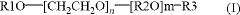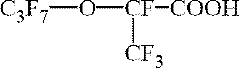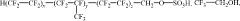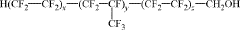How to Discover New Catalytic Solutions with Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Catalysis Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), has emerged as a powerful catalyst in organic synthesis and industrial processes. Its exceptional acidity, surpassing that of 100% sulfuric acid by several orders of magnitude, has sparked significant interest in the scientific community for its potential to revolutionize catalytic reactions.
The development of fluoroantimonic acid catalysis can be traced back to the mid-20th century when researchers began exploring superacids for various chemical transformations. Over the decades, its applications have expanded from laboratory-scale reactions to industrial processes, particularly in the petrochemical industry for hydrocarbon isomerization and alkylation reactions.
The primary objective in the field of fluoroantimonic acid catalysis is to harness its extreme acidity to facilitate challenging chemical transformations that are otherwise difficult or impossible with conventional acid catalysts. Researchers aim to develop novel catalytic systems that can activate inert substrates, promote selective bond cleavage, and enable the synthesis of complex organic molecules with high efficiency and selectivity.
One of the key technological trends in this area is the development of supported fluoroantimonic acid catalysts. These systems aim to combine the superacidity of fluoroantimonic acid with the advantages of heterogeneous catalysis, such as ease of separation and recyclability. This approach addresses some of the challenges associated with handling and containing the highly corrosive liquid superacid.
Another significant trend is the exploration of fluoroantimonic acid in combination with other catalytic species, such as transition metals or enzymes, to create hybrid catalytic systems. These synergistic approaches seek to expand the scope of reactions that can be catalyzed and improve overall reaction efficiency and selectivity.
The quest for new catalytic solutions with fluoroantimonic acid is driven by the increasing demand for more efficient and sustainable chemical processes in various industries. Researchers are focusing on developing catalytic systems that can operate under milder conditions, reduce waste generation, and improve atom economy in chemical transformations.
As we look towards the future, the field of fluoroantimonic acid catalysis is poised for significant advancements. The integration of computational modeling and high-throughput experimentation is expected to accelerate the discovery of novel catalytic solutions. Additionally, the development of new materials capable of withstanding the extreme acidity of fluoroantimonic acid will likely open up new possibilities for its application in catalysis.
The development of fluoroantimonic acid catalysis can be traced back to the mid-20th century when researchers began exploring superacids for various chemical transformations. Over the decades, its applications have expanded from laboratory-scale reactions to industrial processes, particularly in the petrochemical industry for hydrocarbon isomerization and alkylation reactions.
The primary objective in the field of fluoroantimonic acid catalysis is to harness its extreme acidity to facilitate challenging chemical transformations that are otherwise difficult or impossible with conventional acid catalysts. Researchers aim to develop novel catalytic systems that can activate inert substrates, promote selective bond cleavage, and enable the synthesis of complex organic molecules with high efficiency and selectivity.
One of the key technological trends in this area is the development of supported fluoroantimonic acid catalysts. These systems aim to combine the superacidity of fluoroantimonic acid with the advantages of heterogeneous catalysis, such as ease of separation and recyclability. This approach addresses some of the challenges associated with handling and containing the highly corrosive liquid superacid.
Another significant trend is the exploration of fluoroantimonic acid in combination with other catalytic species, such as transition metals or enzymes, to create hybrid catalytic systems. These synergistic approaches seek to expand the scope of reactions that can be catalyzed and improve overall reaction efficiency and selectivity.
The quest for new catalytic solutions with fluoroantimonic acid is driven by the increasing demand for more efficient and sustainable chemical processes in various industries. Researchers are focusing on developing catalytic systems that can operate under milder conditions, reduce waste generation, and improve atom economy in chemical transformations.
As we look towards the future, the field of fluoroantimonic acid catalysis is poised for significant advancements. The integration of computational modeling and high-throughput experimentation is expected to accelerate the discovery of novel catalytic solutions. Additionally, the development of new materials capable of withstanding the extreme acidity of fluoroantimonic acid will likely open up new possibilities for its application in catalysis.
Market Analysis for Superacid Catalysts
The market for superacid catalysts, particularly those involving fluoroantimonic acid, has shown significant growth potential in recent years. This growth is primarily driven by the increasing demand for efficient catalytic processes in various industries, including petrochemicals, pharmaceuticals, and fine chemicals. Fluoroantimonic acid, being one of the strongest known superacids, offers unique catalytic properties that make it attractive for a wide range of applications.
