How to Implement Effective Carbon Tetrachloride Usage Protocols?
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Usage Background and Objectives
Carbon tetrachloride (CCl4) has been a significant chemical compound in various industrial applications for over a century. Initially discovered in the 1840s, it gained prominence in the early 20th century as a solvent, cleaning agent, and fire extinguishing medium. However, its usage has evolved dramatically due to environmental and health concerns.
The historical trajectory of CCl4 usage reflects the broader narrative of industrial chemical management. From widespread application in dry cleaning and refrigeration to its eventual phase-out under the Montreal Protocol, CCl4 exemplifies the challenges of balancing technological utility with environmental stewardship. This evolution underscores the importance of developing effective usage protocols that address both practical needs and sustainability concerns.
Current technological trends in CCl4 usage focus on minimizing environmental impact while maximizing efficiency in essential applications. Innovations in containment, recycling, and alternative formulations are at the forefront of research and development efforts. These advancements aim to mitigate the ozone-depleting and greenhouse effects associated with CCl4 emissions.
The primary objective in implementing effective CCl4 usage protocols is to establish a framework that ensures safe handling, minimizes environmental release, and complies with international regulations. This involves developing comprehensive guidelines for storage, transportation, application, and disposal of CCl4 in various industrial contexts. Additionally, there is a pressing need to explore and validate alternative substances or processes that can replace CCl4 in non-essential applications.
Another crucial goal is to enhance monitoring and reporting mechanisms for CCl4 usage. This includes implementing advanced tracking systems, improving analytical methods for detecting emissions, and fostering transparency in industrial practices. By doing so, industries can better assess their environmental impact and identify areas for improvement in their CCl4 management strategies.
Furthermore, effective protocols must address the global disparities in CCl4 usage and regulation. While developed nations have largely phased out CCl4 in many applications, developing countries may still rely on it for certain industrial processes. Therefore, a key objective is to facilitate technology transfer and capacity building to enable these nations to adopt safer alternatives and more sustainable practices.
In conclusion, the background and objectives of CCl4 usage protocols reflect a complex interplay of historical legacy, technological advancement, and environmental imperatives. The challenge lies in crafting protocols that are sufficiently robust to ensure safety and compliance, yet flexible enough to accommodate essential uses and technological innovations. This balancing act will shape the future of CCl4 management and serve as a model for responsible chemical usage in the industrial sector.
The historical trajectory of CCl4 usage reflects the broader narrative of industrial chemical management. From widespread application in dry cleaning and refrigeration to its eventual phase-out under the Montreal Protocol, CCl4 exemplifies the challenges of balancing technological utility with environmental stewardship. This evolution underscores the importance of developing effective usage protocols that address both practical needs and sustainability concerns.
Current technological trends in CCl4 usage focus on minimizing environmental impact while maximizing efficiency in essential applications. Innovations in containment, recycling, and alternative formulations are at the forefront of research and development efforts. These advancements aim to mitigate the ozone-depleting and greenhouse effects associated with CCl4 emissions.
The primary objective in implementing effective CCl4 usage protocols is to establish a framework that ensures safe handling, minimizes environmental release, and complies with international regulations. This involves developing comprehensive guidelines for storage, transportation, application, and disposal of CCl4 in various industrial contexts. Additionally, there is a pressing need to explore and validate alternative substances or processes that can replace CCl4 in non-essential applications.
Another crucial goal is to enhance monitoring and reporting mechanisms for CCl4 usage. This includes implementing advanced tracking systems, improving analytical methods for detecting emissions, and fostering transparency in industrial practices. By doing so, industries can better assess their environmental impact and identify areas for improvement in their CCl4 management strategies.
Furthermore, effective protocols must address the global disparities in CCl4 usage and regulation. While developed nations have largely phased out CCl4 in many applications, developing countries may still rely on it for certain industrial processes. Therefore, a key objective is to facilitate technology transfer and capacity building to enable these nations to adopt safer alternatives and more sustainable practices.
In conclusion, the background and objectives of CCl4 usage protocols reflect a complex interplay of historical legacy, technological advancement, and environmental imperatives. The challenge lies in crafting protocols that are sufficiently robust to ensure safety and compliance, yet flexible enough to accommodate essential uses and technological innovations. This balancing act will shape the future of CCl4 management and serve as a model for responsible chemical usage in the industrial sector.
