How to Develop Effective Protocols for Carbon Tetrachloride Disposal?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Disposal Background and Objectives
Carbon tetrachloride (CCl4) disposal has become a critical environmental and health concern due to its persistent nature and harmful effects on the ozone layer and human health. The evolution of CCl4 disposal techniques has been driven by increasing awareness of its toxicity and the need for more sustainable waste management practices.
Historically, CCl4 was widely used as a solvent, cleaning agent, and in the production of refrigerants. However, its production and use have been phased out in many countries since the late 1980s due to its ozone-depleting properties and potential carcinogenicity. This shift has led to a growing need for effective disposal methods for existing stockpiles and waste containing CCl4.
The primary objective of developing effective protocols for CCl4 disposal is to minimize environmental impact and protect human health. This involves creating comprehensive guidelines that address the entire lifecycle of CCl4 waste, from identification and collection to treatment and final disposal. These protocols must ensure the complete destruction of CCl4 molecules while preventing the formation of equally harmful by-products.
Current disposal methods range from incineration and chemical treatment to advanced oxidation processes. Each approach has its advantages and limitations, necessitating a thorough evaluation to determine the most suitable technique for different scenarios. The development of effective protocols must consider factors such as disposal efficiency, cost-effectiveness, scalability, and environmental sustainability.
Another crucial aspect of CCl4 disposal protocols is the establishment of stringent safety measures. Given the volatile and toxic nature of CCl4, handling and disposal procedures must prioritize worker safety and prevent accidental releases into the environment. This includes developing proper containment strategies, personal protective equipment requirements, and emergency response plans.
The technological evolution in CCl4 disposal is closely linked to advancements in analytical techniques for detecting and quantifying CCl4 in various matrices. Improved detection methods enable more accurate monitoring of disposal processes and help ensure compliance with environmental regulations. As such, the development of effective protocols must incorporate state-of-the-art analytical tools and methodologies.
International cooperation plays a vital role in addressing the global challenge of CCl4 disposal. Collaborative efforts between countries, research institutions, and industry stakeholders are essential for sharing best practices, harmonizing standards, and developing innovative solutions. The ultimate goal is to create a unified approach to CCl4 disposal that can be adapted to diverse regional contexts while maintaining high environmental and safety standards.
Historically, CCl4 was widely used as a solvent, cleaning agent, and in the production of refrigerants. However, its production and use have been phased out in many countries since the late 1980s due to its ozone-depleting properties and potential carcinogenicity. This shift has led to a growing need for effective disposal methods for existing stockpiles and waste containing CCl4.
The primary objective of developing effective protocols for CCl4 disposal is to minimize environmental impact and protect human health. This involves creating comprehensive guidelines that address the entire lifecycle of CCl4 waste, from identification and collection to treatment and final disposal. These protocols must ensure the complete destruction of CCl4 molecules while preventing the formation of equally harmful by-products.
Current disposal methods range from incineration and chemical treatment to advanced oxidation processes. Each approach has its advantages and limitations, necessitating a thorough evaluation to determine the most suitable technique for different scenarios. The development of effective protocols must consider factors such as disposal efficiency, cost-effectiveness, scalability, and environmental sustainability.
Another crucial aspect of CCl4 disposal protocols is the establishment of stringent safety measures. Given the volatile and toxic nature of CCl4, handling and disposal procedures must prioritize worker safety and prevent accidental releases into the environment. This includes developing proper containment strategies, personal protective equipment requirements, and emergency response plans.
The technological evolution in CCl4 disposal is closely linked to advancements in analytical techniques for detecting and quantifying CCl4 in various matrices. Improved detection methods enable more accurate monitoring of disposal processes and help ensure compliance with environmental regulations. As such, the development of effective protocols must incorporate state-of-the-art analytical tools and methodologies.
International cooperation plays a vital role in addressing the global challenge of CCl4 disposal. Collaborative efforts between countries, research institutions, and industry stakeholders are essential for sharing best practices, harmonizing standards, and developing innovative solutions. The ultimate goal is to create a unified approach to CCl4 disposal that can be adapted to diverse regional contexts while maintaining high environmental and safety standards.
