Quantify Redox Reaction Rates in Iron-Air Cells
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air Battery Technology Background and Objectives
Iron-air batteries represent a promising energy storage technology that has garnered significant attention in recent years due to their potential for low-cost, long-duration energy storage applications. The technology dates back to the 1970s when initial research was conducted, but it was largely abandoned due to technical limitations and the rise of lithium-ion batteries. However, the growing need for grid-scale energy storage solutions has revitalized interest in iron-air battery technology over the past decade.
The fundamental principle of iron-air batteries relies on the redox reaction between iron and oxygen. During discharge, iron is oxidized to iron oxide while oxygen from the air is reduced, generating electricity. During charging, this process is reversed. This chemistry offers theoretical energy densities of approximately 1,200 Wh/kg, significantly higher than current lithium-ion technologies, though practical implementations currently achieve much lower values.
The evolution of iron-air battery technology has been marked by several key advancements. Early versions suffered from poor cycle life and efficiency due to hydrogen evolution side reactions and electrode degradation. Recent innovations in electrode materials, electrolyte formulations, and cell architectures have addressed many of these challenges, leading to improved performance metrics and renewed commercial interest.
A critical aspect of iron-air battery development involves quantifying redox reaction rates, which directly impact power density, efficiency, and overall battery performance. Understanding these reaction kinetics is essential for optimizing electrode designs and electrolyte compositions. Current research aims to develop more precise methodologies for measuring and modeling these reaction rates under various operating conditions.
The primary technical objectives in this field include enhancing reaction kinetics to improve power density, minimizing parasitic reactions to increase efficiency, developing stable electrode structures to extend cycle life, and creating scalable manufacturing processes to reduce costs. Achieving these objectives requires sophisticated analytical techniques to quantify reaction rates and mechanisms at the electrode-electrolyte interface.
Recent advances in operando characterization methods, including synchrotron-based X-ray techniques and electrochemical impedance spectroscopy, have enabled more detailed insights into the complex redox processes occurring within iron-air cells. These techniques allow researchers to observe reaction dynamics in real-time, providing crucial data for optimizing cell performance and validating theoretical models.
The ultimate goal of current research efforts is to develop iron-air battery systems that can deliver reliable, efficient, and cost-effective energy storage at grid scale, with particular emphasis on long-duration applications (10+ hours) where lithium-ion technologies are less economically viable. Quantifying and enhancing redox reaction rates represents a fundamental step toward achieving this ambitious objective.
The fundamental principle of iron-air batteries relies on the redox reaction between iron and oxygen. During discharge, iron is oxidized to iron oxide while oxygen from the air is reduced, generating electricity. During charging, this process is reversed. This chemistry offers theoretical energy densities of approximately 1,200 Wh/kg, significantly higher than current lithium-ion technologies, though practical implementations currently achieve much lower values.
The evolution of iron-air battery technology has been marked by several key advancements. Early versions suffered from poor cycle life and efficiency due to hydrogen evolution side reactions and electrode degradation. Recent innovations in electrode materials, electrolyte formulations, and cell architectures have addressed many of these challenges, leading to improved performance metrics and renewed commercial interest.
A critical aspect of iron-air battery development involves quantifying redox reaction rates, which directly impact power density, efficiency, and overall battery performance. Understanding these reaction kinetics is essential for optimizing electrode designs and electrolyte compositions. Current research aims to develop more precise methodologies for measuring and modeling these reaction rates under various operating conditions.
The primary technical objectives in this field include enhancing reaction kinetics to improve power density, minimizing parasitic reactions to increase efficiency, developing stable electrode structures to extend cycle life, and creating scalable manufacturing processes to reduce costs. Achieving these objectives requires sophisticated analytical techniques to quantify reaction rates and mechanisms at the electrode-electrolyte interface.
Recent advances in operando characterization methods, including synchrotron-based X-ray techniques and electrochemical impedance spectroscopy, have enabled more detailed insights into the complex redox processes occurring within iron-air cells. These techniques allow researchers to observe reaction dynamics in real-time, providing crucial data for optimizing cell performance and validating theoretical models.
The ultimate goal of current research efforts is to develop iron-air battery systems that can deliver reliable, efficient, and cost-effective energy storage at grid scale, with particular emphasis on long-duration applications (10+ hours) where lithium-ion technologies are less economically viable. Quantifying and enhancing redox reaction rates represents a fundamental step toward achieving this ambitious objective.