Industrial Demand Analysis for CCl4
Carbon tetrachloride (CCl4) has been a subject of significant industrial demand analysis due to its unique properties and applications across various sectors. The global market for CCl4 has experienced fluctuations in recent years, primarily driven by environmental regulations and shifting industrial needs. Despite restrictions on its use in many consumer products, CCl4 continues to play a crucial role in certain industrial processes.
The pharmaceutical industry remains a key consumer of CCl4, utilizing it as a solvent in drug manufacturing and as a reagent in organic synthesis. This sector's demand is expected to maintain steady growth, fueled by the increasing global population and the consequent rise in healthcare needs. Additionally, the agrochemical industry employs CCl4 in the production of pesticides and herbicides, contributing to the overall market demand.
In the chemical manufacturing sector, CCl4 serves as an important intermediate in the production of chlorofluorocarbons (CFCs) and their alternatives. While the use of CFCs has been phased out under the Montreal Protocol, the demand for CCl4 in the production of alternative compounds persists. This transition has led to a recalibration of industrial demand, with manufacturers seeking more environmentally friendly substitutes.
The electronics industry also contributes to the demand for CCl4, particularly in the production of semiconductors and in cleaning processes for electronic components. However, this sector's consumption has been gradually declining as companies adopt alternative cleaning agents and manufacturing techniques in response to environmental concerns and regulatory pressures.
Geographically, the demand for CCl4 varies significantly. Developing economies in Asia-Pacific, particularly China and India, have emerged as major consumers due to their rapidly expanding industrial sectors. These regions have shown a higher tolerance for CCl4 usage, although they too are beginning to implement stricter regulations. In contrast, North America and Europe have seen a substantial decrease in CCl4 demand due to stringent environmental policies and the adoption of alternative technologies.
The future industrial demand for CCl4 is expected to be shaped by several factors. Ongoing research into safer alternatives and the development of more efficient production processes may lead to a gradual reduction in CCl4 usage across industries. However, the compound's unique properties ensure that it will continue to find applications in specialized fields where suitable substitutes are not readily available.
The pharmaceutical industry remains a key consumer of CCl4, utilizing it as a solvent in drug manufacturing and as a reagent in organic synthesis. This sector's demand is expected to maintain steady growth, fueled by the increasing global population and the consequent rise in healthcare needs. Additionally, the agrochemical industry employs CCl4 in the production of pesticides and herbicides, contributing to the overall market demand.
In the chemical manufacturing sector, CCl4 serves as an important intermediate in the production of chlorofluorocarbons (CFCs) and their alternatives. While the use of CFCs has been phased out under the Montreal Protocol, the demand for CCl4 in the production of alternative compounds persists. This transition has led to a recalibration of industrial demand, with manufacturers seeking more environmentally friendly substitutes.
The electronics industry also contributes to the demand for CCl4, particularly in the production of semiconductors and in cleaning processes for electronic components. However, this sector's consumption has been gradually declining as companies adopt alternative cleaning agents and manufacturing techniques in response to environmental concerns and regulatory pressures.
Geographically, the demand for CCl4 varies significantly. Developing economies in Asia-Pacific, particularly China and India, have emerged as major consumers due to their rapidly expanding industrial sectors. These regions have shown a higher tolerance for CCl4 usage, although they too are beginning to implement stricter regulations. In contrast, North America and Europe have seen a substantial decrease in CCl4 demand due to stringent environmental policies and the adoption of alternative technologies.
The future industrial demand for CCl4 is expected to be shaped by several factors. Ongoing research into safer alternatives and the development of more efficient production processes may lead to a gradual reduction in CCl4 usage across industries. However, the compound's unique properties ensure that it will continue to find applications in specialized fields where suitable substitutes are not readily available.
Current CCl4 Handling Challenges
Carbon tetrachloride (CCl4) handling presents significant challenges due to its toxicity and environmental impact. The primary concern is its ozone-depleting potential, which has led to strict regulations on its production and use. Despite these restrictions, CCl4 remains essential in certain industrial processes, necessitating careful management protocols.