In the petrochemical industry, superacid catalysts are extensively used in processes such as alkylation, isomerization, and cracking. The global petrochemical market is projected to expand steadily, creating a robust demand for advanced catalytic solutions. Fluoroantimonic acid-based catalysts have demonstrated superior performance in these applications, potentially leading to increased market adoption.
The pharmaceutical sector represents another key market for superacid catalysts. As the industry continues to seek more efficient synthesis routes for complex molecules, the demand for highly active and selective catalysts is rising. Fluoroantimonic acid's exceptional acidity makes it a promising candidate for challenging organic transformations, potentially revolutionizing certain drug synthesis processes.
Fine chemical manufacturing is also a significant consumer of superacid catalysts. The industry's focus on developing high-value specialty chemicals necessitates the use of advanced catalytic systems. Fluoroantimonic acid-based catalysts offer the potential for improved yields, selectivity, and reaction rates in various fine chemical syntheses.
However, the market for fluoroantimonic acid catalysts faces certain challenges. The extreme corrosiveness and reactivity of the acid require specialized handling and equipment, which can increase operational costs. Additionally, safety and environmental concerns associated with its use may limit widespread adoption in some sectors.
Despite these challenges, the market outlook remains positive. Ongoing research and development efforts are focused on addressing the limitations of fluoroantimonic acid catalysts, such as developing more stable and easily handled formulations. These advancements are expected to expand the potential applications and market penetration of fluoroantimonic acid-based catalytic solutions.
The Asia-Pacific region is anticipated to be a major growth driver for the superacid catalyst market, fueled by rapid industrialization and increasing investments in chemical manufacturing. North America and Europe are also significant markets, with a focus on high-value applications in specialty chemicals and pharmaceuticals.
As industries continue to prioritize process efficiency and sustainability, the demand for innovative catalytic solutions is likely to grow. Fluoroantimonic acid, with its unique properties, is well-positioned to play a crucial role in meeting these evolving market needs, potentially reshaping various industrial processes and opening new avenues for chemical synthesis.
In the petrochemical industry, superacid catalysts are extensively used in processes such as alkylation, isomerization, and cracking. The global petrochemical market is projected to expand steadily, creating a robust demand for advanced catalytic solutions. Fluoroantimonic acid-based catalysts have demonstrated superior performance in these applications, potentially leading to increased market adoption.
The pharmaceutical sector represents another key market for superacid catalysts. As the industry continues to seek more efficient synthesis routes for complex molecules, the demand for highly active and selective catalysts is rising. Fluoroantimonic acid's exceptional acidity makes it a promising candidate for challenging organic transformations, potentially revolutionizing certain drug synthesis processes.
Fine chemical manufacturing is also a significant consumer of superacid catalysts. The industry's focus on developing high-value specialty chemicals necessitates the use of advanced catalytic systems. Fluoroantimonic acid-based catalysts offer the potential for improved yields, selectivity, and reaction rates in various fine chemical syntheses.
However, the market for fluoroantimonic acid catalysts faces certain challenges. The extreme corrosiveness and reactivity of the acid require specialized handling and equipment, which can increase operational costs. Additionally, safety and environmental concerns associated with its use may limit widespread adoption in some sectors.
Despite these challenges, the market outlook remains positive. Ongoing research and development efforts are focused on addressing the limitations of fluoroantimonic acid catalysts, such as developing more stable and easily handled formulations. These advancements are expected to expand the potential applications and market penetration of fluoroantimonic acid-based catalytic solutions.
The Asia-Pacific region is anticipated to be a major growth driver for the superacid catalyst market, fueled by rapid industrialization and increasing investments in chemical manufacturing. North America and Europe are also significant markets, with a focus on high-value applications in specialty chemicals and pharmaceuticals.
As industries continue to prioritize process efficiency and sustainability, the demand for innovative catalytic solutions is likely to grow. Fluoroantimonic acid, with its unique properties, is well-positioned to play a crucial role in meeting these evolving market needs, potentially reshaping various industrial processes and opening new avenues for chemical synthesis.
Current Challenges in Fluoroantimonic Acid Applications
Fluoroantimonic acid, known as the world's strongest superacid, presents significant challenges in its applications for discovering new catalytic solutions. One of the primary obstacles is its extreme corrosiveness, which limits the materials that can be used in its handling and storage. This property makes it difficult to conduct experiments and develop new catalytic processes safely and efficiently.