Market Analysis for CCl4 Disposal Solutions
The market for Carbon Tetrachloride (CCl4) disposal solutions has been experiencing significant growth due to increasing environmental regulations and a growing awareness of the harmful effects of this chemical. CCl4, once widely used in various industrial applications, has been phased out in many countries due to its ozone-depleting properties and potential health hazards. This has created a substantial demand for effective disposal methods.
The global market for hazardous waste management, which includes CCl4 disposal, was valued at $27.4 billion in 2020 and is projected to reach $44.5 billion by 2028, growing at a CAGR of 6.2% from 2021 to 2028. Within this broader market, CCl4 disposal solutions represent a niche but crucial segment.
Key drivers of the CCl4 disposal market include stringent environmental regulations, particularly in developed countries, and the ongoing cleanup of legacy contamination sites. The Stockholm Convention on Persistent Organic Pollutants, which aims to eliminate or restrict the production and use of persistent organic pollutants, has been a significant factor in driving the demand for CCl4 disposal solutions.
The market is segmented based on disposal methods, including incineration, chemical treatment, and advanced oxidation processes. Incineration currently holds the largest market share due to its effectiveness in completely destroying CCl4. However, chemical treatment methods are gaining traction due to their lower environmental impact and cost-effectiveness for smaller-scale operations.
Geographically, North America and Europe dominate the CCl4 disposal market, accounting for over 60% of the global market share. This is primarily due to strict environmental regulations and the presence of well-established waste management infrastructure. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization and increasing environmental awareness in countries like China and India.
The market landscape is characterized by a mix of large waste management companies and specialized environmental service providers. Key players in this space include Clean Harbors, Veolia, Suez, and Waste Management Inc. These companies are investing heavily in research and development to improve disposal efficiency and reduce environmental impact.
Challenges in the CCl4 disposal market include high initial investment costs for advanced disposal technologies and the complexity of handling and transporting this hazardous substance. Additionally, the limited availability of specialized disposal facilities in some regions poses a significant barrier to market growth.
Looking ahead, the market for CCl4 disposal solutions is expected to continue its growth trajectory, driven by ongoing environmental remediation efforts and the implementation of stricter regulations worldwide. Emerging technologies, such as plasma-based destruction methods and supercritical water oxidation, are likely to reshape the market landscape in the coming years, offering more efficient and environmentally friendly disposal options.
The global market for hazardous waste management, which includes CCl4 disposal, was valued at $27.4 billion in 2020 and is projected to reach $44.5 billion by 2028, growing at a CAGR of 6.2% from 2021 to 2028. Within this broader market, CCl4 disposal solutions represent a niche but crucial segment.
Key drivers of the CCl4 disposal market include stringent environmental regulations, particularly in developed countries, and the ongoing cleanup of legacy contamination sites. The Stockholm Convention on Persistent Organic Pollutants, which aims to eliminate or restrict the production and use of persistent organic pollutants, has been a significant factor in driving the demand for CCl4 disposal solutions.
The market is segmented based on disposal methods, including incineration, chemical treatment, and advanced oxidation processes. Incineration currently holds the largest market share due to its effectiveness in completely destroying CCl4. However, chemical treatment methods are gaining traction due to their lower environmental impact and cost-effectiveness for smaller-scale operations.
Geographically, North America and Europe dominate the CCl4 disposal market, accounting for over 60% of the global market share. This is primarily due to strict environmental regulations and the presence of well-established waste management infrastructure. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization and increasing environmental awareness in countries like China and India.
The market landscape is characterized by a mix of large waste management companies and specialized environmental service providers. Key players in this space include Clean Harbors, Veolia, Suez, and Waste Management Inc. These companies are investing heavily in research and development to improve disposal efficiency and reduce environmental impact.
Challenges in the CCl4 disposal market include high initial investment costs for advanced disposal technologies and the complexity of handling and transporting this hazardous substance. Additionally, the limited availability of specialized disposal facilities in some regions poses a significant barrier to market growth.
Looking ahead, the market for CCl4 disposal solutions is expected to continue its growth trajectory, driven by ongoing environmental remediation efforts and the implementation of stricter regulations worldwide. Emerging technologies, such as plasma-based destruction methods and supercritical water oxidation, are likely to reshape the market landscape in the coming years, offering more efficient and environmentally friendly disposal options.
