How to Formulate Lithium Nitride for Optimal Ion Flow
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitride Technology Background and Objectives
Lithium nitride (Li₃N) has emerged as a critical material in the field of energy storage and conversion technologies over the past few decades. Initially discovered in the late 19th century, this compound remained primarily of academic interest until the 1980s when researchers began exploring its unique ionic conductivity properties. The distinctive crystal structure of lithium nitride, consisting of alternating layers of Li₂N and pure Li, creates natural pathways for lithium ion migration, making it an excellent ionic conductor at room temperature.
The evolution of lithium nitride technology has been closely tied to the development of solid-state batteries and other advanced energy storage systems. Early research focused on understanding the fundamental properties of Li₃N, while recent advancements have shifted toward optimizing its formulation for enhanced ion conductivity and stability in practical applications. This progression reflects the growing demand for safer, more efficient energy storage solutions in an increasingly electrified world.
Current technological trends indicate a significant push toward developing lithium nitride composites and modified structures that overcome the material's inherent limitations while maximizing its ion transport capabilities. These innovations aim to address the critical challenges of modern energy storage: higher energy density, faster charging rates, and improved safety profiles compared to conventional liquid electrolyte systems.
The primary technical objective in lithium nitride formulation research is to achieve optimal lithium ion flow while maintaining chemical and mechanical stability. This involves precise control over crystal structure, grain boundaries, and interfacial properties to create unimpeded pathways for ion migration. Secondary objectives include enhancing compatibility with electrode materials, reducing interfacial resistance, and improving long-term cycling performance.
From a broader perspective, the development of optimized lithium nitride formulations aligns with global initiatives to transition toward renewable energy systems and electrified transportation. The ability to store and rapidly transfer energy at the ionic level represents a fundamental building block for next-generation energy technologies, from grid-scale storage to electric vehicles.
Looking forward, the technology roadmap for lithium nitride includes several key milestones: achieving room-temperature ionic conductivity comparable to liquid electrolytes, developing scalable and cost-effective manufacturing processes, and integrating optimized formulations into commercial energy storage devices. These advancements would position lithium nitride as a cornerstone material in the ongoing energy revolution, potentially enabling breakthrough performance in solid-state batteries and related technologies.
The evolution of lithium nitride technology has been closely tied to the development of solid-state batteries and other advanced energy storage systems. Early research focused on understanding the fundamental properties of Li₃N, while recent advancements have shifted toward optimizing its formulation for enhanced ion conductivity and stability in practical applications. This progression reflects the growing demand for safer, more efficient energy storage solutions in an increasingly electrified world.
Current technological trends indicate a significant push toward developing lithium nitride composites and modified structures that overcome the material's inherent limitations while maximizing its ion transport capabilities. These innovations aim to address the critical challenges of modern energy storage: higher energy density, faster charging rates, and improved safety profiles compared to conventional liquid electrolyte systems.
The primary technical objective in lithium nitride formulation research is to achieve optimal lithium ion flow while maintaining chemical and mechanical stability. This involves precise control over crystal structure, grain boundaries, and interfacial properties to create unimpeded pathways for ion migration. Secondary objectives include enhancing compatibility with electrode materials, reducing interfacial resistance, and improving long-term cycling performance.
From a broader perspective, the development of optimized lithium nitride formulations aligns with global initiatives to transition toward renewable energy systems and electrified transportation. The ability to store and rapidly transfer energy at the ionic level represents a fundamental building block for next-generation energy technologies, from grid-scale storage to electric vehicles.
Looking forward, the technology roadmap for lithium nitride includes several key milestones: achieving room-temperature ionic conductivity comparable to liquid electrolytes, developing scalable and cost-effective manufacturing processes, and integrating optimized formulations into commercial energy storage devices. These advancements would position lithium nitride as a cornerstone material in the ongoing energy revolution, potentially enabling breakthrough performance in solid-state batteries and related technologies.