The high reactivity of fluoroantimonic acid also poses challenges in controlling and directing its catalytic activity. While this reactivity is beneficial for certain reactions, it can lead to unwanted side reactions or rapid degradation of substrates, making it challenging to achieve selective and targeted catalysis.
Another significant challenge is the environmental and safety concerns associated with fluoroantimonic acid. Its highly toxic and hazardous nature requires stringent safety protocols and specialized equipment, which can hinder widespread research and industrial applications. This also raises concerns about the scalability of processes involving fluoroantimonic acid, as larger-scale operations would require even more robust safety measures.
The stability of fluoroantimonic acid under various reaction conditions is another area of concern. Its sensitivity to moisture and tendency to decompose in the presence of many organic compounds limit the range of potential applications and complicate the design of catalytic systems.
Furthermore, the mechanism of catalysis involving fluoroantimonic acid is not fully understood in many cases. This lack of fundamental knowledge makes it challenging to predict and optimize catalytic performance, hindering the rational design of new catalytic solutions.
The cost and availability of fluoroantimonic acid also present obstacles to its widespread use in catalytic research. Its production requires specialized facilities and precursor materials, making it expensive and sometimes difficult to obtain in the quantities needed for extensive research programs.
Lastly, the compatibility of fluoroantimonic acid with other catalytic components and support materials is limited. This restricts the development of hybrid catalytic systems or supported catalysts, which are often crucial for enhancing catalytic efficiency and selectivity.
Addressing these challenges requires interdisciplinary approaches, combining expertise in materials science, chemical engineering, and safety protocols. Innovations in containment materials, reaction engineering, and in-situ characterization techniques are needed to unlock the full potential of fluoroantimonic acid in discovering new catalytic solutions.
The high reactivity of fluoroantimonic acid also poses challenges in controlling and directing its catalytic activity. While this reactivity is beneficial for certain reactions, it can lead to unwanted side reactions or rapid degradation of substrates, making it challenging to achieve selective and targeted catalysis.
Another significant challenge is the environmental and safety concerns associated with fluoroantimonic acid. Its highly toxic and hazardous nature requires stringent safety protocols and specialized equipment, which can hinder widespread research and industrial applications. This also raises concerns about the scalability of processes involving fluoroantimonic acid, as larger-scale operations would require even more robust safety measures.
The stability of fluoroantimonic acid under various reaction conditions is another area of concern. Its sensitivity to moisture and tendency to decompose in the presence of many organic compounds limit the range of potential applications and complicate the design of catalytic systems.
Furthermore, the mechanism of catalysis involving fluoroantimonic acid is not fully understood in many cases. This lack of fundamental knowledge makes it challenging to predict and optimize catalytic performance, hindering the rational design of new catalytic solutions.
The cost and availability of fluoroantimonic acid also present obstacles to its widespread use in catalytic research. Its production requires specialized facilities and precursor materials, making it expensive and sometimes difficult to obtain in the quantities needed for extensive research programs.
Lastly, the compatibility of fluoroantimonic acid with other catalytic components and support materials is limited. This restricts the development of hybrid catalytic systems or supported catalysts, which are often crucial for enhancing catalytic efficiency and selectivity.
Addressing these challenges requires interdisciplinary approaches, combining expertise in materials science, chemical engineering, and safety protocols. Innovations in containment materials, reaction engineering, and in-situ characterization techniques are needed to unlock the full potential of fluoroantimonic acid in discovering new catalytic solutions.
Existing Fluoroantimonic Acid Catalytic Solutions
01 Synthesis and applications of fluoroantimonic acid
Fluoroantimonic acid is synthesized and used as a super acid catalyst in various chemical reactions. It is particularly effective in hydrocarbon processing, such as isomerization and alkylation reactions. The acid's extreme acidity makes it useful for catalyzing reactions that are difficult to achieve with conventional acids.- Synthesis and applications of fluoroantimonic acid: Fluoroantimonic acid is synthesized and used as a super acid catalyst in various chemical reactions. It is particularly effective in hydrocarbon processing, such as isomerization and alkylation reactions. The acid's extreme acidity makes it useful for catalyzing reactions that are difficult with conventional acids.
- Fluoroantimonic acid in polymer production: Fluoroantimonic acid catalytic solutions are employed in polymer synthesis and modification. They can initiate polymerization reactions, facilitate polymer cross-linking, and enable the production of specialized polymers with unique properties. The acid's strong catalytic activity allows for efficient polymer processing under milder conditions.
