increasingly demand automated, rapid, and cost-effective methods for assessing tartaric acid stability under heat exposure. Traditional methods requiring specialized laboratory equipment and trained personnel are being supplemented or replaced by portable testing devices and predictive algorithms. This shift is driven by industry needs for faster decision-making, reduced testing costs, and integration with existing quality management systems.
Current Stability Assessment Methods and Challenges
The assessment of tartaric acid stability under heat exposure conditions currently employs several established methodologies, each with specific advantages and limitations. The wine industry predominantly utilizes the cold stability test, where samples are refrigerated at -4°C for 4-6 days to observe crystal formation. While effective for cold stability assessment, this method inadequately addresses heat-induced instability mechanisms, which operate through different chemical pathways.
Thermal cycling tests have emerged as more relevant approaches, subjecting tartaric acid solutions to controlled temperature fluctuations between 4°C and 40°C. These tests better simulate real-world storage and transportation conditions but suffer from poor standardization across the industry, leading to inconsistent results and interpretation challenges.
Spectroscopic methods, particularly FTIR (Fourier Transform Infrared) spectroscopy, enable rapid analysis of tartaric acid content and potential stability issues. However, the complex spectral interference from other wine components often compromises accuracy when assessing heat-specific stability parameters. This limitation becomes particularly pronounced in red wines with high phenolic content.
Conductivity measurements offer another assessment avenue by monitoring the electrical conductivity changes during controlled heating. While this approach provides real-time data on ionic changes related to tartaric acid stability, it fails to distinguish between different types of precipitating compounds, limiting its diagnostic specificity.
The mini-contact test, which measures the precipitation time after adding potassium bitartrate crystals to samples under controlled temperature conditions, has been adapted for heat stability assessment. However, its predictive value diminishes at elevated temperatures due to accelerated reaction kinetics that don't necessarily correlate with real-world stability behavior.
Significant challenges persist across all current methodologies. First, there's a fundamental disconnect between laboratory testing conditions and actual commercial storage environments, creating reliability gaps in stability predictions. Second, the multifactorial nature of tartaric acid stability—influenced by pH, alcohol content, metal ions, and macromolecular interactions—complicates the development of universally applicable assessment protocols.
Additionally, the time-intensive nature of most reliable tests conflicts with industry needs for rapid quality control decisions. This has led to compromises where faster but less accurate methods are often preferred in commercial settings, potentially increasing the risk of stability issues in final products exposed to heat during distribution and storage.
Thermal cycling tests have emerged as more relevant approaches, subjecting tartaric acid solutions to controlled temperature fluctuations between 4°C and 40°C. These tests better simulate real-world storage and transportation conditions but suffer from poor standardization across the industry, leading to inconsistent results and interpretation challenges.
Spectroscopic methods, particularly FTIR (Fourier Transform Infrared) spectroscopy, enable rapid analysis of tartaric acid content and potential stability issues. However, the complex spectral interference from other wine components often compromises accuracy when assessing heat-specific stability parameters. This limitation becomes particularly pronounced in red wines with high phenolic content.
Conductivity measurements offer another assessment avenue by monitoring the electrical conductivity changes during controlled heating. While this approach provides real-time data on ionic changes related to tartaric acid stability, it fails to distinguish between different types of precipitating compounds, limiting its diagnostic specificity.
The mini-contact test, which measures the precipitation time after adding potassium bitartrate crystals to samples under controlled temperature conditions, has been adapted for heat stability assessment. However, its predictive value diminishes at elevated temperatures due to accelerated reaction kinetics that don't necessarily correlate with real-world stability behavior.
Significant challenges persist across all current methodologies. First, there's a fundamental disconnect between laboratory testing conditions and actual commercial storage environments, creating reliability gaps in stability predictions. Second, the multifactorial nature of tartaric acid stability—influenced by pH, alcohol content, metal ions, and macromolecular interactions—complicates the development of universally applicable assessment protocols.
Additionally, the time-intensive nature of most reliable tests conflicts with industry needs for rapid quality control decisions. This has led to compromises where faster but less accurate methods are often preferred in commercial settings, potentially increasing the risk of stability issues in final products exposed to heat during distribution and storage.