Current CCl4 Disposal Challenges
Carbon tetrachloride (CCl4) disposal presents significant challenges due to its environmental and health hazards. The primary difficulty lies in its persistence and potential for ozone depletion. CCl4 is highly stable, with a long atmospheric lifetime, making it a potent greenhouse gas and ozone-depleting substance. This stability also complicates its breakdown during disposal processes.
Current disposal methods often involve incineration, which can lead to the formation of harmful by-products if not properly controlled. The high temperatures required for complete destruction of CCl4 (above 1200°C) make the process energy-intensive and costly. Additionally, there's a risk of incomplete combustion, potentially releasing toxic compounds into the environment.
Chemical treatment methods, such as base-catalyzed decomposition, face challenges in achieving complete degradation of CCl4. The process often requires precise control of reaction conditions and can be sensitive to impurities in the waste stream. Scaling up these chemical treatments for industrial-scale disposal remains a significant hurdle.
Landfill disposal of CCl4-contaminated materials is no longer considered a viable option due to the risk of soil and groundwater contamination. The high volatility of CCl4 can lead to vapor intrusion in nearby structures, posing health risks to local populations.
Another challenge is the proper handling and transportation of CCl4 waste. Its volatility and toxicity require specialized containment and safety measures, increasing the complexity and cost of disposal operations. Accidental releases during transport or handling can have severe environmental and health consequences.
The global nature of CCl4 production and use complicates disposal efforts. Inconsistent regulations across different countries can lead to improper disposal practices in regions with less stringent environmental laws. This creates a need for international cooperation and standardized protocols for CCl4 disposal.
Monitoring and verification of CCl4 destruction pose additional challenges. Accurate measurement of destruction efficiency is crucial to ensure compliance with environmental regulations. However, the low concentrations of CCl4 in some waste streams can make detection and quantification difficult, requiring sophisticated analytical techniques.
Developing effective disposal protocols also requires addressing the legacy of historical CCl4 contamination. Many sites worldwide have soil and groundwater contaminated with CCl4 from past industrial activities. Remediation of these sites presents unique challenges, often requiring long-term, costly cleanup efforts.
In conclusion, the disposal of carbon tetrachloride faces multifaceted challenges, ranging from technical difficulties in destruction processes to regulatory and global coordination issues. Overcoming these challenges requires innovative technologies, improved international cooperation, and a comprehensive approach to waste management and site remediation.
Current disposal methods often involve incineration, which can lead to the formation of harmful by-products if not properly controlled. The high temperatures required for complete destruction of CCl4 (above 1200°C) make the process energy-intensive and costly. Additionally, there's a risk of incomplete combustion, potentially releasing toxic compounds into the environment.
Chemical treatment methods, such as base-catalyzed decomposition, face challenges in achieving complete degradation of CCl4. The process often requires precise control of reaction conditions and can be sensitive to impurities in the waste stream. Scaling up these chemical treatments for industrial-scale disposal remains a significant hurdle.
Landfill disposal of CCl4-contaminated materials is no longer considered a viable option due to the risk of soil and groundwater contamination. The high volatility of CCl4 can lead to vapor intrusion in nearby structures, posing health risks to local populations.
Another challenge is the proper handling and transportation of CCl4 waste. Its volatility and toxicity require specialized containment and safety measures, increasing the complexity and cost of disposal operations. Accidental releases during transport or handling can have severe environmental and health consequences.
The global nature of CCl4 production and use complicates disposal efforts. Inconsistent regulations across different countries can lead to improper disposal practices in regions with less stringent environmental laws. This creates a need for international cooperation and standardized protocols for CCl4 disposal.
Monitoring and verification of CCl4 destruction pose additional challenges. Accurate measurement of destruction efficiency is crucial to ensure compliance with environmental regulations. However, the low concentrations of CCl4 in some waste streams can make detection and quantification difficult, requiring sophisticated analytical techniques.
