How to Improve Electrolytic Conductivity in Iron-Air Systems
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air Battery Technology Background and Objectives
Iron-air batteries represent a significant advancement in energy storage technology, emerging as a promising alternative to conventional lithium-ion batteries. The concept of iron-air batteries dates back to the 1970s, but recent technological breakthroughs have revitalized interest in this technology. These batteries operate on the principle of iron oxidation and reduction, utilizing the abundant and low-cost iron as the anode material and oxygen from ambient air as the cathode active material.
The evolution of iron-air battery technology has been marked by several key developments. Initially, these batteries suffered from poor cycle life and low energy efficiency due to hydrogen evolution and iron electrode passivation. However, advancements in electrode materials, electrolyte compositions, and cell designs have significantly improved their performance characteristics over the past decade.
Current research trends in iron-air battery technology focus on enhancing electrolytic conductivity, which is crucial for improving overall battery efficiency and power density. The conductivity of the electrolyte directly impacts the battery's internal resistance, rate capability, and energy efficiency. Researchers are exploring various approaches, including novel electrolyte formulations, additives, and electrode architectures to address this challenge.
The primary technical objectives in improving electrolytic conductivity in iron-air systems include developing electrolytes with higher ionic conductivity, reducing interfacial resistance between electrodes and electrolyte, and maintaining stable conductivity throughout the battery's operational life. Additionally, researchers aim to enhance the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) kinetics at the air electrode, which are critical for the battery's overall performance.
From a broader perspective, iron-air batteries align with the global push towards sustainable and renewable energy solutions. Their potential advantages include high theoretical energy density (approximately 1,200 Wh/kg), use of abundant and environmentally friendly materials, and significantly lower cost compared to lithium-ion batteries. These attributes position iron-air batteries as a promising technology for grid-scale energy storage applications, particularly for integrating intermittent renewable energy sources like solar and wind into the electrical grid.
The development trajectory suggests that with continued research and innovation in electrolytic conductivity enhancement, iron-air batteries could achieve commercial viability within the next decade, potentially revolutionizing the energy storage landscape and accelerating the transition to a more sustainable energy future.
The evolution of iron-air battery technology has been marked by several key developments. Initially, these batteries suffered from poor cycle life and low energy efficiency due to hydrogen evolution and iron electrode passivation. However, advancements in electrode materials, electrolyte compositions, and cell designs have significantly improved their performance characteristics over the past decade.
Current research trends in iron-air battery technology focus on enhancing electrolytic conductivity, which is crucial for improving overall battery efficiency and power density. The conductivity of the electrolyte directly impacts the battery's internal resistance, rate capability, and energy efficiency. Researchers are exploring various approaches, including novel electrolyte formulations, additives, and electrode architectures to address this challenge.
The primary technical objectives in improving electrolytic conductivity in iron-air systems include developing electrolytes with higher ionic conductivity, reducing interfacial resistance between electrodes and electrolyte, and maintaining stable conductivity throughout the battery's operational life. Additionally, researchers aim to enhance the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) kinetics at the air electrode, which are critical for the battery's overall performance.
From a broader perspective, iron-air batteries align with the global push towards sustainable and renewable energy solutions. Their potential advantages include high theoretical energy density (approximately 1,200 Wh/kg), use of abundant and environmentally friendly materials, and significantly lower cost compared to lithium-ion batteries. These attributes position iron-air batteries as a promising technology for grid-scale energy storage applications, particularly for integrating intermittent renewable energy sources like solar and wind into the electrical grid.
The development trajectory suggests that with continued research and innovation in electrolytic conductivity enhancement, iron-air batteries could achieve commercial viability within the next decade, potentially revolutionizing the energy storage landscape and accelerating the transition to a more sustainable energy future.
Market Analysis for Iron-Air Energy Storage Solutions
The iron-air energy storage market is experiencing significant growth as the global energy sector shifts towards renewable sources. This transition necessitates efficient, large-scale energy storage solutions to address the intermittency challenges of renewable energy generation. Iron-air batteries, with their promising combination of abundant materials, high energy density, and relatively low environmental impact, are positioned to capture a substantial portion of this expanding market.