Established Thermal Stability Evaluation Protocols
01 Stabilization methods for tartaric acid in formulations
Various methods can be employed to stabilize tartaric acid in different formulations. These include controlling pH levels, adding specific buffer systems, and incorporating stabilizing agents. The stability of tartaric acid can be enhanced by maintaining optimal environmental conditions and using compatible excipients that prevent degradation. These stabilization techniques are particularly important in pharmaceutical and food applications where tartaric acid's integrity needs to be maintained over time.- Stabilization methods for tartaric acid in formulations: Various methods can be employed to stabilize tartaric acid in different formulations. These include controlling pH levels, adding specific buffer systems, and incorporating stabilizing agents. The stability of tartaric acid can be enhanced by maintaining optimal environmental conditions and using compatible excipients that prevent degradation. These stabilization techniques are particularly important in pharmaceutical and food applications where tartaric acid's integrity must be maintained over time.
- Tartaric acid stability in food and beverage applications: In food and beverage applications, tartaric acid stability is crucial for maintaining product quality and shelf life. Factors affecting stability include temperature, light exposure, and interaction with other ingredients. Specific formulation techniques can be used to prevent degradation in acidic food environments. Stabilized tartaric acid formulations help maintain consistent flavor profiles and acidity levels in products such as wines, fruit juices, and confectionery items.
- Chemical modifications to improve tartaric acid stability: Chemical modifications can be made to the tartaric acid molecule to enhance its stability under various conditions. These modifications include salt formation, esterification, and complexation with other compounds. Modified forms of tartaric acid demonstrate improved resistance to degradation factors such as heat, oxidation, and hydrolysis. These chemically stabilized forms find applications in pharmaceuticals, cosmetics, and industrial processes where standard tartaric acid might be too unstable.
- Tartaric acid stability in pharmaceutical formulations: The stability of tartaric acid in pharmaceutical formulations is essential for drug efficacy and safety. Specific stabilization techniques include selection of compatible excipients, appropriate packaging materials, and controlled manufacturing processes. Stability studies are conducted under various conditions to determine shelf life and storage requirements. Pharmaceutical applications often require tartaric acid to maintain stability across a range of temperatures and humidity levels to ensure consistent therapeutic outcomes.
- Industrial processing methods affecting tartaric acid stability: Industrial processing methods significantly impact tartaric acid stability during production and storage. Techniques such as controlled crystallization, purification processes, and specialized drying methods can enhance stability. The presence of metal ions, particularly iron and copper, can catalyze degradation reactions and must be controlled. Manufacturing parameters including temperature, pressure, and processing time need careful optimization to maintain tartaric acid integrity throughout industrial production cycles.
02 Tartaric acid stability in food and beverage applications
In food and beverage applications, tartaric acid stability is crucial for maintaining product quality and shelf life. Factors affecting stability include temperature, light exposure, and interaction with other ingredients. Specific formulation techniques can be used to prevent degradation in acidic food environments. Stabilized tartaric acid formulations help maintain consistent flavor profiles and acidity levels in products such as wines, fruit juices, and confectionery items.Expand Specific Solutions03 Chemical modifications to improve tartaric acid stability
Chemical modifications of tartaric acid can significantly improve its stability under various conditions. These modifications include esterification, salt formation, and complexation with other compounds. Modified forms of tartaric acid show enhanced resistance to degradation factors such as heat, oxidation, and hydrolysis. These chemically stabilized derivatives find applications in pharmaceuticals, cosmetics, and industrial processes where standard tartaric acid might be too unstable.Expand Specific Solutions04 Tartaric acid stability in pharmaceutical formulations
The stability of tartaric acid in pharmaceutical formulations is essential for drug efficacy and safety. Specific stabilization techniques include selection of compatible excipients, appropriate packaging materials, and controlled manufacturing processes. Stability studies are conducted under various conditions to determine shelf life and storage requirements. Pharmaceutical applications often require tartaric acid to maintain stability across a range of pH values and in the presence of other active ingredients.Expand Specific Solutions05 Environmental factors affecting tartaric acid stability
Environmental factors significantly impact tartaric acid stability. These include temperature, humidity, light exposure, and presence of metal ions. Controlling these factors is essential for maintaining tartaric acid integrity during storage and use. High temperatures can accelerate degradation, while certain metal ions can catalyze oxidation reactions. Protective measures such as controlled storage conditions, light-resistant packaging, and addition of chelating agents can help mitigate these environmental challenges.Expand Specific Solutions
Key Industry Players and Research Institutions
The tartaric acid stability assessment market is currently in a growth phase, with increasing demand driven by wine, pharmaceutical, and food industries requiring reliable stability testing methods during heat exposure. The global market for wine stabilization technologies alone exceeds $300 million annually, with tartaric stability testing representing a significant segment. Technologically, this field has evolved from basic cold stability tests to sophisticated analytical methods, with companies like Genzyme, Novozymes, and AbbVie leading innovation through proprietary stabilization technologies. Lonza and Sumitomo Chemical have developed advanced analytical instruments for precise measurement, while pharmaceutical leaders including Novartis and Pfizer have integrated tartaric stability testing into quality control protocols. The technology continues to mature with emerging spectroscopic and chromatographic methods enhancing precision and efficiency.
