How to Comprehend Carbon Tetrachloride's Scientific Foundations?
CCl4 Background & Objectives
Carbon tetrachloride (CCl4) has a rich scientific history dating back to its discovery in 1839 by French chemist Henri Victor Regnault. This compound, consisting of one carbon atom bonded to four chlorine atoms, has played a significant role in various industrial applications and scientific research. The evolution of our understanding of CCl4 has been closely tied to advancements in chemical theory and analytical techniques.
The primary objective in comprehending the scientific foundations of carbon tetrachloride is to gain a comprehensive understanding of its physical and chemical properties, molecular structure, and behavior under different conditions. This knowledge is crucial for assessing its environmental impact, potential applications, and developing safer alternatives or remediation strategies.
From a structural perspective, CCl4 exemplifies the tetrahedral geometry of carbon compounds, making it an excellent model for studying molecular symmetry and bonding. Its non-polar nature and high electronegativity of chlorine atoms contribute to its unique properties, such as its ability to dissolve non-polar substances and its high volatility.
The reactivity of carbon tetrachloride has been a subject of extensive research, particularly its involvement in free radical reactions. Understanding these reactions is essential for explaining its role in atmospheric chemistry and its potential for ozone depletion. Additionally, the compound's behavior in different solvents and its interactions with other molecules provide valuable insights into intermolecular forces and solution chemistry.
Spectroscopic studies of CCl4 have contributed significantly to our understanding of molecular vibrations and rotations. Its simple structure makes it an ideal candidate for infrared and Raman spectroscopy, helping scientists refine their understanding of molecular energy levels and transition probabilities.
The toxicological profile of carbon tetrachloride has been a driving force behind much of the research into its scientific foundations. Elucidating its mechanisms of toxicity, particularly its effects on the liver and kidneys, has been crucial for developing safety protocols and exploring potential medical applications or treatments for exposure.
In recent years, the focus has shifted towards understanding the environmental fate and transport of CCl4. This includes studying its persistence in various environmental compartments, its degradation pathways, and its role in global biogeochemical cycles. Such knowledge is essential for developing effective strategies to mitigate its environmental impact and comply with international regulations on ozone-depleting substances.
Industrial Applications & Demand
Carbon tetrachloride, once widely used in various industries, has seen a significant shift in its applications and demand due to environmental and health concerns. The chemical's unique properties, including its non-flammability and excellent solvency, initially made it a popular choice in many industrial processes. However, its usage has been severely restricted in recent years.
Historically, carbon tetrachloride found extensive use as a cleaning agent and degreaser in the metal industry. Its ability to dissolve oils and greases made it invaluable in machinery maintenance and parts cleaning. The dry cleaning industry also relied heavily on carbon tetrachloride before the introduction of less harmful alternatives. In the agricultural sector, it was used as a fumigant for grain storage and as a pesticide.
The chemical industry utilized carbon tetrachloride as a feedstock for the production of chlorofluorocarbons (CFCs) and other chlorinated compounds. It also served as a solvent in various chemical processes and as a raw material in the synthesis of pharmaceutical products. In the electronics industry, carbon tetrachloride was employed in the manufacture of semiconductors and as a cleaning agent for electronic components.
Despite its versatility, the demand for carbon tetrachloride has drastically declined due to international regulations aimed at protecting the ozone layer and human health. The Montreal Protocol, implemented in 1989, phased out the production and consumption of ozone-depleting substances, including carbon tetrachloride. This led to a sharp decrease in its use for CFC production and other applications.
Currently, the primary remaining industrial application of carbon tetrachloride is as a feedstock for the production of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs), which are considered more environmentally friendly alternatives to CFCs. However, even this use is subject to strict controls and reporting requirements under international agreements.
The scientific community continues to study carbon tetrachloride's atmospheric concentrations and environmental impact. Research efforts focus on understanding its long-term effects on the ozone layer and identifying any remaining sources of emissions. This ongoing scientific interest drives a small but persistent demand for the compound in laboratory settings for analytical and research purposes.
In the medical field, carbon tetrachloride is still used in some diagnostic procedures, albeit in very limited quantities. Its ability to absorb X-rays makes it useful as a contrast medium in certain radiological examinations. However, safer alternatives are increasingly preferred, further reducing the demand in this sector.
As industries adapt to regulatory pressures and environmental concerns, the search for safer substitutes has intensified. This has led to the development of new technologies and processes that aim to replicate the beneficial properties of carbon tetrachloride without its harmful effects. The ongoing transition away from carbon tetrachloride underscores the dynamic nature of industrial chemistry and the continuous need for innovation in response to environmental and health imperatives.
Current Status & Challenges
Carbon tetrachloride (CCl4) has been a subject of extensive research and industrial application for decades. However, its current status and challenges are marked by a complex interplay of scientific understanding, environmental concerns, and regulatory measures.