Market Analysis for Iron-Air Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, with iron-air battery technology emerging as a promising solution for grid-scale applications. Current market projections indicate that the grid-scale energy storage market will reach approximately $15 billion by 2025, with a compound annual growth rate of 20-25%. Within this expanding landscape, iron-air technology is positioned to capture significant market share due to its cost advantages and sustainability profile.
Market demand for iron-air energy storage is primarily driven by three key factors: the increasing integration of renewable energy sources, regulatory mandates for grid stability, and the push for decarbonization across energy sectors. As renewable penetration increases, the intermittency challenges create substantial demand for long-duration energy storage solutions where iron-air cells excel compared to lithium-ion alternatives.
Geographically, North America currently represents the largest potential market for iron-air technology, with substantial investments in grid modernization and renewable integration. The European market follows closely, propelled by aggressive climate targets and energy transition policies. The Asia-Pacific region, particularly China and India, presents enormous growth potential due to rapidly expanding electricity demand and renewable capacity additions.
Customer segmentation reveals three primary market opportunities: utility-scale storage operators seeking cost-effective solutions for grid balancing; renewable energy developers requiring storage to enhance project economics; and commercial/industrial customers looking to reduce peak demand charges and increase energy resilience.
Competitive analysis shows that iron-air technology faces competition from multiple storage technologies. Lithium-ion batteries dominate the short-duration market but become cost-prohibitive for longer durations. Flow batteries offer similar duration capabilities but at higher costs. Pumped hydro and compressed air energy storage provide the lowest cost per kWh but face significant geographical constraints. Iron-air solutions occupy a strategic middle ground, offering long duration capabilities (100+ hours) at costs potentially below $20/kWh for storage capacity.
Economic modeling suggests that iron-air systems could achieve levelized cost of storage (LCOS) between $0.05-0.08/kWh for long-duration applications, significantly undercutting lithium-ion alternatives that typically range from $0.15-0.30/kWh for similar durations. This cost advantage derives primarily from the abundance and low cost of iron as the active material.
Market barriers include technology maturity concerns, performance uncertainty regarding cycle life and efficiency, and integration challenges with existing grid infrastructure. However, the compelling value proposition of iron-air technology—combining low cost, abundant materials, safety, and long duration—positions it favorably for widespread adoption as these barriers are addressed through continued research and demonstration projects.
Market demand for iron-air energy storage is primarily driven by three key factors: the increasing integration of renewable energy sources, regulatory mandates for grid stability, and the push for decarbonization across energy sectors. As renewable penetration increases, the intermittency challenges create substantial demand for long-duration energy storage solutions where iron-air cells excel compared to lithium-ion alternatives.
Geographically, North America currently represents the largest potential market for iron-air technology, with substantial investments in grid modernization and renewable integration. The European market follows closely, propelled by aggressive climate targets and energy transition policies. The Asia-Pacific region, particularly China and India, presents enormous growth potential due to rapidly expanding electricity demand and renewable capacity additions.
Customer segmentation reveals three primary market opportunities: utility-scale storage operators seeking cost-effective solutions for grid balancing; renewable energy developers requiring storage to enhance project economics; and commercial/industrial customers looking to reduce peak demand charges and increase energy resilience.
Competitive analysis shows that iron-air technology faces competition from multiple storage technologies. Lithium-ion batteries dominate the short-duration market but become cost-prohibitive for longer durations. Flow batteries offer similar duration capabilities but at higher costs. Pumped hydro and compressed air energy storage provide the lowest cost per kWh but face significant geographical constraints. Iron-air solutions occupy a strategic middle ground, offering long duration capabilities (100+ hours) at costs potentially below $20/kWh for storage capacity.
Economic modeling suggests that iron-air systems could achieve levelized cost of storage (LCOS) between $0.05-0.08/kWh for long-duration applications, significantly undercutting lithium-ion alternatives that typically range from $0.15-0.30/kWh for similar durations. This cost advantage derives primarily from the abundance and low cost of iron as the active material.
Market barriers include technology maturity concerns, performance uncertainty regarding cycle life and efficiency, and integration challenges with existing grid infrastructure. However, the compelling value proposition of iron-air technology—combining low cost, abundant materials, safety, and long duration—positions it favorably for widespread adoption as these barriers are addressed through continued research and demonstration projects.