Lonza Ltd.
Technical Solution: Lonza has developed a comprehensive tartaric acid stability assessment protocol that combines thermal cycling tests with advanced analytical techniques. Their approach utilizes High-Performance Liquid Chromatography (HPLC) coupled with Mass Spectrometry to monitor degradation products formed during heat exposure. The company employs controlled temperature chambers that can simulate various environmental conditions (40°C/75% RH, 50°C/ambient humidity) for accelerated stability testing. Lonza's method incorporates differential scanning calorimetry (DSC) to determine the thermal transition points of tartaric acid and its formulations, establishing critical temperature thresholds for stability. Their protocol includes monitoring pH changes during heating, as tartaric acid degradation typically causes pH shifts that can be early indicators of instability. Additionally, Lonza has developed proprietary stabilizing excipients that can be incorporated with tartaric acid to enhance its thermal stability in pharmaceutical formulations.
Strengths: Highly sensitive analytical methods capable of detecting degradation products at very low concentrations; comprehensive approach combining multiple analytical techniques; established correlation between accelerated and real-time stability data. Weaknesses: Equipment-intensive methodology requiring specialized analytical instruments; longer testing cycles compared to some competitors; higher cost of implementation for smaller operations.
Novartis AG
Technical Solution: Novartis has pioneered an integrated approach to tartaric acid stability assessment focusing on pharmaceutical applications. Their methodology employs isothermal microcalorimetry to detect subtle thermal events during heat exposure, providing early warning of potential instability. The company has developed a proprietary stability-indicating HPLC method specifically optimized for tartaric acid and its stereoisomers, capable of separating and quantifying degradation products with high precision. Novartis utilizes Fourier Transform Infrared Spectroscopy (FTIR) with attenuated total reflectance to monitor structural changes in tartaric acid during thermal stress without sample preparation. Their protocol incorporates multiple temperature points (30°C, 40°C, 50°C, and 60°C) with controlled humidity conditions to generate comprehensive Arrhenius plots, enabling accurate shelf-life predictions. Additionally, Novartis has developed a rapid screening method using Near-Infrared (NIR) spectroscopy coupled with chemometric analysis to quickly assess tartaric acid stability in various formulation matrices.
Strengths: Highly predictive stability models based on extensive historical data; rapid screening capabilities for formulation development; sophisticated data analysis algorithms for detecting subtle stability trends. Weaknesses: Methods optimized primarily for pharmaceutical applications with less validation in food/beverage contexts; requires significant investment in specialized analytical equipment; higher complexity requiring advanced user training.
Critical Research Findings on Heat-Induced Degradation
Stable hyaluronan/steroid formulation
PatentActiveUS8273725B2
Innovation
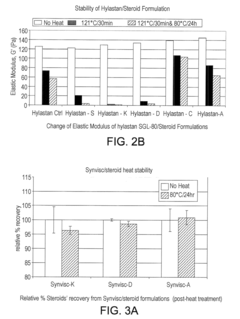
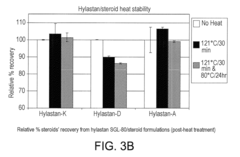
- The combination of hyaluronic acid-related components (HARCs) with triamcinolone hexacetonide (TAH) retains chemical integrity and provides stable viscoelastic properties even after heat sterilization, maintaining suitable shelf life and stability, as compared to compositions without TAH.