Current market projections indicate that the global grid-scale energy storage market will reach approximately 150 GWh by 2025, with a compound annual growth rate exceeding 30%. Within this broader market, iron-air technology is gaining traction due to its cost advantages compared to lithium-ion alternatives. The estimated cost of iron-air storage systems ranges from $20-40 per kWh, significantly lower than lithium-ion systems which typically cost $100-200 per kWh.
The primary market segments for iron-air energy storage include utility-scale grid storage, microgrid applications, and renewable energy integration. Utility companies are particularly interested in these systems for load leveling and peak shaving applications, where the long duration storage capabilities of iron-air batteries provide distinct advantages over competing technologies.
Geographically, North America currently leads in iron-air technology adoption, with several pilot projects underway in the United States. Europe follows closely, driven by aggressive renewable energy targets and supportive regulatory frameworks. The Asia-Pacific region, particularly China and India, represents the fastest-growing market due to rapid renewable energy deployment and increasing grid stability requirements.
Key market drivers include declining renewable energy costs, increasing grid instability concerns, and supportive government policies promoting clean energy technologies. Regulatory incentives for energy storage, such as investment tax credits and capacity payments, are further accelerating market growth in several regions.
Market barriers include technological immaturity, particularly regarding electrolytic conductivity challenges, competition from established storage technologies, and limited commercial-scale demonstration projects. The technology's relatively low round-trip efficiency compared to some alternatives also presents a market adoption challenge that requires addressing through continued research and development.
Customer demand is primarily focused on systems offering 10+ hours of storage duration, scalability to multi-megawatt installations, and lifecycle costs below $0.05 per kWh-cycle. Meeting these requirements while improving electrolytic conductivity will be crucial for iron-air systems to achieve widespread commercial adoption and realize their full market potential.
Current market projections indicate that the global grid-scale energy storage market will reach approximately 150 GWh by 2025, with a compound annual growth rate exceeding 30%. Within this broader market, iron-air technology is gaining traction due to its cost advantages compared to lithium-ion alternatives. The estimated cost of iron-air storage systems ranges from $20-40 per kWh, significantly lower than lithium-ion systems which typically cost $100-200 per kWh.
The primary market segments for iron-air energy storage include utility-scale grid storage, microgrid applications, and renewable energy integration. Utility companies are particularly interested in these systems for load leveling and peak shaving applications, where the long duration storage capabilities of iron-air batteries provide distinct advantages over competing technologies.
Geographically, North America currently leads in iron-air technology adoption, with several pilot projects underway in the United States. Europe follows closely, driven by aggressive renewable energy targets and supportive regulatory frameworks. The Asia-Pacific region, particularly China and India, represents the fastest-growing market due to rapid renewable energy deployment and increasing grid stability requirements.
Key market drivers include declining renewable energy costs, increasing grid instability concerns, and supportive government policies promoting clean energy technologies. Regulatory incentives for energy storage, such as investment tax credits and capacity payments, are further accelerating market growth in several regions.
Market barriers include technological immaturity, particularly regarding electrolytic conductivity challenges, competition from established storage technologies, and limited commercial-scale demonstration projects. The technology's relatively low round-trip efficiency compared to some alternatives also presents a market adoption challenge that requires addressing through continued research and development.
Customer demand is primarily focused on systems offering 10+ hours of storage duration, scalability to multi-megawatt installations, and lifecycle costs below $0.05 per kWh-cycle. Meeting these requirements while improving electrolytic conductivity will be crucial for iron-air systems to achieve widespread commercial adoption and realize their full market potential.
Current Electrolytic Conductivity Challenges in Iron-Air Systems
Iron-air batteries represent a promising energy storage technology due to their high theoretical energy density, low cost, and environmental friendliness. However, the practical implementation of these systems faces significant challenges, particularly in terms of electrolytic conductivity. Current iron-air systems suffer from several conductivity-related issues that limit their commercial viability and performance.