Current Challenges in Redox Reaction Rate Quantification
Despite significant advancements in iron-air battery technology, accurately quantifying redox reaction rates remains one of the most challenging aspects of research and development in this field. The complex nature of the iron electrode's redox chemistry presents multiple measurement difficulties that impede precise characterization and optimization of these energy storage systems.
A primary challenge lies in the multi-phase nature of the reactions occurring at the iron electrode. The iron undergoes transitions between metallic iron, various iron oxides, and hydroxides during charge-discharge cycles, creating a heterogeneous reaction environment. This heterogeneity makes it difficult to isolate and measure individual reaction steps, as multiple processes occur simultaneously across different phases.
The presence of parasitic reactions, particularly hydrogen evolution during charging, further complicates rate measurements. These side reactions consume charge that would otherwise contribute to the main redox process, leading to overestimation of reaction rates if not properly accounted for. Current analytical techniques struggle to differentiate between charge consumed by the desired iron redox reactions versus these competing processes.
Mass transport limitations represent another significant obstacle. The formation of passive layers on electrode surfaces and the poor solubility of iron species in alkaline electrolytes create diffusion barriers that mask the intrinsic kinetics of the redox reactions. Researchers face difficulties in decoupling these mass transport effects from the actual reaction kinetics.
Time-dependent phenomena add another layer of complexity. Iron electrodes undergo morphological and compositional changes during cycling, altering reaction pathways and rates over time. Conventional steady-state measurement techniques fail to capture these dynamic aspects, leading to incomplete understanding of long-term performance.
Standardization issues further hinder progress in this field. Various research groups employ different electrode preparations, cell configurations, and measurement protocols, making direct comparisons between studies challenging. This lack of standardized methodologies creates inconsistencies in reported reaction rates and mechanisms.
Advanced in-situ characterization techniques, while promising, face limitations when applied to iron-air systems. The opaque nature of electrodes, high alkalinity of electrolytes, and sensitivity of iron species to ambient conditions restrict the application of many spectroscopic and microscopic techniques that could otherwise provide valuable kinetic information.
The mathematical modeling of these complex systems presents additional challenges. Current models often rely on simplifications that fail to capture the full complexity of the reaction mechanisms, leading to discrepancies between theoretical predictions and experimental observations. More sophisticated models require parameters that are difficult to measure experimentally, creating a circular problem in validation efforts.
A primary challenge lies in the multi-phase nature of the reactions occurring at the iron electrode. The iron undergoes transitions between metallic iron, various iron oxides, and hydroxides during charge-discharge cycles, creating a heterogeneous reaction environment. This heterogeneity makes it difficult to isolate and measure individual reaction steps, as multiple processes occur simultaneously across different phases.
The presence of parasitic reactions, particularly hydrogen evolution during charging, further complicates rate measurements. These side reactions consume charge that would otherwise contribute to the main redox process, leading to overestimation of reaction rates if not properly accounted for. Current analytical techniques struggle to differentiate between charge consumed by the desired iron redox reactions versus these competing processes.
Mass transport limitations represent another significant obstacle. The formation of passive layers on electrode surfaces and the poor solubility of iron species in alkaline electrolytes create diffusion barriers that mask the intrinsic kinetics of the redox reactions. Researchers face difficulties in decoupling these mass transport effects from the actual reaction kinetics.
Time-dependent phenomena add another layer of complexity. Iron electrodes undergo morphological and compositional changes during cycling, altering reaction pathways and rates over time. Conventional steady-state measurement techniques fail to capture these dynamic aspects, leading to incomplete understanding of long-term performance.
Standardization issues further hinder progress in this field. Various research groups employ different electrode preparations, cell configurations, and measurement protocols, making direct comparisons between studies challenging. This lack of standardized methodologies creates inconsistencies in reported reaction rates and mechanisms.
Advanced in-situ characterization techniques, while promising, face limitations when applied to iron-air systems. The opaque nature of electrodes, high alkalinity of electrolytes, and sensitivity of iron species to ambient conditions restrict the application of many spectroscopic and microscopic techniques that could otherwise provide valuable kinetic information.