- Use in electrochemical applications: Fluoroantimonic acid solutions find applications in electrochemical processes. They can be used in battery electrolytes, fuel cells, and other electrochemical devices to enhance performance. The acid's unique properties contribute to improved conductivity and electrochemical stability in these applications.
- Fluoroantimonic acid in materials processing: Catalytic solutions containing fluoroantimonic acid are utilized in various materials processing techniques. These include surface treatments, etching processes, and the modification of advanced materials. The acid's strong reactivity enables efficient material transformations and surface modifications.
- Safety and handling of fluoroantimonic acid solutions: Due to the extreme reactivity of fluoroantimonic acid, specialized safety measures and handling procedures are necessary. This includes the development of containment systems, neutralization methods, and protective equipment for working with the acid. Proper storage, transportation, and disposal protocols are crucial for safe utilization of fluoroantimonic acid catalytic solutions.
02 Fluoroantimonic acid in polymer production
Fluoroantimonic acid catalytic solutions are employed in the production and modification of polymers. The acid's strong catalytic properties enable efficient polymerization reactions, cross-linking, and polymer chain modifications. This application is particularly useful in the production of high-performance plastics and specialty polymers.Expand Specific Solutions03 Use in electrochemical applications
Fluoroantimonic acid solutions find applications in electrochemical processes. They are used in the development of advanced battery technologies, fuel cells, and other electrochemical devices. The acid's unique properties contribute to improved electrode performance and electrolyte efficiency.Expand Specific Solutions04 Fluoroantimonic acid in materials processing
The catalytic solutions of fluoroantimonic acid are utilized in various materials processing applications. These include surface treatments, etching processes, and the modification of advanced materials. The acid's strong reactivity allows for precise control in nanomaterial synthesis and surface engineering.Expand Specific Solutions05 Safety and handling of fluoroantimonic acid solutions
Due to the extreme reactivity of fluoroantimonic acid, specialized safety measures and handling procedures are necessary. This includes the development of containment systems, neutralization methods, and protective equipment. Research focuses on improving the safety profile of fluoroantimonic acid catalytic solutions while maintaining their effectiveness.Expand Specific Solutions
Key Players in Superacid Catalyst Research
The development of new catalytic solutions using fluoroantimonic acid is in an early stage, with significant potential for growth. The market size is expanding as industries seek more efficient and sustainable chemical processes. Technologically, it's still evolving, with varying levels of maturity among key players. Companies like BASF SE, ExxonMobil Technology & Engineering Co., and Honeywell International Technologies Ltd. are at the forefront, leveraging their extensive R&D capabilities. Academic institutions such as Nagoya University and Yale University are contributing fundamental research. Emerging players from China, like Zhejiang Shangyu Lixing Chemical Co., Ltd and Nanjing Tech University, are also making strides, indicating a globally competitive landscape with diverse technological approaches and applications.
BASF SE
Technical Solution: BASF SE has developed a novel approach to utilizing fluoroantimonic acid as a superacid catalyst in various chemical processes. Their method involves stabilizing the highly reactive acid through the use of specially designed fluorinated organic compounds, allowing for controlled catalysis in organic synthesis. This technique enables the activation of typically unreactive C-H bonds, facilitating the production of high-value chemicals and pharmaceuticals. BASF's process also incorporates a unique recycling system that captures and reuses the acid, minimizing waste and environmental impact.
Strengths: Highly efficient catalysis, enables previously challenging reactions, environmentally conscious design. Weaknesses: Requires specialized handling due to extreme reactivity, potential safety concerns in large-scale applications.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has pioneered a fluoroantimonic acid-based catalytic system for the conversion of light hydrocarbons into more valuable products. Their technology utilizes a proprietary supported catalyst system where fluoroantimonic acid is immobilized on a porous, inert substrate. This approach allows for heterogeneous catalysis, making it easier to separate the catalyst from the reaction products. The company has also developed advanced reactor designs that can withstand the corrosive nature of the superacid while maintaining optimal reaction conditions. This technology has been particularly successful in isomerization reactions and the production of high-octane gasoline components.
Strengths: Improved catalyst handling and separation, enhanced product selectivity. Weaknesses: High initial investment costs, potential for catalyst deactivation over time.
Innovative Approaches in Fluoroantimonic Acid Catalysis
Process to reduce the concentration of fluoroorganic acidic compounds in aqueous dispersions
PatentPendingUS20230312776A1
Innovation
- A process involving the formation of a mixture with a pH value of less than 6, using a dispersion of fluoroorganic polymer particles and a protic solvent, reacting the fluoroorganic acidic compounds with an alkylamine to form a hydrophobic ionic compound, and separating this compound from the mixture into distinct phases for efficient removal.