The primary challenge lies in the electrolyte's ionic conductivity. Conventional aqueous electrolytes used in iron-air systems, typically potassium hydroxide (KOH) solutions, exhibit suboptimal ionic transport properties at the concentrations required for practical operation. This results in high internal resistance, voltage losses, and reduced power density. The trade-off between concentration and conductivity creates a fundamental limitation that has proven difficult to overcome.
Interface resistance presents another critical barrier. The solid-electrolyte interface (SEI) that forms between the iron electrode and the electrolyte often develops high resistance over time, impeding ion transport and contributing to capacity fade. This interface degradation accelerates during cycling, particularly at higher current densities, creating a significant obstacle for long-term stability.
Electrolyte degradation compounds these challenges. During operation, side reactions lead to electrolyte decomposition, resulting in decreased conductivity and formation of insulating byproducts. The carbonate formation from atmospheric CO2 contamination further reduces electrolyte conductivity and alters the electrode surface properties, creating additional resistance pathways.
Temperature sensitivity of the electrolyte conductivity introduces operational constraints. Iron-air systems typically show optimal conductivity within a narrow temperature range, with significant performance drops at lower temperatures due to increased electrolyte viscosity and reduced ion mobility. This temperature dependence limits the practical deployment of these systems in variable climate conditions.
The iron electrode's passivation layer formation presents yet another conductivity challenge. During discharge, iron oxidation products can form insulating layers that impede electron transfer and ion transport, increasing internal resistance. This passivation effect becomes more pronounced with cycling, contributing to capacity fade and performance degradation.
Current research approaches to address these challenges include developing advanced electrolyte formulations with additives to enhance conductivity, exploring alternative electrolyte systems beyond traditional KOH solutions, and engineering electrode structures to minimize interface resistance. Despite these efforts, a comprehensive solution that simultaneously addresses all conductivity challenges while maintaining the cost advantages of iron-air systems remains elusive, highlighting the need for continued innovation in this field.
The primary challenge lies in the electrolyte's ionic conductivity. Conventional aqueous electrolytes used in iron-air systems, typically potassium hydroxide (KOH) solutions, exhibit suboptimal ionic transport properties at the concentrations required for practical operation. This results in high internal resistance, voltage losses, and reduced power density. The trade-off between concentration and conductivity creates a fundamental limitation that has proven difficult to overcome.
Interface resistance presents another critical barrier. The solid-electrolyte interface (SEI) that forms between the iron electrode and the electrolyte often develops high resistance over time, impeding ion transport and contributing to capacity fade. This interface degradation accelerates during cycling, particularly at higher current densities, creating a significant obstacle for long-term stability.
Electrolyte degradation compounds these challenges. During operation, side reactions lead to electrolyte decomposition, resulting in decreased conductivity and formation of insulating byproducts. The carbonate formation from atmospheric CO2 contamination further reduces electrolyte conductivity and alters the electrode surface properties, creating additional resistance pathways.
Temperature sensitivity of the electrolyte conductivity introduces operational constraints. Iron-air systems typically show optimal conductivity within a narrow temperature range, with significant performance drops at lower temperatures due to increased electrolyte viscosity and reduced ion mobility. This temperature dependence limits the practical deployment of these systems in variable climate conditions.
The iron electrode's passivation layer formation presents yet another conductivity challenge. During discharge, iron oxidation products can form insulating layers that impede electron transfer and ion transport, increasing internal resistance. This passivation effect becomes more pronounced with cycling, contributing to capacity fade and performance degradation.
Current research approaches to address these challenges include developing advanced electrolyte formulations with additives to enhance conductivity, exploring alternative electrolyte systems beyond traditional KOH solutions, and engineering electrode structures to minimize interface resistance. Despite these efforts, a comprehensive solution that simultaneously addresses all conductivity challenges while maintaining the cost advantages of iron-air systems remains elusive, highlighting the need for continued innovation in this field.