The mathematical modeling of these complex systems presents additional challenges. Current models often rely on simplifications that fail to capture the full complexity of the reaction mechanisms, leading to discrepancies between theoretical predictions and experimental observations. More sophisticated models require parameters that are difficult to measure experimentally, creating a circular problem in validation efforts.
Existing Methods for Quantifying Redox Kinetics
01 Electrode materials and catalysts for iron-air cells
Various electrode materials and catalysts can significantly impact the redox reaction rates in iron-air cells. Advanced iron-based electrodes with specific compositions and structures can enhance electron transfer and reaction kinetics. Catalysts, particularly those containing transition metals or their oxides, can lower activation energy barriers for the redox reactions, thereby increasing reaction rates and overall cell efficiency. The morphology and surface area of these materials also play crucial roles in determining reaction rates.- Electrode materials for enhancing redox reaction rates: Various electrode materials can significantly impact the redox reaction rates in iron-air cells. Advanced iron-based electrodes with specific compositions and structures can improve electron transfer kinetics. Modifications such as doping with other metals or incorporating carbon-based materials can enhance the catalytic activity and conductivity of the electrodes, leading to faster redox reactions and improved overall cell performance.
- Electrolyte composition effects on reaction kinetics: The composition of the electrolyte plays a crucial role in determining the redox reaction rates in iron-air cells. Optimized electrolyte formulations can facilitate ion transport between electrodes and enhance the overall reaction kinetics. Additives in the electrolyte can prevent unwanted side reactions, reduce passivation of the iron electrode, and maintain stable pH conditions, all contributing to sustained high reaction rates during cell operation.
- Catalysts for accelerating oxygen reduction reactions: Specialized catalysts can be incorporated into iron-air cells to accelerate the oxygen reduction reaction, which is often a rate-limiting step. These catalysts can be based on noble metals, transition metal oxides, or novel nanomaterials that provide high catalytic activity at lower costs. The strategic placement and distribution of these catalysts within the air electrode structure can significantly enhance the redox reaction rates and overall energy efficiency of the cell.
- Temperature and pressure control for reaction optimization: The operating temperature and pressure conditions have substantial effects on the redox reaction rates in iron-air cells. Controlled temperature ranges can accelerate reaction kinetics while preventing degradation of cell components. Similarly, optimized pressure conditions, particularly for the air electrode, can enhance oxygen availability and transport, leading to improved reaction rates. Advanced thermal and pressure management systems can be integrated to maintain optimal conditions throughout cell operation.
- Cell design and architecture for improved mass transport: The physical design and architecture of iron-air cells significantly impact the redox reaction rates through their effect on mass transport processes. Innovative cell designs can minimize diffusion limitations, reduce internal resistance, and optimize the interface between electrodes and electrolyte. Features such as three-dimensional electrode structures, optimized porosity, and strategic flow field designs can enhance reactant accessibility and product removal, thereby increasing the overall reaction rates and power density of the cells.
02 Electrolyte composition effects on redox kinetics
The composition of the electrolyte significantly influences the redox reaction rates in iron-air cells. Additives and pH modifiers can enhance ion mobility and reduce side reactions. Electrolyte formulations with optimized ionic conductivity facilitate faster charge transfer at the electrode-electrolyte interface. Some electrolytes contain specific compounds that prevent iron passivation, which would otherwise slow down reaction rates. The concentration and type of supporting electrolytes also affect the stability and reversibility of the redox reactions.Expand Specific Solutions03 Temperature and pressure effects on reaction kinetics
Operating conditions such as temperature and pressure significantly impact the redox reaction rates in iron-air cells. Higher temperatures generally accelerate reaction kinetics by increasing ion mobility and reducing activation energy barriers. However, excessive temperatures may lead to unwanted side reactions or electrolyte degradation. Pressure variations, particularly of oxygen at the air electrode, affect the oxygen reduction and evolution reactions. Controlled pressure and temperature management systems can optimize reaction rates while maintaining cell stability and longevity.Expand Specific Solutions04 Cell design and architecture for improved reaction rates
The physical design and architecture of iron-air cells significantly influence redox reaction rates. Optimized electrode spacing reduces ion diffusion distances, while enhanced surface area designs increase reaction sites. Flow-through configurations can improve mass transport of reactants and products. Advanced cell designs incorporate features that manage heat generation and dissipation, maintaining optimal temperature for reaction kinetics. Some designs also include specialized components that facilitate oxygen transport to the air electrode, enhancing the overall reaction rate.Expand Specific Solutions05 Cycling protocols and charge-discharge strategies