Developing effective disposal protocols also requires addressing the legacy of historical CCl4 contamination. Many sites worldwide have soil and groundwater contaminated with CCl4 from past industrial activities. Remediation of these sites presents unique challenges, often requiring long-term, costly cleanup efforts.
In conclusion, the disposal of carbon tetrachloride faces multifaceted challenges, ranging from technical difficulties in destruction processes to regulatory and global coordination issues. Overcoming these challenges requires innovative technologies, improved international cooperation, and a comprehensive approach to waste management and site remediation.
Existing CCl4 Disposal Protocols
01 Chemical treatment methods for carbon tetrachloride disposal
Various chemical treatment methods can be employed for the disposal of carbon tetrachloride. These methods include oxidation, reduction, and decomposition processes that convert carbon tetrachloride into less harmful substances. Chemical treatments may involve the use of specific reagents or catalysts to facilitate the breakdown of carbon tetrachloride under controlled conditions.- Chemical treatment methods: Various chemical treatment methods can be employed for the disposal of carbon tetrachloride. These methods may involve reactions that convert carbon tetrachloride into less harmful substances or break it down into simpler compounds. Chemical treatments can include oxidation, reduction, or other transformations that render the carbon tetrachloride less toxic or more easily manageable.
- Thermal decomposition techniques: Thermal decomposition is a method used for disposing of carbon tetrachloride. This process involves heating the compound to high temperatures, causing it to break down into simpler, less harmful components. The technique may be carried out in specialized incinerators or reactors designed to handle hazardous materials safely and efficiently.
- Adsorption and filtration systems: Adsorption and filtration systems can be utilized to remove carbon tetrachloride from contaminated media. These systems may employ activated carbon, zeolites, or other adsorbent materials to capture and concentrate the carbon tetrachloride. Once collected, the concentrated waste can be further treated or disposed of using other methods.
- Biological degradation processes: Biological degradation processes involve the use of microorganisms to break down carbon tetrachloride into less harmful substances. This approach may include the use of specialized bacteria or fungi that can metabolize the compound. Bioremediation techniques can be applied in situ or ex situ, depending on the specific contamination scenario and environmental conditions.
- Containment and long-term storage: In cases where immediate treatment is not feasible, containment and long-term storage methods may be employed for carbon tetrachloride disposal. This approach involves securely storing the compound in specially designed containers or facilities that prevent its release into the environment. Proper monitoring and maintenance of these storage systems are crucial to ensure long-term safety and environmental protection.
02 Thermal decomposition and incineration techniques
Thermal decomposition and incineration are effective methods for disposing of carbon tetrachloride. These processes involve subjecting the compound to high temperatures, which break down the molecular structure and convert it into less harmful byproducts. Specialized incinerators and thermal treatment facilities are used to ensure complete destruction of the carbon tetrachloride while minimizing the release of toxic emissions.Expand Specific Solutions03 Adsorption and filtration systems for carbon tetrachloride removal
Adsorption and filtration systems can be utilized to remove carbon tetrachloride from contaminated media, such as water or air. These systems employ specialized adsorbents or filter materials that selectively capture carbon tetrachloride molecules. The contaminated fluid passes through the adsorption or filtration system, effectively separating the carbon tetrachloride for subsequent disposal or treatment.Expand Specific Solutions04 Biological treatment methods for carbon tetrachloride degradation
Biological treatment methods harness the ability of certain microorganisms to degrade carbon tetrachloride. These approaches involve the use of specialized bacteria or fungi that can metabolize or transform carbon tetrachloride into less harmful compounds. Bioremediation techniques can be applied in situ or ex situ, depending on the specific contamination scenario and environmental conditions.Expand Specific Solutions05 Containment and long-term storage solutions
In cases where immediate treatment or disposal is not feasible, containment and long-term storage solutions can be employed for carbon tetrachloride. These methods involve the use of specialized containers, storage facilities, or geological formations to safely isolate the compound from the environment. Proper monitoring and maintenance protocols are implemented to ensure the integrity of the containment system over extended periods.Expand Specific Solutions
Key Players in CCl4 Disposal Industry
The development of effective protocols for carbon tetrachloride disposal is currently in a transitional phase, with the market showing moderate growth as environmental regulations tighten globally. The technology is approaching maturity, but there's still room for innovation. Key players like Massachusetts Institute of Technology, Trinity College Dublin, and Greenlyte Carbon Technologies GmbH are driving research and development in this field. Companies such as Toho Titanium Co., Ltd. and Solvay SA are likely focusing on industrial applications, while academic institutions are contributing to fundamental research. The market size is expanding, driven by increasing environmental concerns and stricter waste management policies, creating opportunities for both established firms and innovative startups in this sector.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed an innovative electrochemical approach for carbon tetrachloride disposal. Their method employs a specialized electrochemical cell with a porous carbon cathode and a dimensionally stable anode. When an electric current is applied in an aqueous medium, CCl4 undergoes reductive dechlorination at the cathode, progressively removing chlorine atoms and ultimately converting CCl4 to methane or ethane[7]. The process operates at ambient temperature and pressure, achieving a CCl4 removal efficiency of over 99% within 4 hours at optimized conditions[8]. MIT's system also incorporates in-situ generated hydrogen peroxide at the anode, which helps to oxidize any intermediate organic compounds, ensuring more complete mineralization.