Current Approaches to Enhance Electrolytic Conductivity
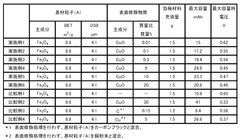
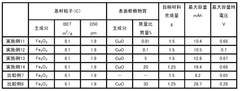
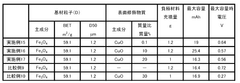
01 Electrolyte compositions for iron-air batteries
Various electrolyte compositions can be used to enhance the conductivity of iron-air battery systems. These electrolytes typically contain alkaline solutions with specific additives that improve ionic transport between the iron electrode and air electrode. The composition of these electrolytes significantly affects the overall performance, efficiency, and lifespan of iron-air batteries by facilitating better charge transfer and reducing internal resistance.- Electrolyte compositions for iron-air batteries: Various electrolyte compositions can be used to enhance the electrolytic conductivity in iron-air systems. These compositions typically include alkaline solutions with specific additives that improve ionic transport and electrochemical reactions. The electrolytes play a crucial role in facilitating the redox reactions at the iron electrode and the air electrode, thereby affecting the overall performance and efficiency of the iron-air system.
- Electrode materials and structures for improved conductivity: The design and composition of electrodes significantly impact the electrolytic conductivity of iron-air systems. Advanced electrode materials and structures can enhance electron transfer and ionic movement. Modifications to the iron electrode, such as specific surface treatments or the addition of conductive materials, can reduce internal resistance and improve overall system performance. Similarly, optimized air electrodes with catalytic properties can facilitate oxygen reduction reactions.
- Measurement and monitoring of conductivity parameters: Various techniques and devices are employed to measure and monitor the electrolytic conductivity in iron-air systems. These methods help in understanding the electrochemical behavior and optimizing the system performance. Conductivity measurements can provide insights into the state of charge, degradation mechanisms, and overall health of the iron-air system, enabling better control and management of the electrochemical processes.
- Temperature effects on iron-air system conductivity: Temperature significantly influences the electrolytic conductivity of iron-air systems. Higher temperatures generally increase ionic mobility and reaction rates, enhancing conductivity. However, excessive temperatures can lead to electrolyte degradation or unwanted side reactions. Understanding and controlling the temperature-conductivity relationship is essential for optimizing the performance and longevity of iron-air systems across various operating conditions.
- Additives and inhibitors for conductivity enhancement: Specific additives and inhibitors can be incorporated into iron-air systems to enhance electrolytic conductivity while preventing unwanted reactions. These compounds can modify the electrochemical interface, reduce passivation of the iron electrode, or improve the wettability of the air electrode. The careful selection and concentration of these additives can significantly impact the overall efficiency, power density, and cycle life of iron-air systems.
02 Electrode materials and structures for improved conductivity
The design and composition of electrodes play a crucial role in the electrolytic conductivity of iron-air systems. Advanced electrode materials, including modified iron compounds, carbon-based materials, and catalytic additives, can significantly enhance electron transfer and ionic conductivity. Structural modifications such as porosity control, surface area optimization, and layered designs contribute to improved electrochemical performance and conductivity in iron-air battery systems.Expand Specific Solutions03 Measurement and monitoring of conductivity parameters
Various techniques and devices are employed to measure and monitor the electrolytic conductivity in iron-air systems. These include impedance spectroscopy, potentiostatic methods, and specialized sensors that can provide real-time data on conductivity parameters. Accurate measurement of conductivity is essential for optimizing battery performance, diagnosing issues, and developing improved iron-air battery systems with enhanced efficiency and longevity.Expand Specific Solutions04 Temperature effects on iron-air system conductivity
Temperature has a significant impact on the electrolytic conductivity of iron-air systems. Research shows that conductivity generally increases with temperature due to enhanced ion mobility, but extreme temperatures can lead to degradation of components and reduced overall performance. Understanding and controlling temperature effects is crucial for maintaining optimal conductivity and ensuring stable operation of iron-air batteries under various environmental conditions.Expand Specific Solutions05 Additives and catalysts for conductivity enhancement