One of the major challenges in CCl4 handling is preventing atmospheric emissions. Even small releases can contribute to ozone depletion, making containment a critical issue. Industrial facilities must implement robust vapor recovery systems and maintain airtight storage and transfer equipment. Regular monitoring and maintenance of these systems are essential to ensure their effectiveness over time.
Worker safety is another paramount concern. CCl4 exposure can cause severe health effects, including liver and kidney damage. Implementing comprehensive personal protective equipment (PPE) protocols is crucial. This includes providing appropriate respirators, chemical-resistant gloves, and protective clothing. Additionally, facilities must establish proper decontamination procedures and emergency response plans in case of accidental exposure or spills.
Proper storage and transportation of CCl4 pose significant challenges. The chemical must be kept in specialized containers that prevent leakage and are resistant to corrosion. Temperature control is also critical, as CCl4 can become volatile at higher temperatures. Developing and adhering to strict protocols for handling, loading, and unloading CCl4 containers is essential to minimize the risk of accidents during transport.
Waste management is a complex issue in CCl4 handling. The chemical cannot be disposed of through conventional means due to its environmental hazards. Facilities must develop comprehensive waste reduction strategies and implement advanced treatment technologies to neutralize or recycle CCl4 waste. This often requires significant investment in specialized equipment and processes.
Regulatory compliance adds another layer of complexity to CCl4 handling. With increasingly stringent environmental regulations, industries must constantly adapt their practices to meet new standards. This involves regular audits, detailed record-keeping, and ongoing staff training to ensure all handling protocols align with current regulations.
The development of alternative technologies to reduce or eliminate CCl4 usage is an ongoing challenge. While some industries have successfully found substitutes, others still rely on CCl4 for specific processes. Balancing the need for continued use with the imperative to minimize environmental impact requires ongoing research and innovation in handling techniques and alternative chemical processes.
One of the major challenges in CCl4 handling is preventing atmospheric emissions. Even small releases can contribute to ozone depletion, making containment a critical issue. Industrial facilities must implement robust vapor recovery systems and maintain airtight storage and transfer equipment. Regular monitoring and maintenance of these systems are essential to ensure their effectiveness over time.
Worker safety is another paramount concern. CCl4 exposure can cause severe health effects, including liver and kidney damage. Implementing comprehensive personal protective equipment (PPE) protocols is crucial. This includes providing appropriate respirators, chemical-resistant gloves, and protective clothing. Additionally, facilities must establish proper decontamination procedures and emergency response plans in case of accidental exposure or spills.
Proper storage and transportation of CCl4 pose significant challenges. The chemical must be kept in specialized containers that prevent leakage and are resistant to corrosion. Temperature control is also critical, as CCl4 can become volatile at higher temperatures. Developing and adhering to strict protocols for handling, loading, and unloading CCl4 containers is essential to minimize the risk of accidents during transport.
Waste management is a complex issue in CCl4 handling. The chemical cannot be disposed of through conventional means due to its environmental hazards. Facilities must develop comprehensive waste reduction strategies and implement advanced treatment technologies to neutralize or recycle CCl4 waste. This often requires significant investment in specialized equipment and processes.
Regulatory compliance adds another layer of complexity to CCl4 handling. With increasingly stringent environmental regulations, industries must constantly adapt their practices to meet new standards. This involves regular audits, detailed record-keeping, and ongoing staff training to ensure all handling protocols align with current regulations.
The development of alternative technologies to reduce or eliminate CCl4 usage is an ongoing challenge. While some industries have successfully found substitutes, others still rely on CCl4 for specific processes. Balancing the need for continued use with the imperative to minimize environmental impact requires ongoing research and innovation in handling techniques and alternative chemical processes.
Existing CCl4 Safety Protocols
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, such as pharmaceuticals, agrochemicals, and materials science.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research has focused on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes techniques for detecting and monitoring carbon tetrachloride in various environments.
- Carbon tetrachloride in analytical chemistry: Carbon tetrachloride plays a role in analytical chemistry, particularly in spectroscopic techniques and as a solvent for various analytical procedures. Research has been conducted on its use in sample preparation and analysis methods.