Heat stability evaluation method of sample
PatentInactiveJP2011043374A
Innovation
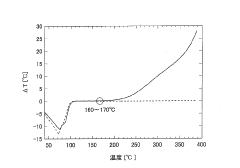
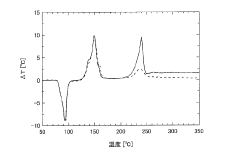
- A method involving thermal stability evaluation that compares ΔT1 (in an oxygen-containing atmosphere) and ΔT2 (in an inert gas atmosphere) to differentiate between thermal decomposition and oxidation reactions by plotting ΔT1 and ΔT2 curves against sample temperature.
Regulatory Standards for Food-Grade Acid Stability
The regulatory landscape for food-grade acid stability, particularly tartaric acid, is governed by several international and regional standards that manufacturers must adhere to. The FDA in the United States classifies tartaric acid as Generally Recognized as Safe (GRAS) under 21 CFR 184.1099, but requires stability testing under various conditions, including heat exposure, to maintain this status. The European Food Safety Authority (EFSA) similarly regulates tartaric acid under E334 designation, with specific requirements for stability assessment outlined in Commission Regulation (EU) No 231/2012.
The Codex Alimentarius Commission has established international standards for food additives, including tartaric acid, which specify acceptable stability parameters under thermal stress. These standards mandate that tartaric acid must maintain at least 99% purity after exposure to temperatures up to 80°C for 4 hours, with degradation products not exceeding established safety thresholds.
In Asia, regulatory frameworks vary significantly. Japan's Ministry of Health, Labour and Welfare enforces strict stability requirements through the Japanese Food Sanitation Law, while China's GB standards (GB 1886.187-2016) outline specific protocols for heat stability assessment of food-grade tartaric acid, requiring stability testing at temperatures ranging from 60°C to 120°C.
Industry-specific regulations also exist, particularly in the wine and beverage sectors. The International Organisation of Vine and Wine (OIV) has established specific guidelines for tartaric acid stability in wine products, including thermal stability requirements that manufacturers must meet to ensure product quality and safety.
The pharmaceutical industry faces even more stringent requirements, with the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) both providing detailed monographs for tartaric acid that include stability testing protocols. These standards typically require demonstration of stability under accelerated conditions (40°C/75% RH) for at least six months.
Testing methodologies for regulatory compliance typically include High-Performance Liquid Chromatography (HPLC), Differential Scanning Calorimetry (DSC), and spectrophotometric analyses. Regulatory bodies increasingly require validation of these methods according to ICH guidelines or equivalent standards to ensure consistency and reliability in stability assessments.
Recent regulatory trends indicate a move toward harmonization of standards across different regions, with the International Council for Harmonisation (ICH) playing a key role in developing unified approaches to stability testing requirements for food additives including tartaric acid.
The Codex Alimentarius Commission has established international standards for food additives, including tartaric acid, which specify acceptable stability parameters under thermal stress. These standards mandate that tartaric acid must maintain at least 99% purity after exposure to temperatures up to 80°C for 4 hours, with degradation products not exceeding established safety thresholds.
In Asia, regulatory frameworks vary significantly. Japan's Ministry of Health, Labour and Welfare enforces strict stability requirements through the Japanese Food Sanitation Law, while China's GB standards (GB 1886.187-2016) outline specific protocols for heat stability assessment of food-grade tartaric acid, requiring stability testing at temperatures ranging from 60°C to 120°C.
Industry-specific regulations also exist, particularly in the wine and beverage sectors. The International Organisation of Vine and Wine (OIV) has established specific guidelines for tartaric acid stability in wine products, including thermal stability requirements that manufacturers must meet to ensure product quality and safety.
The pharmaceutical industry faces even more stringent requirements, with the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) both providing detailed monographs for tartaric acid that include stability testing protocols. These standards typically require demonstration of stability under accelerated conditions (40°C/75% RH) for at least six months.
Testing methodologies for regulatory compliance typically include High-Performance Liquid Chromatography (HPLC), Differential Scanning Calorimetry (DSC), and spectrophotometric analyses. Regulatory bodies increasingly require validation of these methods according to ICH guidelines or equivalent standards to ensure consistency and reliability in stability assessments.
Recent regulatory trends indicate a move toward harmonization of standards across different regions, with the International Council for Harmonisation (ICH) playing a key role in developing unified approaches to stability testing requirements for food additives including tartaric acid.