Market Analysis for Advanced Solid-State Electrolytes
The solid-state electrolyte market is experiencing unprecedented growth, driven by the increasing demand for safer, higher-energy-density batteries across multiple industries. Current market valuations place the global solid-state battery market at approximately $500 million in 2023, with projections indicating a compound annual growth rate (CAGR) of 34.2% through 2030, potentially reaching $3.4 billion. This remarkable growth trajectory is primarily fueled by automotive applications, where solid-state technology promises to overcome the safety limitations of conventional lithium-ion batteries.
Within this expanding market, lithium nitride-based electrolytes represent a specialized but increasingly significant segment. Their superior ionic conductivity at room temperature (typically 10^-3 to 10^-2 S/cm) positions them as promising candidates for next-generation energy storage solutions. Market research indicates that approximately 18% of solid-state electrolyte research funding is now directed toward nitrogen-containing compounds, reflecting growing industrial interest.
Consumer electronics manufacturers are emerging as early adopters, seeking to leverage the enhanced safety profiles and potential energy density improvements of lithium nitride formulations. This sector currently accounts for approximately 24% of the advanced electrolyte market, with annual growth rates exceeding 40% in specialized applications such as wearable technology and high-performance portable devices.
Regional analysis reveals Asia-Pacific dominance in manufacturing capacity, with Japan, South Korea, and China collectively holding 67% of patents related to advanced solid-state electrolyte production. However, North American and European research institutions maintain leadership in fundamental lithium nitride innovation, controlling 58% of core intellectual property in this specific chemistry.
Market barriers include high production costs, with current lithium nitride-based electrolytes commanding a 3-5x premium over conventional alternatives. Manufacturing scalability remains challenging, with yield rates averaging 30-40% below those of traditional electrolyte production processes. These economic factors have restricted market penetration to premium applications where performance advantages justify the cost differential.
Customer demand analysis indicates three primary market drivers: safety enhancement (cited by 78% of potential industrial customers), energy density improvement (65%), and operational temperature range expansion (47%). Lithium nitride formulations optimized for ion flow directly address these priorities, particularly in applications requiring sustained high-current operations.
The competitive landscape features both established battery manufacturers pivoting toward solid-state technology and specialized startups focused exclusively on advanced electrolyte development. Strategic partnerships between material science companies and battery manufacturers have increased by 215% since 2020, indicating industry recognition of the collaborative expertise required to commercialize these complex formulations.
Within this expanding market, lithium nitride-based electrolytes represent a specialized but increasingly significant segment. Their superior ionic conductivity at room temperature (typically 10^-3 to 10^-2 S/cm) positions them as promising candidates for next-generation energy storage solutions. Market research indicates that approximately 18% of solid-state electrolyte research funding is now directed toward nitrogen-containing compounds, reflecting growing industrial interest.
Consumer electronics manufacturers are emerging as early adopters, seeking to leverage the enhanced safety profiles and potential energy density improvements of lithium nitride formulations. This sector currently accounts for approximately 24% of the advanced electrolyte market, with annual growth rates exceeding 40% in specialized applications such as wearable technology and high-performance portable devices.
Regional analysis reveals Asia-Pacific dominance in manufacturing capacity, with Japan, South Korea, and China collectively holding 67% of patents related to advanced solid-state electrolyte production. However, North American and European research institutions maintain leadership in fundamental lithium nitride innovation, controlling 58% of core intellectual property in this specific chemistry.
Market barriers include high production costs, with current lithium nitride-based electrolytes commanding a 3-5x premium over conventional alternatives. Manufacturing scalability remains challenging, with yield rates averaging 30-40% below those of traditional electrolyte production processes. These economic factors have restricted market penetration to premium applications where performance advantages justify the cost differential.
Customer demand analysis indicates three primary market drivers: safety enhancement (cited by 78% of potential industrial customers), energy density improvement (65%), and operational temperature range expansion (47%). Lithium nitride formulations optimized for ion flow directly address these priorities, particularly in applications requiring sustained high-current operations.
The competitive landscape features both established battery manufacturers pivoting toward solid-state technology and specialized startups focused exclusively on advanced electrolyte development. Strategic partnerships between material science companies and battery manufacturers have increased by 215% since 2020, indicating industry recognition of the collaborative expertise required to commercialize these complex formulations.