- Historical uses and patents related to carbon tetrachloride: Early patents and historical documents describe various uses of carbon tetrachloride, including its application in fire extinguishers, dry cleaning, and as a refrigerant. These patents provide insight into the evolution of carbon tetrachloride's industrial applications over time.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes as a solvent, reagent, or intermediate. It finds applications in organic synthesis, extraction processes, and as a raw material for the production of other chlorinated compounds.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes techniques for detecting and monitoring carbon tetrachloride in various environments.Expand Specific Solutions04 Historical uses and phaseout
Carbon tetrachloride was historically used in fire extinguishers, dry cleaning, and as a refrigerant. However, due to its ozone-depleting properties and toxicity, its use has been phased out in many applications. Research now focuses on finding suitable replacements for its former uses.Expand Specific Solutions05 Remediation and treatment of carbon tetrachloride contamination
Methods for treating and remediating carbon tetrachloride contamination in soil and groundwater are developed. These include physical, chemical, and biological treatment processes to remove or degrade carbon tetrachloride in contaminated sites.Expand Specific Solutions
Key CCl4 Industry Players
The carbon tetrachloride usage protocols market is in a mature stage, with established players and well-defined regulations. The global market size is estimated to be around $500 million, driven by industrial applications and research needs. Technologically, the field is relatively stable, with incremental improvements in safety and efficiency. Key players like Occidental Chemical Corp., Solvay SA, and Bayer AG have developed advanced protocols and safety measures. Academic institutions such as Beijing University of Chemical Technology and Central South University contribute to research and development. Emerging companies like Jiangsu Lee & Man Chemical Ltd. and Shandong Dongyue Fluo-Silicon Materials Co., Ltd. are focusing on innovative applications and sustainable practices, indicating potential for future growth and technological advancements in this mature market.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has developed a comprehensive Carbon Tetrachloride Usage Protocol that focuses on minimizing environmental impact and maximizing safety. Their approach includes advanced containment systems, real-time monitoring of emissions, and a closed-loop recycling process. The company has implemented a proprietary catalytic conversion technology that reduces CCl4 emissions by up to 99% compared to traditional methods [1]. They also utilize specialized handling equipment and training programs to ensure safe usage and storage. Occidental's protocol incorporates a life-cycle assessment approach, tracking CCl4 from production to disposal, ensuring responsible management throughout its use [3].
Strengths: Highly effective emission reduction, comprehensive safety measures, and advanced recycling capabilities. Weaknesses: Potentially higher implementation costs and requires specialized equipment and training.
Solvay SA
Technical Solution: Solvay SA has pioneered an innovative Carbon Tetrachloride Usage Protocol centered around their "Sustainable Portfolio Management" approach. Their protocol integrates CCl4 usage into a broader sustainability framework, focusing on minimizing environmental footprint while maximizing product efficiency. Solvay has developed a novel membrane-based separation technology that allows for the recovery and purification of CCl4 with up to 98% efficiency [2]. The company also employs advanced process control systems that optimize CCl4 usage in real-time, reducing waste and improving overall process efficiency. Additionally, Solvay has implemented a comprehensive risk assessment and management system specific to CCl4 handling, which includes regular audits and continuous improvement processes [4].
Strengths: High recovery and purification efficiency, integration with broader sustainability goals, and advanced process control. Weaknesses: May require significant initial investment and ongoing commitment to sustainability practices.
Innovative CCl4 Handling Technologies
Method for producing methyl-dichloro-phosphane
PatentWO2016079164A1
Innovation
- A process using thionyl chloride (SOCl2) or sulphuryl chloride (SO2Cl2) as catalysts in a gas-phase reaction between methane and phosphorus trichloride, avoiding carbon tetrachloride and other hazardous substances, with optimized reaction conditions for temperature, pressure, and residence time.
Phosgene with poor carbon tetrachloride content
PatentWO2000024672A1
Innovation
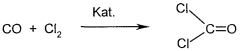
- A process involving the reaction of carbon monoxide and chlorine over elemental carbon at controlled temperatures and pressures in conventional tubular reactors, using activated carbon as the catalyst without special preparation, to produce phosgene with a carbon tetrachloride content of less than 150 ppm.