Recovery method of acid from fluoronitric acid waste liquid
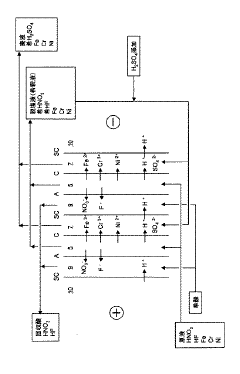
PatentActiveJP2009039672A
Innovation
- An electrodialysis process using a specific membrane configuration with anion and cation exchange membranes, along with a monovalent selective cation exchange membrane, directly treats nitric acid hydrofluoric acid waste liquid to recover high-purity acids in fewer steps by electrodialysis without neutralization or filtration, utilizing sulfuric acid as a proton source to compensate for proton loss.
Safety and Handling Protocols for Fluoroantimonic Acid
Fluoroantimonic acid is one of the strongest known superacids, with a Hammett acidity function estimated at -21.6. Due to its extreme corrosiveness and reactivity, handling this substance requires stringent safety protocols and specialized equipment. When working with fluoroantimonic acid, researchers must prioritize personal protection equipment (PPE) including chemical-resistant suits, gloves, and full-face respirators with appropriate filters.
All operations involving fluoroantimonic acid must be conducted in a properly functioning fume hood or glove box to contain hazardous vapors. The work area should be equipped with safety showers, eyewash stations, and spill containment materials specifically designed for hydrofluoric acid. Proper storage is crucial, utilizing containers made of PTFE (polytetrafluoroethylene) or other fluoropolymers that can withstand the acid's corrosive nature.
Researchers must be thoroughly trained in the proper handling techniques and emergency procedures before working with fluoroantimonic acid. This includes understanding the unique hazards associated with hydrofluoric acid, which is a component of fluoroantimonic acid. HF can cause severe, delayed burns and systemic toxicity through skin absorption.
When conducting experiments, it is essential to use only compatible materials and equipment. Glass and most metals are rapidly attacked by fluoroantimonic acid, so specialized apparatus made from fluoropolymers or noble metals like platinum must be employed. Dilution and neutralization procedures must be carefully planned and executed, as the acid reacts violently with water and many common bases.
Waste management is another critical aspect of safety protocols. All waste containing fluoroantimonic acid must be treated as hazardous and disposed of according to strict guidelines. This often involves neutralization with appropriate agents under controlled conditions before disposal through authorized channels.
Regular safety audits and equipment checks should be implemented to ensure the integrity of containment systems and PPE. Emergency response plans must be in place and regularly practiced, including procedures for acid spills, personal contamination, and facility evacuation.
Lastly, when exploring new catalytic solutions with fluoroantimonic acid, researchers must consider the scalability and industrial applicability of their safety protocols. While extreme precautions may be feasible in a laboratory setting, the development of safer handling methods or alternative superacid catalysts may be necessary for practical, large-scale applications.
All operations involving fluoroantimonic acid must be conducted in a properly functioning fume hood or glove box to contain hazardous vapors. The work area should be equipped with safety showers, eyewash stations, and spill containment materials specifically designed for hydrofluoric acid. Proper storage is crucial, utilizing containers made of PTFE (polytetrafluoroethylene) or other fluoropolymers that can withstand the acid's corrosive nature.
Researchers must be thoroughly trained in the proper handling techniques and emergency procedures before working with fluoroantimonic acid. This includes understanding the unique hazards associated with hydrofluoric acid, which is a component of fluoroantimonic acid. HF can cause severe, delayed burns and systemic toxicity through skin absorption.
When conducting experiments, it is essential to use only compatible materials and equipment. Glass and most metals are rapidly attacked by fluoroantimonic acid, so specialized apparatus made from fluoropolymers or noble metals like platinum must be employed. Dilution and neutralization procedures must be carefully planned and executed, as the acid reacts violently with water and many common bases.
Waste management is another critical aspect of safety protocols. All waste containing fluoroantimonic acid must be treated as hazardous and disposed of according to strict guidelines. This often involves neutralization with appropriate agents under controlled conditions before disposal through authorized channels.
Regular safety audits and equipment checks should be implemented to ensure the integrity of containment systems and PPE. Emergency response plans must be in place and regularly practiced, including procedures for acid spills, personal contamination, and facility evacuation.