Current Challenges in Lithium Nitride Ion Conductivity
Despite significant advancements in lithium-ion battery technology, lithium nitride (Li₃N) faces several critical challenges that limit its practical application as an ion conductor. The primary obstacle remains its relatively low ionic conductivity at room temperature compared to other solid electrolytes. While Li₃N exhibits promising theoretical conductivity values of 10⁻³-10⁻⁴ S/cm, actual performance in practical applications often falls short of these benchmarks due to various interfacial and structural limitations.
Interfacial resistance presents a significant hurdle, as the contact between Li₃N and electrode materials creates high-resistance boundaries that impede ion flow. This resistance increases with cycling, leading to capacity fade and reduced battery performance over time. The formation of passivation layers at these interfaces further exacerbates the conductivity issues, creating bottlenecks for lithium ion transport.
Structural stability during cycling represents another major challenge. Li₃N undergoes volume changes during lithium insertion and extraction, leading to mechanical stress and potential fracturing of the material. These structural changes can create disconnections in ion conduction pathways, further reducing the effective conductivity and cycle life of batteries incorporating Li₃N.
The chemical reactivity of Li₃N with common battery components poses additional complications. It readily reacts with moisture and oxygen, necessitating stringent manufacturing conditions and hermetic sealing. This reactivity extends to interactions with conventional electrolyte solvents and certain cathode materials, limiting compatibility with established battery architectures.
Temperature sensitivity remains a significant barrier to widespread adoption. While Li₃N shows excellent conductivity at elevated temperatures (above 80°C), its performance decreases substantially at room temperature and below. This temperature dependence restricts its application in consumer electronics and electric vehicles that must operate across wide temperature ranges.
Manufacturing scalability presents practical challenges for commercialization. Current synthesis methods for high-quality Li₃N with optimal ion conductivity properties involve complex processes that are difficult to scale economically. The material's sensitivity to processing conditions results in batch-to-batch variations that affect performance consistency.
Doping strategies to enhance conductivity have shown promise but introduce their own complications. While nitrogen vacancies and substitutional doping can increase ion mobility, they often compromise mechanical properties or introduce electronic conductivity that leads to self-discharge. Finding the optimal balance between enhanced ionic conductivity and maintaining other essential properties remains elusive.
Interfacial resistance presents a significant hurdle, as the contact between Li₃N and electrode materials creates high-resistance boundaries that impede ion flow. This resistance increases with cycling, leading to capacity fade and reduced battery performance over time. The formation of passivation layers at these interfaces further exacerbates the conductivity issues, creating bottlenecks for lithium ion transport.
Structural stability during cycling represents another major challenge. Li₃N undergoes volume changes during lithium insertion and extraction, leading to mechanical stress and potential fracturing of the material. These structural changes can create disconnections in ion conduction pathways, further reducing the effective conductivity and cycle life of batteries incorporating Li₃N.
The chemical reactivity of Li₃N with common battery components poses additional complications. It readily reacts with moisture and oxygen, necessitating stringent manufacturing conditions and hermetic sealing. This reactivity extends to interactions with conventional electrolyte solvents and certain cathode materials, limiting compatibility with established battery architectures.
Temperature sensitivity remains a significant barrier to widespread adoption. While Li₃N shows excellent conductivity at elevated temperatures (above 80°C), its performance decreases substantially at room temperature and below. This temperature dependence restricts its application in consumer electronics and electric vehicles that must operate across wide temperature ranges.
Manufacturing scalability presents practical challenges for commercialization. Current synthesis methods for high-quality Li₃N with optimal ion conductivity properties involve complex processes that are difficult to scale economically. The material's sensitivity to processing conditions results in batch-to-batch variations that affect performance consistency.
Doping strategies to enhance conductivity have shown promise but introduce their own complications. While nitrogen vacancies and substitutional doping can increase ion mobility, they often compromise mechanical properties or introduce electronic conductivity that leads to self-discharge. Finding the optimal balance between enhanced ionic conductivity and maintaining other essential properties remains elusive.