Various additives and catalysts can be incorporated into iron-air systems to enhance electrolytic conductivity. These include metal oxides, conductive polymers, and ionic compounds that facilitate electron transfer and ion transport. Catalysts at the air electrode improve oxygen reduction reaction kinetics, while specific additives in the electrolyte can prevent iron passivation and maintain high conductivity throughout the battery lifecycle, resulting in improved energy efficiency and power density.Expand Specific Solutions
Leading Companies and Research Institutions in Iron-Air Technology
The iron-air battery market is in an early growth phase, characterized by increasing R&D investments but limited commercial deployment. The global market size is projected to expand significantly as renewable energy storage demands grow, with estimates reaching several billion dollars by 2030. Technologically, electrolytic conductivity improvements remain a critical challenge, with varying maturity levels across key players. Form Energy leads with innovative long-duration iron-air battery technology, while established corporations like BASF, Panasonic, and Volkswagen are leveraging their materials expertise. Research institutions including Tianjin University and Arizona State University contribute fundamental scientific advancements, creating a competitive landscape balanced between startups, industrial conglomerates, and academic institutions all working to overcome conductivity limitations.
Form Energy, Inc.
Technical Solution: Form Energy has developed a pioneering iron-air battery technology that addresses electrolytic conductivity challenges through a novel aqueous electrolyte system. Their approach utilizes specially formulated alkaline electrolytes with proprietary additives that enhance ionic conductivity while minimizing parasitic reactions. The company's technology employs a multi-layer electrode architecture with optimized porosity to facilitate efficient ion transport throughout the iron electrode structure. Form Energy's system incorporates advanced surface modification techniques for iron particles, creating pathways for improved electron transfer at the electrode-electrolyte interface. Their batteries operate at ambient temperatures and leverage reversible rust formation (Fe ⟷ Fe2O3) chemistry, with electrolyte formulations specifically designed to maintain conductivity over thousands of charge-discharge cycles while preventing electrode passivation.
Strengths: Achieves extremely low-cost energy storage ($20/kWh) with abundant materials; electrolyte formulation enables multi-day storage capabilities with minimal self-discharge. Weaknesses: Lower power density compared to lithium-ion systems; requires careful management of iron electrode morphology changes during cycling to maintain conductivity pathways.
BASF Corp.
Technical Solution: BASF has developed advanced electrolyte formulations for iron-air systems that significantly enhance ionic conductivity through proprietary salt combinations and solvent systems. Their approach focuses on tailored electrolyte additives that form stable solid-electrolyte interphase (SEI) layers on iron electrodes, preventing passivation while maintaining high conductivity. BASF's technology incorporates specialized conductive polymers that create three-dimensional networks within the electrode structure, providing continuous pathways for electron transport. The company utilizes nano-engineered iron particles with controlled surface chemistry to optimize the electrode-electrolyte interface. Their electrolyte systems are designed to operate across wide temperature ranges (-20°C to 60°C) while maintaining stable conductivity properties. BASF has also developed advanced membrane separators with optimized pore structures that enhance ion transport while preventing iron species crossover.
Strengths: Leverages extensive chemical expertise to create highly stable electrolyte systems; formulations significantly reduce capacity fade over extended cycling. Weaknesses: Higher production costs associated with specialty additives; some formulations may have limited temperature operating windows that restrict application in extreme environments.
Key Patents and Research on Iron-Air Electrolyte Innovations
Additive for iron-air batteries
PatentPendingUS20250140990A1
Innovation
- An alkaline electrolyte with a total hydroxide concentration greater than 1 molar, containing a trivalent element such as aluminum, sulfur, and tin, is used to improve the performance of iron-air batteries.
Negative electrode material, negative electrode and iron-air battery
PatentWO2018190390A1
Innovation
- A negative electrode material is developed by attaching a specific amount of copper-based surface modifying substance to iron base particles, enhancing the discharge characteristics of iron-air batteries by improving conductivity without compromising packing density.