Environmental Impact of Tartaric Acid Processing
The processing of tartaric acid, while essential for various industries, carries significant environmental implications that warrant careful consideration. Traditional extraction methods from wine lees and grape pomace involve chemical processes that generate substantial waste streams containing organic residues and chemical reagents. These effluents, when improperly managed, can contaminate local water bodies due to their high biochemical oxygen demand (BOD) and chemical oxygen demand (COD) levels, potentially disrupting aquatic ecosystems.
Energy consumption represents another critical environmental concern in tartaric acid production. The purification process typically requires multiple heating and cooling cycles, particularly during crystallization phases where temperature stability assessment becomes crucial. This energy-intensive approach contributes to greenhouse gas emissions, with studies indicating that producing one ton of tartaric acid can generate approximately 2.5-3 tons of CO2 equivalent emissions depending on the energy source utilized.
Water usage in tartaric acid processing presents additional sustainability challenges. The industry consumes significant volumes of water for extraction, purification, and cleaning operations. Recent data suggests that conventional processing methods require 40-60 cubic meters of water per ton of tartaric acid produced, placing pressure on local water resources in production regions.
Chemical inputs used during stability testing and processing, including various acids and solvents, pose potential environmental hazards if released untreated. These substances can persist in the environment and potentially bioaccumulate in organisms. Modern regulatory frameworks increasingly require manufacturers to implement proper chemical management systems and treatment protocols to mitigate these risks.
Land use changes associated with vineyard expansion for tartaric acid source materials also merit attention. The conversion of natural habitats to agricultural land for grape cultivation can reduce biodiversity and alter local ecosystems. However, sustainable vineyard management practices can help minimize these impacts while still providing raw materials for tartaric acid production.
Recent innovations in green chemistry approaches offer promising pathways to reduce the environmental footprint of tartaric acid processing. These include enzyme-assisted extraction methods, which operate at lower temperatures and reduce chemical inputs, thereby decreasing energy requirements for stability testing and processing. Additionally, closed-loop systems that recycle water and recover solvents are gaining traction, potentially reducing resource consumption by 30-40% compared to conventional methods.
Carbon footprint certification programs are emerging as valuable tools for manufacturers to quantify and communicate their environmental performance. These initiatives encourage continuous improvement in processing efficiency and stability testing methodologies, driving the industry toward more sustainable practices that balance economic viability with environmental responsibility.
Energy consumption represents another critical environmental concern in tartaric acid production. The purification process typically requires multiple heating and cooling cycles, particularly during crystallization phases where temperature stability assessment becomes crucial. This energy-intensive approach contributes to greenhouse gas emissions, with studies indicating that producing one ton of tartaric acid can generate approximately 2.5-3 tons of CO2 equivalent emissions depending on the energy source utilized.
Water usage in tartaric acid processing presents additional sustainability challenges. The industry consumes significant volumes of water for extraction, purification, and cleaning operations. Recent data suggests that conventional processing methods require 40-60 cubic meters of water per ton of tartaric acid produced, placing pressure on local water resources in production regions.
Chemical inputs used during stability testing and processing, including various acids and solvents, pose potential environmental hazards if released untreated. These substances can persist in the environment and potentially bioaccumulate in organisms. Modern regulatory frameworks increasingly require manufacturers to implement proper chemical management systems and treatment protocols to mitigate these risks.
Land use changes associated with vineyard expansion for tartaric acid source materials also merit attention. The conversion of natural habitats to agricultural land for grape cultivation can reduce biodiversity and alter local ecosystems. However, sustainable vineyard management practices can help minimize these impacts while still providing raw materials for tartaric acid production.
Recent innovations in green chemistry approaches offer promising pathways to reduce the environmental footprint of tartaric acid processing. These include enzyme-assisted extraction methods, which operate at lower temperatures and reduce chemical inputs, thereby decreasing energy requirements for stability testing and processing. Additionally, closed-loop systems that recycle water and recover solvents are gaining traction, potentially reducing resource consumption by 30-40% compared to conventional methods.
Carbon footprint certification programs are emerging as valuable tools for manufacturers to quantify and communicate their environmental performance. These initiatives encourage continuous improvement in processing efficiency and stability testing methodologies, driving the industry toward more sustainable practices that balance economic viability with environmental responsibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!