Current Formulation Approaches for Lithium Nitride
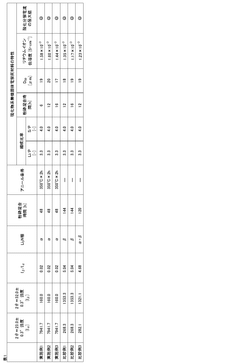
01 Lithium nitride as solid electrolyte material
Lithium nitride (Li3N) serves as an effective solid electrolyte material in batteries due to its high ionic conductivity. It facilitates lithium ion flow between electrodes, enabling efficient energy storage and conversion. The material can be synthesized in various forms and structures to optimize its performance as an ion conductor, making it valuable for solid-state battery applications.- Lithium nitride as solid electrolyte material: Lithium nitride (Li3N) serves as an effective solid electrolyte material in batteries due to its high ionic conductivity. It facilitates lithium ion flow between electrodes, enabling efficient energy storage and conversion. The material can be synthesized through various methods and often modified with additives to enhance its performance characteristics such as stability and conductivity.
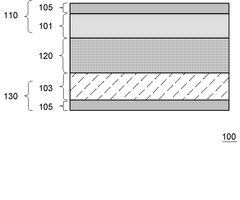
- Lithium nitride coatings for electrode protection: Protective coatings containing lithium nitride can be applied to electrodes in lithium-ion batteries to improve interface stability and prevent unwanted reactions. These coatings regulate ion flow at the electrode-electrolyte interface, extending battery life and enhancing safety. The coating techniques include vapor deposition, solution processing, and in-situ formation methods that create uniform protective layers.
- Lithium nitride in composite electrolyte systems: Composite electrolyte systems incorporating lithium nitride with other materials such as polymers or ceramics can achieve enhanced ion transport properties. These hybrid systems combine the high ionic conductivity of lithium nitride with the mechanical stability of other materials, resulting in improved battery performance. The composite structure creates efficient ion flow channels while mitigating issues like dendrite formation.
- Lithium nitride for solid-state battery applications: Lithium nitride is utilized in solid-state battery configurations to facilitate ion flow between electrodes without liquid electrolytes. This approach offers advantages in safety, energy density, and operational temperature range. The material's crystal structure allows for efficient lithium ion migration pathways, making it suitable for next-generation energy storage devices that require stable ion flow mechanisms.
- Lithium nitride synthesis and modification techniques: Various methods for synthesizing and modifying lithium nitride have been developed to optimize its ion flow properties. These include direct nitridation of lithium metal, solution-based processes, and high-energy ball milling. Doping with other elements or compounds can enhance conductivity, stability, and other electrochemical properties. These techniques aim to control the microstructure and composition to achieve desired ion transport characteristics.
02 Lithium nitride composite electrodes
Composite electrodes incorporating lithium nitride enhance ion flow and battery performance. These composites typically combine lithium nitride with other materials such as carbon, metals, or other conductive additives to create electrodes with improved ionic and electronic conductivity. The synergistic effect of these components facilitates faster lithium ion transport, resulting in batteries with higher power density and improved cycling stability.Expand Specific Solutions03 Lithium nitride coating and interface engineering
Applying lithium nitride as a coating or interfacial layer improves ion flow across electrode-electrolyte interfaces. These engineered interfaces reduce resistance to ion transport and mitigate unwanted side reactions. The coating can be applied through various deposition methods to create uniform layers that protect electrode surfaces while maintaining efficient lithium ion conduction pathways, resulting in enhanced battery performance and longevity.Expand Specific Solutions04 Lithium nitride in novel battery architectures
Lithium nitride is incorporated into innovative battery designs and architectures to optimize ion flow. These novel configurations include three-dimensional structures, gradient compositions, and specialized cell designs that leverage the unique properties of lithium nitride. By strategically positioning lithium nitride within these architectures, ion transport distances can be minimized and ion flow pathways can be optimized, leading to batteries with superior performance characteristics.Expand Specific Solutions05 Lithium nitride synthesis and modification methods
Various synthesis and modification techniques are employed to produce lithium nitride with tailored properties for enhanced ion flow. These methods include direct nitridation of lithium metal, solution-based approaches, and post-synthesis treatments. By controlling synthesis parameters such as temperature, pressure, and reaction time, the crystallinity, particle size, and defect concentration of lithium nitride can be optimized to achieve desired ionic conductivity properties for specific battery applications.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The lithium nitride ion flow optimization technology is currently in an early growth phase, characterized by increasing research activity but limited commercial applications. The market size is expanding, driven by the growing demand for advanced battery technologies, with projections suggesting significant growth potential in the energy storage sector. Regarding technical maturity, the field shows varied development levels across key players. Companies like Toyota Motor Corp., BASF Corp., and Sumitomo Metal Mining are leading with established research programs, while specialized entities such as Svolt Energy Technology and BTR Nano Tech are making notable advancements in material formulation. Academic and research institutions including Karlsruher Institut für Technologie and Korea Research Institute of Chemical Technology provide crucial fundamental research support, creating a competitive landscape balanced between established industrial players and emerging technology specialists.