Specific cycling protocols and charge-discharge strategies can optimize redox reaction rates in iron-air cells. Pulsed charging techniques can reduce electrode passivation and enhance reaction kinetics. Rest periods between cycles allow for reactant redistribution and prevent concentration polarization. Controlled current densities during operation prevent localized reaction hotspots that could lead to uneven reaction rates across the electrode surface. Advanced battery management systems that adapt charging parameters based on cell state can maintain optimal reaction conditions throughout the battery lifecycle.Expand Specific Solutions
Leading Research Institutions and Companies in Iron-Air Technology
The iron-air battery technology market is in an early growth phase, characterized by significant research activity but limited commercial deployment. The global market for iron-air energy storage is projected to expand rapidly as renewable energy integration demands increase, with estimates suggesting a multi-billion dollar opportunity by 2030. Academic institutions (University of Western Ontario, Northwestern University, Tsinghua University) are driving fundamental research on redox reaction kinetics, while corporate players are approaching commercialization from diverse angles. Companies like POSCO Holdings and JFE Steel bring metallurgical expertise, while Battelle Memorial Institute and Dalian Institute of Chemical Physics contribute advanced electrochemical research capabilities. The technology remains at mid-maturity, with significant improvements in reaction rate quantification and electrode design needed before widespread commercial adoption.
Northwestern University
Technical Solution: Northwestern University has developed advanced in-situ characterization techniques to quantify redox reaction rates in iron-air cells. Their approach combines operando X-ray absorption spectroscopy (XAS) and transmission electron microscopy (TEM) to monitor iron oxidation states during charge-discharge cycles in real-time. The research team has implemented a multi-scale modeling framework that correlates electrochemical impedance spectroscopy data with reaction kinetics parameters, enabling precise measurement of electron transfer rates at the iron electrode-electrolyte interface. Their methodology includes the development of specialized cell designs with reference electrodes that allow for the isolation and quantification of specific redox reactions occurring at the iron electrode. Northwestern's researchers have also pioneered the use of isotope labeling combined with mass spectrometry to track oxygen participation in the redox processes, providing unprecedented insights into reaction mechanisms and rate-limiting steps.
Strengths: Superior in-situ characterization capabilities allowing for real-time monitoring of reaction kinetics without disrupting cell operation. Their multi-technique approach provides comprehensive understanding of both surface and bulk processes. Weaknesses: The specialized equipment required for their characterization techniques limits widespread adoption, and their methods may introduce artifacts due to modified cell geometries needed for in-situ measurements.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute of Chemical Physics has established a comprehensive methodology for quantifying redox reaction rates in iron-air batteries using electrochemical analysis coupled with advanced spectroscopic techniques. Their approach employs rotating disk electrode (RDE) measurements to determine reaction kinetics parameters such as exchange current density and transfer coefficients specific to iron electrodes in alkaline environments. The institute has developed novel pulse techniques that can separate the contributions of different reaction steps in the complex iron redox process. Their researchers have created specialized protocols for Tafel analysis that account for the unique multi-step electron transfer processes in iron-air systems. Additionally, they've pioneered the application of in-situ Mössbauer spectroscopy specifically optimized for iron-air cells, allowing direct observation of iron oxidation state changes during operation. This technique provides quantitative data on reaction intermediates and their formation rates, critical for understanding the complete redox mechanism.
Strengths: Exceptional expertise in spectroelectrochemical techniques specifically tailored for iron-based systems, with particular strength in Mössbauer spectroscopy that directly probes iron species. Their methods provide detailed kinetic parameters essential for cell optimization. Weaknesses: Their approaches often require highly controlled laboratory conditions that may not translate directly to practical cell environments, and some techniques have limited temporal resolution for capturing fastest reaction steps.
Key Scientific Advances in Electrochemical Measurement
Reducing agent composition for iron oxide and/or iron hydroxide
PatentPendingEP4293137A1
Innovation
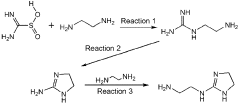
- A reducing agent composition comprising thiourea dioxide, a water-soluble metal chelating agent, and an amine compound, specifically an amine with a 1,2-diamine structure, is used to rapidly generate dithionous acid and suppress deactivation by oxygen, enhancing the dissolution rate of iron oxide and hydroxide.