Material Science Advancements for Iron-Air Electrolytes
Recent advancements in material science have significantly contributed to improving the electrolytic conductivity in iron-air systems. The development of novel electrolyte materials has been a focal point for researchers aiming to enhance the performance of iron-air batteries. Traditional aqueous electrolytes containing potassium hydroxide have been modified with additives such as lithium hydroxide and sodium sulfide, resulting in improved ionic conductivity and reduced electrode passivation.
Nanostructured materials have emerged as promising candidates for electrolyte enhancement. Carbon-based nanomaterials, including graphene and carbon nanotubes, have demonstrated exceptional capabilities in facilitating electron transfer and ionic movement within the electrolyte. These materials provide increased surface area and create efficient pathways for ion transport, thereby reducing internal resistance and enhancing overall conductivity.
Polymer-based electrolytes represent another significant advancement in this field. Researchers have developed composite polymer electrolytes incorporating ionic liquids and ceramic nanoparticles, which exhibit superior conductivity compared to conventional electrolytes. These materials offer advantages such as improved mechanical stability, wider electrochemical windows, and enhanced safety profiles, addressing several limitations of traditional aqueous electrolytes.
The integration of metal-organic frameworks (MOFs) into electrolyte systems has shown remarkable potential. MOFs possess highly ordered porous structures that can be tailored to optimize ion transport while maintaining structural integrity. Studies have demonstrated that MOF-modified electrolytes can significantly enhance the conductivity in iron-air systems while simultaneously improving the stability of iron electrodes during cycling.
Surface modification techniques have been employed to optimize the interface between electrodes and electrolytes. Treatments such as plasma etching, chemical vapor deposition, and atomic layer deposition have been utilized to create specialized surface structures that promote ion transfer and reduce interfacial resistance. These modifications have proven effective in mitigating the formation of passivation layers that typically hinder conductivity in iron-air systems.
Computational modeling and simulation tools have accelerated the discovery and optimization of new electrolyte materials. Machine learning algorithms combined with density functional theory calculations have enabled researchers to predict the properties of potential electrolyte compositions before experimental validation. This approach has led to the identification of several promising material combinations that demonstrate enhanced conductivity under various operating conditions.
Nanostructured materials have emerged as promising candidates for electrolyte enhancement. Carbon-based nanomaterials, including graphene and carbon nanotubes, have demonstrated exceptional capabilities in facilitating electron transfer and ionic movement within the electrolyte. These materials provide increased surface area and create efficient pathways for ion transport, thereby reducing internal resistance and enhancing overall conductivity.
Polymer-based electrolytes represent another significant advancement in this field. Researchers have developed composite polymer electrolytes incorporating ionic liquids and ceramic nanoparticles, which exhibit superior conductivity compared to conventional electrolytes. These materials offer advantages such as improved mechanical stability, wider electrochemical windows, and enhanced safety profiles, addressing several limitations of traditional aqueous electrolytes.
The integration of metal-organic frameworks (MOFs) into electrolyte systems has shown remarkable potential. MOFs possess highly ordered porous structures that can be tailored to optimize ion transport while maintaining structural integrity. Studies have demonstrated that MOF-modified electrolytes can significantly enhance the conductivity in iron-air systems while simultaneously improving the stability of iron electrodes during cycling.
Surface modification techniques have been employed to optimize the interface between electrodes and electrolytes. Treatments such as plasma etching, chemical vapor deposition, and atomic layer deposition have been utilized to create specialized surface structures that promote ion transfer and reduce interfacial resistance. These modifications have proven effective in mitigating the formation of passivation layers that typically hinder conductivity in iron-air systems.
Computational modeling and simulation tools have accelerated the discovery and optimization of new electrolyte materials. Machine learning algorithms combined with density functional theory calculations have enabled researchers to predict the properties of potential electrolyte compositions before experimental validation. This approach has led to the identification of several promising material combinations that demonstrate enhanced conductivity under various operating conditions.