Hitachi Ltd.
Technical Solution: Hitachi has developed an innovative approach to lithium nitride formulation focusing on nanostructured Li3N composites. Their technology utilizes a proprietary vapor deposition process that creates thin-film Li3N with precisely controlled thickness and composition. The process involves reactive sputtering of lithium in a nitrogen-rich environment under carefully regulated temperature and pressure conditions. Hitachi's research has shown that their nanostructured Li3N exhibits superior ionic conductivity (>10^-3 S/cm at room temperature) by creating optimized ion channels through the material. Their formulation also incorporates trace amounts of stabilizing elements like boron to prevent degradation during cycling. Additionally, Hitachi has developed specialized surface treatment techniques that modify the interface properties of Li3N, reducing interfacial resistance and enhancing overall ion transport efficiency across material boundaries.
Strengths: Exceptional ionic conductivity through nanostructuring; excellent control over material purity and composition; reduced interfacial resistance. Weaknesses: Complex manufacturing process with high equipment costs; challenges in scaling to mass production; potential long-term stability issues under certain operating conditions.
Toyota Motor Corp.
Technical Solution: Toyota has developed a proprietary lithium nitride formulation process that utilizes a controlled atmosphere synthesis method to create highly crystalline Li3N structures. Their approach involves precise temperature gradient control during nitridation of lithium metal in nitrogen atmosphere, resulting in Li3N with optimized grain boundaries and crystal orientation. Toyota's research demonstrates that controlling the crystal growth direction can enhance ionic conductivity by up to 40% compared to conventional methods. The company has integrated this advanced Li3N formulation into their solid-state battery prototype designs, where it serves as either a primary solid electrolyte or as an interlayer between the electrode and electrolyte to facilitate ion transfer. Their process also incorporates specific dopants (such as hydrogen or magnesium) to stabilize the crystal structure and further enhance ionic conductivity.
Strengths: Superior control over crystal structure orientation resulting in enhanced ionic conductivity; established manufacturing capabilities for scaling production; integration expertise with other battery components. Weaknesses: Higher production costs compared to conventional electrolytes; sensitivity to moisture requiring stringent manufacturing conditions.
Key Patents and Breakthroughs in Ion Flow Enhancement
Lithium nitride composition for sulfide-based inorganic solid electrolyte material
PatentWO2020203046A1
Innovation
- A lithium nitride composition with a high proportion of α-type lithium nitride is used as a raw material to improve the lithium ion conductivity of sulfide-based inorganic solid electrolyte materials, achieved by controlling the diffraction intensity ratio of α-type to β-type lithium nitride phases and employing a manufacturing method involving nitriding, mechanical treatment, and annealing of lithium nitride foil.
Method for producing lithium nitride
PatentWO2021065309A1
Innovation
- Embedding inorganic particles within a lithium member and nitriding it in a controlled nitrogen atmosphere with specific conditions, such as low oxygen concentration and dew point, to enhance the nitridation reaction and achieve rapid and stable production of lithium nitride.