The scientific community has made significant strides in comprehending the chemical properties and behavior of carbon tetrachloride. Its molecular structure, physical characteristics, and reactivity are well-documented, providing a solid foundation for further research and applications. Nevertheless, ongoing studies continue to reveal new aspects of its interactions with various substances and environments, particularly in atmospheric and aquatic systems.
One of the primary challenges in the field of carbon tetrachloride research is its environmental impact. Despite being phased out as an industrial solvent and refrigerant due to its ozone-depleting properties, CCl4 persists in the environment due to its long atmospheric lifetime. This persistence has led to continued efforts to understand its long-term effects on global climate and ecosystems.
The discrepancy between observed atmospheric concentrations of carbon tetrachloride and reported emissions presents a significant scientific puzzle. Researchers are working to identify and quantify unknown sources of CCl4, which may include unreported industrial emissions, natural sources, or previously unrecognized chemical processes in the atmosphere.
In the realm of analytical chemistry, the detection and quantification of carbon tetrachloride in various matrices remain challenging. While advanced techniques such as gas chromatography-mass spectrometry (GC-MS) offer high sensitivity, there is an ongoing need for more rapid, cost-effective, and field-deployable methods for environmental monitoring and industrial quality control.
The toxicological aspects of carbon tetrachloride continue to be an area of active research. While its acute toxicity is well-established, the long-term effects of low-level exposure and its potential role in chronic diseases are still being investigated. This research is crucial for refining occupational safety standards and environmental regulations.
From a technological perspective, the development of remediation techniques for CCl4-contaminated sites presents both challenges and opportunities. Current methods, such as pump-and-treat systems and in situ chemical oxidation, have limitations in terms of efficiency and cost-effectiveness. Innovative approaches, including bioremediation and nanomaterial-based treatments, are being explored to address these limitations.
The global distribution of carbon tetrachloride research and technological development is uneven. While developed countries have made significant progress in phasing out CCl4 use and implementing stringent controls, many developing nations face challenges in monitoring, regulation, and remediation efforts. This disparity highlights the need for international collaboration and knowledge transfer in addressing the global impact of carbon tetrachloride.
Synthesis & Production Methods
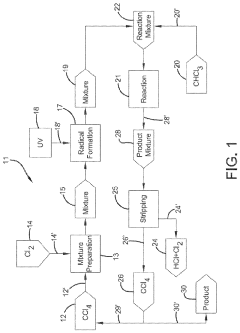
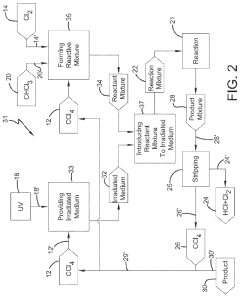
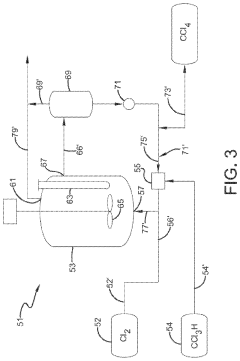
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical reactions, distillation processes, and other purification techniques to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is used in various chemical processes as a solvent, reagent, or intermediate. It plays a role in organic synthesis, extraction processes, and as a raw material for the production of other chlorinated compounds.
- Environmental and safety considerations: Due to its environmental impact and health hazards, there are methods and systems developed for the safe handling, storage, and disposal of carbon tetrachloride. This includes techniques for detecting leaks, reducing emissions, and treating contaminated areas.
- Alternatives and replacements for carbon tetrachloride: Research into alternatives and replacements for carbon tetrachloride in various applications, due to its ozone-depleting properties and toxicity. This includes the development of new compounds or processes that can perform similar functions with reduced environmental impact.
- Historical uses and early patents related to carbon tetrachloride: Early patents and historical uses of carbon tetrachloride, including its applications in fire extinguishers, dry cleaning, and as a refrigerant. These documents provide insight into the initial discoveries and industrial applications of the compound.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes as a solvent, reagent, or intermediate. It finds applications in organic synthesis, extraction processes, and as a raw material for the production of other chlorinated compounds.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes techniques for detecting and monitoring carbon tetrachloride in various environments.Expand Specific Solutions04 Historical uses and patents
Early patents and historical documents describe various applications of carbon tetrachloride, including its use as a fire extinguishing agent, cleaning solvent, and in the production of refrigerants. These patents provide insight into the compound's industrial importance over time.Expand Specific Solutions05 Analytical methods involving carbon tetrachloride
Carbon tetrachloride is used in various analytical methods and laboratory techniques. This includes its application in spectroscopic studies, as a solvent for NMR spectroscopy, and in the development of new analytical procedures for chemical analysis.Expand Specific Solutions
Key Industry Players Analysis
The carbon tetrachloride market is in a mature phase, with a relatively stable global demand. The market size is estimated to be in the range of $200-300 million annually. Technologically, carbon tetrachloride production is well-established, with key players like Occidental Chemical Corp., Tronox LLC, and BASF Corp. having significant expertise. However, due to environmental concerns and regulations, there's a shift towards developing alternatives and more sustainable production methods. Companies like DuPont de Nemours, Inc. and Sumitomo Chemical Co., Ltd. are investing in research for safer substitutes and improved manufacturing processes to address these challenges.