Strengths: High removal efficiency, low temperature operation, potential for integration with renewable energy sources. Weaknesses: Requires specialized electrodes, potential for electrode fouling over time.
Greenlyte Carbon Technologies GmbH
Technical Solution: Greenlyte Carbon Technologies has developed a supercritical water oxidation (SCWO) process for carbon tetrachloride disposal. Their system operates at temperatures above 374°C and pressures exceeding 22.1 MPa, conditions at which water becomes a supercritical fluid. In this state, CCl4 becomes fully miscible with water, allowing for rapid and complete oxidation. The process achieves a destruction efficiency of 99.9999% for CCl4 within seconds[9]. Greenlyte's technology also incorporates a proprietary catalyst system that enhances oxidation rates and reduces the formation of corrosive by-products. The company has successfully scaled this technology to handle industrial quantities of CCl4, with a treatment capacity of up to 1000 kg/hour in their largest units[10].
Strengths: Extremely high destruction efficiency, rapid treatment times, ability to handle high concentrations of CCl4. Weaknesses: High capital and operating costs due to extreme conditions, potential for corrosion issues.
Innovative CCl4 Disposal Techniques
Method for dechlorinating carbon tetrachloride and method for producing dechlorinated material
PatentInactiveJP2015113335A
Innovation
- A method involving the use of an ionic liquid represented by a specific general formula to dechlorinate carbon tetrachloride at moderate temperatures and pressures, producing a dechlorinated product without the need for hazardous chemicals, and allowing for the regeneration and reuse of the ionic liquid.
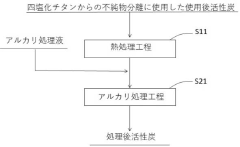
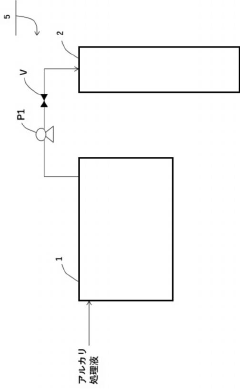
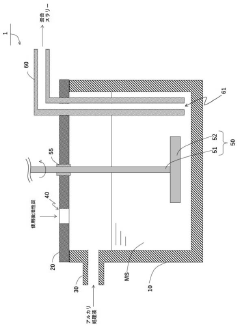
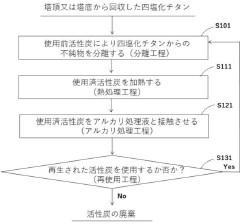
Method of treating used active carbon and method for producing titanium tetrachloride
PatentActiveJP2021031361A
Innovation
- A method involving an alkali treatment step where used activated carbon is contacted with an alkali treatment liquid, followed by a heat treatment step to vaporize residual titanium tetrachloride, using a carrier gas like argon or nitrogen, and then treating with alkali hydroxides to precipitate impurities as solids.
Environmental Impact Assessment
The environmental impact assessment of carbon tetrachloride disposal protocols is a critical component in developing effective and sustainable waste management strategies. Carbon tetrachloride, a potent ozone-depleting substance and potential carcinogen, poses significant risks to both human health and the environment if not properly handled and disposed of.