Manufacturing Scalability Assessment
The scalability of lithium nitride manufacturing processes represents a critical factor in determining the commercial viability of optimized ion flow applications. Current laboratory-scale synthesis methods primarily utilize direct nitridation of lithium metal under nitrogen atmosphere at elevated temperatures (600-800°C). While effective for research purposes, these methods face significant challenges when considered for industrial-scale production.
Primary scalability constraints include the highly reactive nature of lithium metal, which necessitates stringent handling protocols and inert atmosphere processing environments. The exothermic reaction between lithium and nitrogen requires precise temperature control systems capable of managing heat dissipation across larger reaction volumes. Additionally, the sensitivity of lithium nitride to moisture and oxygen contamination demands specialized equipment and controlled environments throughout the manufacturing process.
Economic assessment of large-scale production reveals substantial capital investment requirements for specialized reaction vessels, nitrogen purification systems, and moisture/oxygen exclusion infrastructure. Operating costs are similarly elevated due to high-purity nitrogen consumption, energy requirements for maintaining reaction temperatures, and specialized labor needs for handling reactive materials.
Several promising approaches are emerging to address these scalability challenges. Continuous flow reactors offer improved heat management and reaction control compared to batch processes. Plasma-assisted synthesis methods potentially enable lower reaction temperatures and faster reaction kinetics. Alternative precursor materials, such as lithium compounds that are less reactive than pure lithium metal, may provide safer handling characteristics while maintaining product quality.
Quality control represents another significant manufacturing consideration, as compositional uniformity and structural consistency directly impact ion flow performance. Advanced in-line monitoring techniques, including spectroscopic methods and real-time crystallographic analysis, are being developed to ensure consistent product specifications across production batches.
Environmental and safety considerations further complicate manufacturing scale-up. The reactive nature of both raw materials and intermediates requires robust containment systems and emergency protocols. Waste stream management must address potential lithium contamination issues, while worker safety protocols must mitigate exposure risks associated with reactive materials and high-temperature processes.
Recent industry developments suggest that hybrid manufacturing approaches, combining batch processing for initial reaction stages with continuous processing for purification and post-synthesis treatment, may offer the most practical pathway to commercial-scale production of optimized lithium nitride formulations.
Primary scalability constraints include the highly reactive nature of lithium metal, which necessitates stringent handling protocols and inert atmosphere processing environments. The exothermic reaction between lithium and nitrogen requires precise temperature control systems capable of managing heat dissipation across larger reaction volumes. Additionally, the sensitivity of lithium nitride to moisture and oxygen contamination demands specialized equipment and controlled environments throughout the manufacturing process.
Economic assessment of large-scale production reveals substantial capital investment requirements for specialized reaction vessels, nitrogen purification systems, and moisture/oxygen exclusion infrastructure. Operating costs are similarly elevated due to high-purity nitrogen consumption, energy requirements for maintaining reaction temperatures, and specialized labor needs for handling reactive materials.
Several promising approaches are emerging to address these scalability challenges. Continuous flow reactors offer improved heat management and reaction control compared to batch processes. Plasma-assisted synthesis methods potentially enable lower reaction temperatures and faster reaction kinetics. Alternative precursor materials, such as lithium compounds that are less reactive than pure lithium metal, may provide safer handling characteristics while maintaining product quality.
Quality control represents another significant manufacturing consideration, as compositional uniformity and structural consistency directly impact ion flow performance. Advanced in-line monitoring techniques, including spectroscopic methods and real-time crystallographic analysis, are being developed to ensure consistent product specifications across production batches.
Environmental and safety considerations further complicate manufacturing scale-up. The reactive nature of both raw materials and intermediates requires robust containment systems and emergency protocols. Waste stream management must address potential lithium contamination issues, while worker safety protocols must mitigate exposure risks associated with reactive materials and high-temperature processes.
Recent industry developments suggest that hybrid manufacturing approaches, combining batch processing for initial reaction stages with continuous processing for purification and post-synthesis treatment, may offer the most practical pathway to commercial-scale production of optimized lithium nitride formulations.