Occidental Chemical Corp.
Tronox LLC
Core Chemical Properties Analysis
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Environmental Impact Assessment
Carbon tetrachloride (CCl4) is a synthetic chemical compound that has been widely used in various industrial applications. However, its environmental impact has become a significant concern due to its persistence and potential harm to ecosystems and human health. The environmental impact assessment of carbon tetrachloride reveals several critical areas of concern.
Atmospheric effects are one of the primary environmental impacts of carbon tetrachloride. When released into the air, CCl4 contributes to the depletion of the ozone layer, which protects the Earth from harmful ultraviolet radiation. The compound has a long atmospheric lifetime, estimated at 26 years, allowing it to reach the stratosphere and participate in ozone-depleting reactions. This has led to its regulation under the Montreal Protocol, an international treaty designed to protect the ozone layer.
Water contamination is another significant environmental issue associated with carbon tetrachloride. Due to its high density and low water solubility, CCl4 can form dense non-aqueous phase liquids (DNAPLs) when released into soil or water bodies. These DNAPLs can sink to the bottom of aquifers and slowly dissolve over time, leading to long-term groundwater contamination. This poses risks to aquatic ecosystems and potential human exposure through drinking water sources.
Soil contamination by carbon tetrachloride can have lasting effects on terrestrial ecosystems. The compound can persist in soil for extended periods, potentially affecting soil microorganisms and plant life. It may also volatilize from contaminated soil, leading to air pollution and potential exposure to humans and wildlife through inhalation.
Bioaccumulation and biomagnification of carbon tetrachloride in food chains are additional concerns. Although CCl4 does not tend to bioaccumulate significantly in aquatic organisms, its presence in the environment can lead to chronic exposure for various species. This may result in long-term ecological impacts, particularly in areas with historical contamination from industrial activities.
The global transport of carbon tetrachloride through atmospheric and oceanic circulation patterns contributes to its widespread environmental impact. Even though its production and use have been significantly reduced in many countries, the compound's persistence means that it continues to affect ecosystems far from its original sources of release.
In conclusion, the environmental impact assessment of carbon tetrachloride highlights the compound's far-reaching effects on atmospheric, aquatic, and terrestrial systems. Its ozone-depleting potential, persistence in various environmental compartments, and potential for long-term contamination underscore the importance of continued monitoring and mitigation efforts to address its legacy and prevent further environmental damage.
Safety Regulations & Handling
Carbon tetrachloride is a highly toxic and potentially dangerous chemical compound that requires strict safety regulations and careful handling procedures. The Occupational Safety and Health Administration (OSHA) has established specific guidelines for its use in industrial settings, including permissible exposure limits (PELs) and recommended personal protective equipment (PPE). Workers must be provided with appropriate respiratory protection, impervious gloves, and protective clothing when handling this substance.
Storage of carbon tetrachloride must adhere to specific regulations to prevent accidental release or exposure. It should be kept in tightly sealed containers in a cool, well-ventilated area away from sources of heat or ignition. Incompatible materials, such as strong oxidizers and alkali metals, must be stored separately to avoid potentially hazardous reactions. Regular inspections of storage areas and containers are essential to ensure integrity and prevent leaks.
Transportation of carbon tetrachloride is regulated by the Department of Transportation (DOT) as a hazardous material. It must be properly labeled and packaged in accordance with DOT regulations, including the use of UN-approved containers and appropriate hazard communication labels. Vehicles transporting this chemical must display proper placards and carry necessary documentation, including safety data sheets (SDS) and emergency response information.
Proper disposal of carbon tetrachloride and its waste products is crucial to prevent environmental contamination. The Environmental Protection Agency (EPA) classifies it as a hazardous waste under the Resource Conservation and Recovery Act (RCRA). Disposal must be carried out by licensed hazardous waste management facilities using approved methods such as incineration or chemical treatment.
Emergency response procedures for carbon tetrachloride incidents are critical. Facilities using or storing this chemical must have comprehensive emergency plans in place, including spill containment measures, evacuation procedures, and decontamination protocols. First responders should be trained in handling hazardous materials and equipped with appropriate PPE, including self-contained breathing apparatus (SCBA).
Environmental monitoring is essential when working with carbon tetrachloride. Regular air quality testing should be conducted to ensure that concentrations remain below established safety thresholds. Wastewater and soil testing may also be necessary in areas where the chemical is used or stored to detect potential contamination and implement appropriate remediation measures if needed.