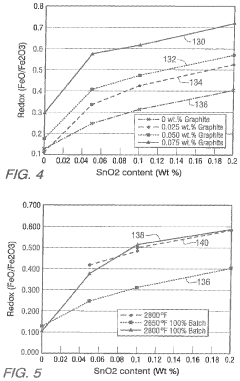
Low iron, high redox ratio, and high iron, high redox ratio, soda-lime-silica glasses and methods of making same
PatentPendingUS20240018032A1
Innovation
- A method involving a basic soda-lime-silica glass with a colorant portion containing total iron as Fe2O3 and tin or tin-containing compounds, where the glass is formed by flowing onto a molten tin bath, allowing for controlled cooling and force application to achieve the desired thickness, thereby maintaining a redox ratio of 0.2 to 0.6 regardless of the furnace type.
Environmental Impact and Sustainability Assessment
Iron-air batteries represent a promising sustainable energy storage solution due to their use of abundant, low-cost materials compared to conventional lithium-ion technologies. The environmental footprint of these systems is significantly lower, with iron being the fourth most abundant element in Earth's crust and readily available worldwide. Life cycle assessments indicate that iron-air cells can achieve up to 70% reduction in greenhouse gas emissions during manufacturing compared to lithium-based alternatives, primarily due to the elimination of rare earth elements and toxic materials in their construction.
The sustainability advantages extend beyond material composition to operational characteristics. The quantification of redox reaction rates reveals that iron-air cells maintain stable performance over thousands of cycles without significant degradation, potentially extending battery lifespans and reducing electronic waste. Furthermore, end-of-life considerations demonstrate superior recyclability, with over 90% of cell components being recoverable through established metallurgical processes, creating a nearly closed-loop material cycle.
Water consumption represents a critical environmental consideration in iron-air technology. Current designs require approximately 2-3 liters of water per kWh of storage capacity, necessitating careful implementation in water-stressed regions. However, research indicates that optimized redox reaction rates can reduce water requirements by up to 40% through improved electrolyte management systems.
Energy density calculations based on redox kinetics demonstrate that iron-air batteries can store renewable energy at approximately one-tenth the carbon intensity of natural gas peaker plants when considering full lifecycle emissions. This positions them as key enablers for grid-scale renewable integration, potentially preventing 0.5-0.7 metric tons of CO2 equivalent per MWh of deployed capacity compared to conventional storage alternatives.
Land use impact assessments show that facilities housing iron-air battery systems require 30-40% less space than comparable lithium-ion installations due to their simpler thermal management requirements and higher volumetric energy density. This spatial efficiency becomes increasingly important as energy storage deployment accelerates in densely populated regions with limited available land.
The quantification of redox reaction rates directly influences the technology's environmental profile by determining charge/discharge efficiency. Current iron-air systems operate at 50-65% round-trip efficiency, which, while lower than some alternatives, is offset by their minimal environmental impact and exceptional longevity. Research targeting catalytic improvements could potentially increase this efficiency to 70-75%, further enhancing their sustainability credentials.
The sustainability advantages extend beyond material composition to operational characteristics. The quantification of redox reaction rates reveals that iron-air cells maintain stable performance over thousands of cycles without significant degradation, potentially extending battery lifespans and reducing electronic waste. Furthermore, end-of-life considerations demonstrate superior recyclability, with over 90% of cell components being recoverable through established metallurgical processes, creating a nearly closed-loop material cycle.
Water consumption represents a critical environmental consideration in iron-air technology. Current designs require approximately 2-3 liters of water per kWh of storage capacity, necessitating careful implementation in water-stressed regions. However, research indicates that optimized redox reaction rates can reduce water requirements by up to 40% through improved electrolyte management systems.
Energy density calculations based on redox kinetics demonstrate that iron-air batteries can store renewable energy at approximately one-tenth the carbon intensity of natural gas peaker plants when considering full lifecycle emissions. This positions them as key enablers for grid-scale renewable integration, potentially preventing 0.5-0.7 metric tons of CO2 equivalent per MWh of deployed capacity compared to conventional storage alternatives.