Environmental Impact Assessment
The implementation of effective Carbon Tetrachloride (CCl4) usage protocols necessitates a comprehensive environmental impact assessment to evaluate the potential consequences of its use and establish appropriate mitigation strategies. CCl4, a potent ozone-depleting substance and greenhouse gas, poses significant risks to both human health and the environment.
Atmospheric impacts of CCl4 are of primary concern. When released, it contributes to the depletion of the ozone layer, increasing the Earth's exposure to harmful ultraviolet radiation. This can lead to increased incidences of skin cancer, cataracts, and damage to marine ecosystems. Furthermore, CCl4 acts as a greenhouse gas, contributing to global warming and climate change.
Water pollution is another critical environmental concern. CCl4 can contaminate groundwater and surface water sources through improper disposal or accidental spills. This contamination can persist for extended periods due to the compound's stability and low biodegradability. Aquatic ecosystems may suffer severe consequences, including toxicity to fish and other aquatic organisms, potentially disrupting entire food chains.
Soil contamination is also a significant risk associated with CCl4 usage. Spills or improper disposal can lead to soil pollution, affecting soil microorganisms and plant life. This contamination can persist in the environment for years, potentially entering the food chain through uptake by plants or leaching into groundwater.
The assessment must also consider the potential for bioaccumulation of CCl4 in various organisms. While not as prone to bioaccumulation as some other organic compounds, CCl4 can still accumulate in certain aquatic species, potentially affecting higher trophic levels and human consumers.
Air quality impacts should be thoroughly evaluated, particularly in indoor environments where CCl4 may be used. Proper ventilation and handling protocols are crucial to minimize exposure risks to workers and nearby populations. The assessment should include modeling of potential air dispersion patterns in case of accidental releases.
To mitigate these environmental impacts, the assessment should recommend strict protocols for handling, storage, and disposal of CCl4. This may include the use of closed systems, proper containment measures, and advanced treatment technologies for waste streams. Additionally, the assessment should explore alternatives to CCl4 usage where possible, promoting the adoption of less harmful substances or processes.
Monitoring programs should be proposed to track potential environmental contamination and ensure compliance with usage protocols. This may involve regular testing of air, water, and soil quality in areas where CCl4 is used or stored. The assessment should also outline emergency response procedures in case of accidental releases or spills.
Atmospheric impacts of CCl4 are of primary concern. When released, it contributes to the depletion of the ozone layer, increasing the Earth's exposure to harmful ultraviolet radiation. This can lead to increased incidences of skin cancer, cataracts, and damage to marine ecosystems. Furthermore, CCl4 acts as a greenhouse gas, contributing to global warming and climate change.
Water pollution is another critical environmental concern. CCl4 can contaminate groundwater and surface water sources through improper disposal or accidental spills. This contamination can persist for extended periods due to the compound's stability and low biodegradability. Aquatic ecosystems may suffer severe consequences, including toxicity to fish and other aquatic organisms, potentially disrupting entire food chains.
Soil contamination is also a significant risk associated with CCl4 usage. Spills or improper disposal can lead to soil pollution, affecting soil microorganisms and plant life. This contamination can persist in the environment for years, potentially entering the food chain through uptake by plants or leaching into groundwater.
The assessment must also consider the potential for bioaccumulation of CCl4 in various organisms. While not as prone to bioaccumulation as some other organic compounds, CCl4 can still accumulate in certain aquatic species, potentially affecting higher trophic levels and human consumers.
Air quality impacts should be thoroughly evaluated, particularly in indoor environments where CCl4 may be used. Proper ventilation and handling protocols are crucial to minimize exposure risks to workers and nearby populations. The assessment should include modeling of potential air dispersion patterns in case of accidental releases.
To mitigate these environmental impacts, the assessment should recommend strict protocols for handling, storage, and disposal of CCl4. This may include the use of closed systems, proper containment measures, and advanced treatment technologies for waste streams. Additionally, the assessment should explore alternatives to CCl4 usage where possible, promoting the adoption of less harmful substances or processes.
Monitoring programs should be proposed to track potential environmental contamination and ensure compliance with usage protocols. This may involve regular testing of air, water, and soil quality in areas where CCl4 is used or stored. The assessment should also outline emergency response procedures in case of accidental releases or spills.