Lastly, when exploring new catalytic solutions with fluoroantimonic acid, researchers must consider the scalability and industrial applicability of their safety protocols. While extreme precautions may be feasible in a laboratory setting, the development of safer handling methods or alternative superacid catalysts may be necessary for practical, large-scale applications.
Environmental Impact of Superacid Catalysts
The environmental impact of superacid catalysts, particularly fluoroantimonic acid, is a critical consideration in the development of new catalytic solutions. These powerful catalysts offer significant advantages in terms of reaction efficiency and selectivity, but their use also raises important environmental concerns that must be carefully addressed.
Fluoroantimonic acid, being one of the strongest known superacids, has the potential to cause severe environmental damage if not properly handled and contained. Its extreme corrosiveness can lead to the degradation of containment materials, potentially resulting in leaks and spills. Such incidents could have devastating effects on soil and water ecosystems, causing long-term contamination and disrupting local biodiversity.
The production and use of superacid catalysts often involve the handling of highly toxic and reactive precursor chemicals. The manufacturing process may generate hazardous waste streams that require specialized treatment and disposal methods. Improper management of these waste products can lead to air and water pollution, posing risks to both human health and the environment.
Furthermore, the high reactivity of superacid catalysts can contribute to the formation of secondary pollutants. In industrial applications, trace amounts of these catalysts may escape into the atmosphere, potentially leading to the formation of acid rain or other harmful atmospheric phenomena. This underscores the need for robust emission control systems and stringent safety protocols in facilities utilizing these catalysts.
The long-term environmental persistence of superacid catalysts and their byproducts is another area of concern. Unlike some conventional catalysts, superacids may not readily degrade in natural environments, leading to potential accumulation in ecosystems. This persistence can result in chronic exposure risks for wildlife and may disrupt natural chemical cycles in affected areas.
However, it is important to note that the environmental impact of superacid catalysts is not uniformly negative. Their high efficiency in catalyzing reactions can lead to reduced energy consumption and waste generation in certain industrial processes. By enabling more selective and efficient chemical transformations, these catalysts may actually contribute to greener chemistry practices when used appropriately.
To mitigate the environmental risks associated with superacid catalysts, ongoing research is focused on developing more environmentally benign alternatives and improving containment and handling technologies. Innovations in catalyst design are exploring ways to maintain high catalytic activity while reducing the overall acidity and corrosiveness of these materials. Additionally, advancements in reactor technology and process engineering are aimed at minimizing the potential for environmental release and maximizing the recyclability of these powerful catalytic agents.
Fluoroantimonic acid, being one of the strongest known superacids, has the potential to cause severe environmental damage if not properly handled and contained. Its extreme corrosiveness can lead to the degradation of containment materials, potentially resulting in leaks and spills. Such incidents could have devastating effects on soil and water ecosystems, causing long-term contamination and disrupting local biodiversity.
The production and use of superacid catalysts often involve the handling of highly toxic and reactive precursor chemicals. The manufacturing process may generate hazardous waste streams that require specialized treatment and disposal methods. Improper management of these waste products can lead to air and water pollution, posing risks to both human health and the environment.
Furthermore, the high reactivity of superacid catalysts can contribute to the formation of secondary pollutants. In industrial applications, trace amounts of these catalysts may escape into the atmosphere, potentially leading to the formation of acid rain or other harmful atmospheric phenomena. This underscores the need for robust emission control systems and stringent safety protocols in facilities utilizing these catalysts.
The long-term environmental persistence of superacid catalysts and their byproducts is another area of concern. Unlike some conventional catalysts, superacids may not readily degrade in natural environments, leading to potential accumulation in ecosystems. This persistence can result in chronic exposure risks for wildlife and may disrupt natural chemical cycles in affected areas.
However, it is important to note that the environmental impact of superacid catalysts is not uniformly negative. Their high efficiency in catalyzing reactions can lead to reduced energy consumption and waste generation in certain industrial processes. By enabling more selective and efficient chemical transformations, these catalysts may actually contribute to greener chemistry practices when used appropriately.
To mitigate the environmental risks associated with superacid catalysts, ongoing research is focused on developing more environmentally benign alternatives and improving containment and handling technologies. Innovations in catalyst design are exploring ways to maintain high catalytic activity while reducing the overall acidity and corrosiveness of these materials. Additionally, advancements in reactor technology and process engineering are aimed at minimizing the potential for environmental release and maximizing the recyclability of these powerful catalytic agents.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!