Environmental Impact and Sustainability of Iron-Air Battery Systems
Iron-air battery systems represent a promising sustainable energy storage solution with significant environmental advantages over conventional battery technologies. The environmental footprint of these systems is notably smaller than lithium-ion batteries, primarily due to the abundance and low extraction impact of iron as the main active material. Iron is the fourth most abundant element in the Earth's crust, requiring significantly less energy-intensive mining operations compared to lithium, cobalt, and other critical battery materials.
The production process of iron-air batteries generates approximately 40% lower greenhouse gas emissions compared to lithium-ion batteries of equivalent capacity. This reduction stems from simpler manufacturing processes and lower temperature requirements during production. Additionally, the water consumption associated with iron-air battery manufacturing is estimated to be 30-50% lower than conventional battery technologies, contributing to their overall environmental sustainability.
From a life-cycle perspective, iron-air systems demonstrate exceptional sustainability credentials. The materials used are non-toxic and pose minimal environmental hazards during operation. The absence of rare earth elements and conflict minerals in their composition further enhances their ethical production profile. At end-of-life, these systems offer superior recyclability, with up to 90% of components being recoverable through established metallurgical processes.
The operational phase of iron-air batteries presents additional environmental benefits. Their long cycle life—potentially exceeding 10,000 cycles under optimal conditions—reduces the frequency of replacement and associated resource consumption. When paired with renewable energy sources, these systems enable greater integration of intermittent renewables into the grid, potentially displacing fossil fuel generation and further reducing carbon emissions.
Land use considerations also favor iron-air systems, particularly for grid-scale applications. Their higher energy density compared to some alternative grid storage technologies translates to smaller physical footprints for equivalent storage capacity. This characteristic makes them particularly suitable for urban and space-constrained environments.
Challenges remain in optimizing the environmental performance of iron-air systems, particularly regarding electrolyte management and potential iron leaching in improper disposal scenarios. Improving electrolytic conductivity must be approached with sustainability considerations, avoiding solutions that might introduce toxic additives or compromise the system's recyclability. Research into bio-derived electrolyte additives and water-based electrolyte systems shows promise for further reducing environmental impact while enhancing conductivity performance.
The production process of iron-air batteries generates approximately 40% lower greenhouse gas emissions compared to lithium-ion batteries of equivalent capacity. This reduction stems from simpler manufacturing processes and lower temperature requirements during production. Additionally, the water consumption associated with iron-air battery manufacturing is estimated to be 30-50% lower than conventional battery technologies, contributing to their overall environmental sustainability.
From a life-cycle perspective, iron-air systems demonstrate exceptional sustainability credentials. The materials used are non-toxic and pose minimal environmental hazards during operation. The absence of rare earth elements and conflict minerals in their composition further enhances their ethical production profile. At end-of-life, these systems offer superior recyclability, with up to 90% of components being recoverable through established metallurgical processes.
The operational phase of iron-air batteries presents additional environmental benefits. Their long cycle life—potentially exceeding 10,000 cycles under optimal conditions—reduces the frequency of replacement and associated resource consumption. When paired with renewable energy sources, these systems enable greater integration of intermittent renewables into the grid, potentially displacing fossil fuel generation and further reducing carbon emissions.
Land use considerations also favor iron-air systems, particularly for grid-scale applications. Their higher energy density compared to some alternative grid storage technologies translates to smaller physical footprints for equivalent storage capacity. This characteristic makes them particularly suitable for urban and space-constrained environments.
Challenges remain in optimizing the environmental performance of iron-air systems, particularly regarding electrolyte management and potential iron leaching in improper disposal scenarios. Improving electrolytic conductivity must be approached with sustainability considerations, avoiding solutions that might introduce toxic additives or compromise the system's recyclability. Research into bio-derived electrolyte additives and water-based electrolyte systems shows promise for further reducing environmental impact while enhancing conductivity performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!