Land use impact assessments show that facilities housing iron-air battery systems require 30-40% less space than comparable lithium-ion installations due to their simpler thermal management requirements and higher volumetric energy density. This spatial efficiency becomes increasingly important as energy storage deployment accelerates in densely populated regions with limited available land.
The quantification of redox reaction rates directly influences the technology's environmental profile by determining charge/discharge efficiency. Current iron-air systems operate at 50-65% round-trip efficiency, which, while lower than some alternatives, is offset by their minimal environmental impact and exceptional longevity. Research targeting catalytic improvements could potentially increase this efficiency to 70-75%, further enhancing their sustainability credentials.
Scalability and Commercial Implementation Roadmap
The scalability of iron-air cell technology represents a critical pathway from laboratory research to commercial implementation. Current laboratory-scale quantification methods for redox reaction rates must be adapted for industrial production environments. This transition requires standardized measurement protocols that can be integrated into manufacturing processes while maintaining accuracy and reliability.
Manufacturing scale-up presents several technical challenges that must be addressed sequentially. Initial pilot production facilities will need to implement in-line monitoring systems capable of real-time assessment of redox kinetics. These systems must balance precision with throughput requirements, potentially utilizing automated electrochemical impedance spectroscopy (EIS) stations integrated directly into production lines.
Material consistency becomes paramount at commercial scale, as variations in iron electrode composition can significantly impact reaction rates. Implementation of statistical process control methods specifically calibrated for redox reaction metrics will be essential for quality assurance. This necessitates the development of rapid testing protocols that correlate with long-term cell performance.
The commercial roadmap can be divided into three distinct phases. The near-term phase (1-2 years) should focus on translating laboratory quantification methods to pilot production environments, establishing baseline performance metrics, and developing preliminary quality control standards. Mid-term implementation (3-5 years) will require refinement of high-throughput testing methodologies and integration with manufacturing execution systems to enable data-driven optimization of production parameters.
Long-term commercial viability (5+ years) depends on the establishment of industry-wide standards for redox rate quantification in iron-air cells. This standardization will facilitate supply chain development and enable meaningful comparison between different manufacturers' products. The development of these standards should be pursued through industry consortia and regulatory engagement.
Cost considerations for implementation must balance capital expenditure for advanced measurement equipment against the value derived from improved product consistency and performance. Initial investments in sophisticated electrochemical measurement systems may be substantial but can be justified through reduced warranty claims and enhanced product differentiation based on verified performance metrics.
Ultimately, successful commercial implementation will require cross-disciplinary collaboration between electrochemists, process engineers, and quality assurance specialists to develop practical, reliable methods for quantifying redox reaction rates at industrial scale.
Manufacturing scale-up presents several technical challenges that must be addressed sequentially. Initial pilot production facilities will need to implement in-line monitoring systems capable of real-time assessment of redox kinetics. These systems must balance precision with throughput requirements, potentially utilizing automated electrochemical impedance spectroscopy (EIS) stations integrated directly into production lines.
Material consistency becomes paramount at commercial scale, as variations in iron electrode composition can significantly impact reaction rates. Implementation of statistical process control methods specifically calibrated for redox reaction metrics will be essential for quality assurance. This necessitates the development of rapid testing protocols that correlate with long-term cell performance.
The commercial roadmap can be divided into three distinct phases. The near-term phase (1-2 years) should focus on translating laboratory quantification methods to pilot production environments, establishing baseline performance metrics, and developing preliminary quality control standards. Mid-term implementation (3-5 years) will require refinement of high-throughput testing methodologies and integration with manufacturing execution systems to enable data-driven optimization of production parameters.
Long-term commercial viability (5+ years) depends on the establishment of industry-wide standards for redox rate quantification in iron-air cells. This standardization will facilitate supply chain development and enable meaningful comparison between different manufacturers' products. The development of these standards should be pursued through industry consortia and regulatory engagement.
Cost considerations for implementation must balance capital expenditure for advanced measurement equipment against the value derived from improved product consistency and performance. Initial investments in sophisticated electrochemical measurement systems may be substantial but can be justified through reduced warranty claims and enhanced product differentiation based on verified performance metrics.
Ultimately, successful commercial implementation will require cross-disciplinary collaboration between electrochemists, process engineers, and quality assurance specialists to develop practical, reliable methods for quantifying redox reaction rates at industrial scale.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!