When assessing the environmental impact of carbon tetrachloride disposal, it is essential to consider multiple factors across various ecosystems. Atmospheric release of carbon tetrachloride can contribute to ozone depletion, potentially increasing harmful ultraviolet radiation reaching the Earth's surface. This can have far-reaching consequences for terrestrial and aquatic ecosystems, affecting plant growth, animal populations, and overall biodiversity.
Soil contamination is another crucial aspect to evaluate. Improper disposal methods may lead to carbon tetrachloride leaching into the soil, potentially contaminating groundwater sources and affecting soil microorganisms. This can disrupt local ecosystems and pose long-term risks to agricultural productivity and human health through contaminated food and water supplies.
Aquatic ecosystems are particularly vulnerable to carbon tetrachloride contamination. The compound's persistence in water bodies can lead to bioaccumulation in aquatic organisms, potentially causing toxicity throughout the food chain. This can result in reduced species diversity, altered ecosystem functions, and potential human health risks through consumption of contaminated fish and shellfish.
The assessment should also consider the potential for long-range transport of carbon tetrachloride through air and water currents. This aspect is crucial in understanding the global impact of local disposal practices and developing international protocols for managing this persistent organic pollutant.
Evaluating the carbon footprint associated with different disposal methods is another important consideration. Incineration, a common disposal technique for hazardous chemicals, may contribute to greenhouse gas emissions if not properly controlled. Comparing the environmental impacts of various disposal methods, including chemical treatment, incineration, and advanced oxidation processes, is essential in determining the most environmentally friendly approach.
Furthermore, the assessment should include an analysis of potential accidents or spills during the disposal process. This involves evaluating the risks associated with transportation, storage, and handling of carbon tetrachloride, as well as the potential environmental consequences of accidental releases.
Lastly, the environmental impact assessment should consider the long-term monitoring requirements for disposal sites. This includes evaluating the need for ongoing soil and groundwater testing, air quality monitoring, and ecological surveys to ensure the continued effectiveness of the disposal protocols and to detect any unforeseen environmental impacts that may emerge over time.
When assessing the environmental impact of carbon tetrachloride disposal, it is essential to consider multiple factors across various ecosystems. Atmospheric release of carbon tetrachloride can contribute to ozone depletion, potentially increasing harmful ultraviolet radiation reaching the Earth's surface. This can have far-reaching consequences for terrestrial and aquatic ecosystems, affecting plant growth, animal populations, and overall biodiversity.
Soil contamination is another crucial aspect to evaluate. Improper disposal methods may lead to carbon tetrachloride leaching into the soil, potentially contaminating groundwater sources and affecting soil microorganisms. This can disrupt local ecosystems and pose long-term risks to agricultural productivity and human health through contaminated food and water supplies.
Aquatic ecosystems are particularly vulnerable to carbon tetrachloride contamination. The compound's persistence in water bodies can lead to bioaccumulation in aquatic organisms, potentially causing toxicity throughout the food chain. This can result in reduced species diversity, altered ecosystem functions, and potential human health risks through consumption of contaminated fish and shellfish.
The assessment should also consider the potential for long-range transport of carbon tetrachloride through air and water currents. This aspect is crucial in understanding the global impact of local disposal practices and developing international protocols for managing this persistent organic pollutant.
Evaluating the carbon footprint associated with different disposal methods is another important consideration. Incineration, a common disposal technique for hazardous chemicals, may contribute to greenhouse gas emissions if not properly controlled. Comparing the environmental impacts of various disposal methods, including chemical treatment, incineration, and advanced oxidation processes, is essential in determining the most environmentally friendly approach.
Furthermore, the assessment should include an analysis of potential accidents or spills during the disposal process. This involves evaluating the risks associated with transportation, storage, and handling of carbon tetrachloride, as well as the potential environmental consequences of accidental releases.
Lastly, the environmental impact assessment should consider the long-term monitoring requirements for disposal sites. This includes evaluating the need for ongoing soil and groundwater testing, air quality monitoring, and ecological surveys to ensure the continued effectiveness of the disposal protocols and to detect any unforeseen environmental impacts that may emerge over time.