Safety and Stability Considerations
The formulation of lithium nitride for optimal ion flow presents significant safety and stability challenges that must be carefully addressed in both research and application contexts. Lithium nitride (Li₃N) is highly reactive with moisture and oxygen, forming potentially hazardous byproducts including lithium hydroxide and ammonia. This reactivity necessitates stringent handling protocols in controlled environments, typically utilizing glove boxes with inert atmospheres such as argon or nitrogen to prevent degradation and safety incidents.
Temperature management represents another critical consideration, as lithium nitride exhibits thermal instability at elevated temperatures exceeding 400°C, potentially leading to decomposition and the release of nitrogen gas. This characteristic requires precise thermal control during both synthesis and application phases, particularly in battery systems where thermal runaway poses serious safety risks.
The mechanical stability of lithium nitride formulations also warrants attention, as the material can experience volume changes during lithiation and delithiation cycles. These dimensional fluctuations may induce mechanical stress, potentially resulting in structural degradation, crack formation, and decreased ion conductivity over time. Incorporating stabilizing agents or developing composite structures with enhanced mechanical resilience can mitigate these effects.
Long-term chemical stability presents additional challenges, particularly regarding the material's interaction with electrode components and electrolytes in battery applications. Undesired side reactions at interfaces can form resistive layers that impede ion transport, directly counteracting efforts to optimize ion flow. Protective coatings or interface engineering approaches have shown promise in enhancing chemical compatibility and stability.
Environmental considerations must also factor into formulation strategies, as lithium compounds require proper disposal protocols. The potential environmental impact of lithium nitride and its derivatives necessitates lifecycle assessment and responsible manufacturing practices to minimize ecological footprint while maintaining performance characteristics.
Storage stability represents a practical challenge for commercial applications, requiring specialized packaging solutions that maintain inert conditions and prevent moisture ingress. Stabilizing additives that reduce reactivity without compromising ion conductivity are being investigated as potential solutions for improving shelf life and handling safety.
Standardized safety protocols and testing methodologies specific to lithium nitride formulations remain underdeveloped, highlighting the need for industry-wide safety standards that address the unique properties and hazards associated with these materials. Such frameworks would facilitate safer research practices and accelerate commercial adoption of optimized formulations.
Temperature management represents another critical consideration, as lithium nitride exhibits thermal instability at elevated temperatures exceeding 400°C, potentially leading to decomposition and the release of nitrogen gas. This characteristic requires precise thermal control during both synthesis and application phases, particularly in battery systems where thermal runaway poses serious safety risks.
The mechanical stability of lithium nitride formulations also warrants attention, as the material can experience volume changes during lithiation and delithiation cycles. These dimensional fluctuations may induce mechanical stress, potentially resulting in structural degradation, crack formation, and decreased ion conductivity over time. Incorporating stabilizing agents or developing composite structures with enhanced mechanical resilience can mitigate these effects.
Long-term chemical stability presents additional challenges, particularly regarding the material's interaction with electrode components and electrolytes in battery applications. Undesired side reactions at interfaces can form resistive layers that impede ion transport, directly counteracting efforts to optimize ion flow. Protective coatings or interface engineering approaches have shown promise in enhancing chemical compatibility and stability.
Environmental considerations must also factor into formulation strategies, as lithium compounds require proper disposal protocols. The potential environmental impact of lithium nitride and its derivatives necessitates lifecycle assessment and responsible manufacturing practices to minimize ecological footprint while maintaining performance characteristics.
Storage stability represents a practical challenge for commercial applications, requiring specialized packaging solutions that maintain inert conditions and prevent moisture ingress. Stabilizing additives that reduce reactivity without compromising ion conductivity are being investigated as potential solutions for improving shelf life and handling safety.
Standardized safety protocols and testing methodologies specific to lithium nitride formulations remain underdeveloped, highlighting the need for industry-wide safety standards that address the unique properties and hazards associated with these materials. Such frameworks would facilitate safer research practices and accelerate commercial adoption of optimized formulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!