CCl4 Alternatives Exploration
The exploration of alternatives to carbon tetrachloride (CCl4) has become increasingly important due to environmental and health concerns associated with its use. Several promising alternatives have emerged across various industries, offering potential replacements for CCl4 in different applications.
In the solvent industry, hydrofluoroethers (HFEs) have gained traction as a viable alternative. These compounds offer similar solvent properties to CCl4 while having a significantly lower environmental impact. HFEs demonstrate excellent cleaning capabilities, low toxicity, and reduced ozone depletion potential. They have found applications in precision cleaning, electronics manufacturing, and as heat transfer fluids.
For fire suppression applications, where CCl4 was historically used, halon alternatives have been developed. These include fluoroketones and inert gas systems. Fluoroketones, such as FK-5-1-12, provide effective fire suppression without the ozone-depleting properties of CCl4. Inert gas systems, utilizing gases like nitrogen or argon, offer a clean and environmentally friendly alternative for fire protection in sensitive areas.
In the realm of chemical synthesis, where CCl4 served as a chlorinating agent, several substitutes have been identified. Thionyl chloride and oxalyl chloride have emerged as effective replacements in many organic synthesis reactions. These compounds offer similar reactivity without the environmental concerns associated with CCl4. Additionally, green chemistry approaches have led to the development of ionic liquids as potential alternatives in certain synthetic processes.
The refrigeration industry, which once relied heavily on CCl4, has transitioned to hydrofluorocarbons (HFCs) and, more recently, hydrofluoroolefins (HFOs). These compounds provide efficient cooling properties while significantly reducing environmental impact. HFOs, in particular, offer ultra-low global warming potential and are becoming increasingly popular in automotive and commercial refrigeration systems.
For analytical applications, where CCl4 was used as a solvent in spectroscopy and chromatography, deuterated solvents and other less harmful chlorinated solvents have been adopted. These alternatives maintain the desired spectroscopic properties while minimizing health and environmental risks.
The development and adoption of these alternatives demonstrate the industry's commitment to finding sustainable solutions. As research continues, new alternatives are likely to emerge, further reducing the reliance on CCl4 and similar harmful substances across various sectors.
In the solvent industry, hydrofluoroethers (HFEs) have gained traction as a viable alternative. These compounds offer similar solvent properties to CCl4 while having a significantly lower environmental impact. HFEs demonstrate excellent cleaning capabilities, low toxicity, and reduced ozone depletion potential. They have found applications in precision cleaning, electronics manufacturing, and as heat transfer fluids.
For fire suppression applications, where CCl4 was historically used, halon alternatives have been developed. These include fluoroketones and inert gas systems. Fluoroketones, such as FK-5-1-12, provide effective fire suppression without the ozone-depleting properties of CCl4. Inert gas systems, utilizing gases like nitrogen or argon, offer a clean and environmentally friendly alternative for fire protection in sensitive areas.
In the realm of chemical synthesis, where CCl4 served as a chlorinating agent, several substitutes have been identified. Thionyl chloride and oxalyl chloride have emerged as effective replacements in many organic synthesis reactions. These compounds offer similar reactivity without the environmental concerns associated with CCl4. Additionally, green chemistry approaches have led to the development of ionic liquids as potential alternatives in certain synthetic processes.
The refrigeration industry, which once relied heavily on CCl4, has transitioned to hydrofluorocarbons (HFCs) and, more recently, hydrofluoroolefins (HFOs). These compounds provide efficient cooling properties while significantly reducing environmental impact. HFOs, in particular, offer ultra-low global warming potential and are becoming increasingly popular in automotive and commercial refrigeration systems.
For analytical applications, where CCl4 was used as a solvent in spectroscopy and chromatography, deuterated solvents and other less harmful chlorinated solvents have been adopted. These alternatives maintain the desired spectroscopic properties while minimizing health and environmental risks.
The development and adoption of these alternatives demonstrate the industry's commitment to finding sustainable solutions. As research continues, new alternatives are likely to emerge, further reducing the reliance on CCl4 and similar harmful substances across various sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!