Regulatory Framework for CCl4 Disposal
The regulatory framework for Carbon Tetrachloride (CCl4) disposal is a complex and multifaceted system designed to ensure the safe and environmentally responsible handling of this hazardous substance. At the international level, the Montreal Protocol on Substances that Deplete the Ozone Layer plays a crucial role in regulating CCl4 production and consumption. This treaty, ratified by 197 countries, has been instrumental in phasing out the use of CCl4 and other ozone-depleting substances.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing CCl4 disposal. The Resource Conservation and Recovery Act (RCRA) provides the legal framework for the management of hazardous waste, including CCl4. Under RCRA, CCl4 is classified as a listed hazardous waste (U211) and must be handled, stored, and disposed of according to strict guidelines.
The Toxic Substances Control Act (TSCA) also plays a significant role in regulating CCl4. This act gives the EPA authority to require reporting, record-keeping, and testing requirements, as well as restrictions relating to chemical substances and mixtures, including CCl4. The Clean Air Act further regulates CCl4 emissions and sets standards for air quality.
At the state level, many jurisdictions have implemented their own regulations that complement or exceed federal standards. These state-specific rules often address local environmental concerns and may impose additional requirements for CCl4 disposal. Facilities handling CCl4 must comply with both federal and state regulations, which can vary significantly across different regions.
Internationally, the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal governs the international transport and disposal of hazardous wastes, including CCl4. This convention aims to reduce the movement of hazardous waste between nations, particularly from developed to less developed countries.
The regulatory landscape for CCl4 disposal is continually evolving, with ongoing efforts to strengthen environmental protections and improve disposal methods. Recent trends include increased focus on sustainable disposal techniques, stricter reporting requirements, and enhanced monitoring of environmental impacts. As scientific understanding of CCl4's environmental effects grows, regulations are likely to become more stringent, necessitating continuous adaptation by industries involved in CCl4 handling and disposal.
Compliance with these regulations requires a comprehensive understanding of the legal framework and ongoing vigilance to stay abreast of regulatory changes. Organizations dealing with CCl4 must implement robust management systems, maintain detailed records, and regularly train personnel to ensure adherence to all applicable laws and regulations. The complexity of the regulatory environment underscores the need for specialized expertise in hazardous waste management and environmental compliance.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing CCl4 disposal. The Resource Conservation and Recovery Act (RCRA) provides the legal framework for the management of hazardous waste, including CCl4. Under RCRA, CCl4 is classified as a listed hazardous waste (U211) and must be handled, stored, and disposed of according to strict guidelines.
The Toxic Substances Control Act (TSCA) also plays a significant role in regulating CCl4. This act gives the EPA authority to require reporting, record-keeping, and testing requirements, as well as restrictions relating to chemical substances and mixtures, including CCl4. The Clean Air Act further regulates CCl4 emissions and sets standards for air quality.
At the state level, many jurisdictions have implemented their own regulations that complement or exceed federal standards. These state-specific rules often address local environmental concerns and may impose additional requirements for CCl4 disposal. Facilities handling CCl4 must comply with both federal and state regulations, which can vary significantly across different regions.
Internationally, the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal governs the international transport and disposal of hazardous wastes, including CCl4. This convention aims to reduce the movement of hazardous waste between nations, particularly from developed to less developed countries.
The regulatory landscape for CCl4 disposal is continually evolving, with ongoing efforts to strengthen environmental protections and improve disposal methods. Recent trends include increased focus on sustainable disposal techniques, stricter reporting requirements, and enhanced monitoring of environmental impacts. As scientific understanding of CCl4's environmental effects grows, regulations are likely to become more stringent, necessitating continuous adaptation by industries involved in CCl4 handling and disposal.
Compliance with these regulations requires a comprehensive understanding of the legal framework and ongoing vigilance to stay abreast of regulatory changes. Organizations dealing with CCl4 must implement robust management systems, maintain detailed records, and regularly train personnel to ensure adherence to all applicable laws and regulations. The complexity of the regulatory environment underscores the need for specialized expertise in hazardous waste management and environmental compliance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!