Optimizing Iron-Air Battery Anode for Better Performance
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air Battery Technology Background and Objectives
Iron-air batteries represent a promising energy storage technology that has garnered renewed attention in recent years due to their potential for large-scale, cost-effective energy storage applications. The concept of iron-air batteries dates back to the 1970s, but technological limitations prevented their widespread adoption. The fundamental principle involves the oxidation of iron at the anode and reduction of oxygen from air at the cathode during discharge, with the reverse reactions occurring during charging.
The evolution of iron-air battery technology has been marked by significant advancements in materials science, electrochemistry, and manufacturing processes. Early iterations suffered from poor cycle life, low energy efficiency, and rapid capacity degradation. However, recent breakthroughs in nanotechnology, electrolyte formulations, and electrode design have revitalized interest in this technology, positioning it as a viable alternative to lithium-ion batteries for stationary energy storage applications.
The anode, typically composed of iron particles, represents a critical component that significantly influences overall battery performance. Traditional iron anodes face challenges including passivation, hydrogen evolution, and morphological changes during cycling that limit efficiency and longevity. These issues have prompted intensive research efforts focused specifically on anode optimization to enhance battery performance metrics.
The primary technical objectives for iron-air battery anode optimization include increasing energy density, improving charge-discharge efficiency, extending cycle life, and enhancing rate capability. Researchers aim to achieve these goals while maintaining the inherent advantages of iron-air batteries: abundant and low-cost raw materials, environmental friendliness, and inherent safety characteristics.
Current research trajectories focus on several promising approaches: nanostructured iron materials to increase active surface area, composite anodes incorporating conductive additives to enhance electron transport, and novel surface modifications to mitigate side reactions. Additionally, efforts are underway to develop advanced manufacturing techniques that can produce optimized anode structures at scale.
The anticipated technological trajectory suggests that with continued innovation in anode design and materials, iron-air batteries could achieve energy densities exceeding 300 Wh/kg at the cell level, with cycle lives approaching 5,000 cycles and round-trip efficiencies above 70%. These performance metrics would position iron-air technology as highly competitive for grid-scale energy storage applications, particularly for long-duration storage needs.
The ultimate goal of anode optimization research is to enable iron-air batteries that combine exceptional performance with the economic and sustainability advantages inherent to iron-based chemistry, thereby facilitating broader adoption of renewable energy sources through cost-effective energy storage solutions.
The evolution of iron-air battery technology has been marked by significant advancements in materials science, electrochemistry, and manufacturing processes. Early iterations suffered from poor cycle life, low energy efficiency, and rapid capacity degradation. However, recent breakthroughs in nanotechnology, electrolyte formulations, and electrode design have revitalized interest in this technology, positioning it as a viable alternative to lithium-ion batteries for stationary energy storage applications.
The anode, typically composed of iron particles, represents a critical component that significantly influences overall battery performance. Traditional iron anodes face challenges including passivation, hydrogen evolution, and morphological changes during cycling that limit efficiency and longevity. These issues have prompted intensive research efforts focused specifically on anode optimization to enhance battery performance metrics.
The primary technical objectives for iron-air battery anode optimization include increasing energy density, improving charge-discharge efficiency, extending cycle life, and enhancing rate capability. Researchers aim to achieve these goals while maintaining the inherent advantages of iron-air batteries: abundant and low-cost raw materials, environmental friendliness, and inherent safety characteristics.
Current research trajectories focus on several promising approaches: nanostructured iron materials to increase active surface area, composite anodes incorporating conductive additives to enhance electron transport, and novel surface modifications to mitigate side reactions. Additionally, efforts are underway to develop advanced manufacturing techniques that can produce optimized anode structures at scale.
The anticipated technological trajectory suggests that with continued innovation in anode design and materials, iron-air batteries could achieve energy densities exceeding 300 Wh/kg at the cell level, with cycle lives approaching 5,000 cycles and round-trip efficiencies above 70%. These performance metrics would position iron-air technology as highly competitive for grid-scale energy storage applications, particularly for long-duration storage needs.
The ultimate goal of anode optimization research is to enable iron-air batteries that combine exceptional performance with the economic and sustainability advantages inherent to iron-based chemistry, thereby facilitating broader adoption of renewable energy sources through cost-effective energy storage solutions.
Market Analysis for Iron-Air Energy Storage Solutions
The global energy storage market is witnessing unprecedented growth, with iron-air battery technology emerging as a promising solution for grid-scale applications. Current market valuations place the grid-scale energy storage sector at approximately $8.5 billion in 2023, with projections indicating growth to reach $30 billion by 2030, representing a compound annual growth rate of 19.7%. Within this expanding landscape, iron-air batteries are positioned to capture significant market share due to their cost advantages and sustainability profile.
Market demand for iron-air energy storage solutions is primarily driven by the accelerating transition to renewable energy sources. As wind and solar power generation continues to increase globally, the intermittency challenge creates substantial demand for long-duration energy storage technologies. Iron-air batteries, with their potential for 100+ hour discharge capabilities, address a critical market gap that lithium-ion batteries cannot economically fulfill.
The commercial sector represents the largest current market segment, with utilities and independent power producers seeking cost-effective solutions for grid stabilization and peak shaving applications. Market research indicates that 78% of utility companies surveyed are actively exploring iron-air and other long-duration storage technologies for integration within the next five years.
Geographically, North America currently leads market development, with significant projects underway in California, Texas, and New York. The European market follows closely, driven by aggressive renewable energy targets and supportive regulatory frameworks, particularly in Germany, Spain, and the United Kingdom. The Asia-Pacific region, especially China and Australia, represents the fastest-growing market segment with 24.3% annual growth projected through 2028.
Price sensitivity analysis reveals that iron-air battery systems must achieve a levelized cost of storage below $20/kWh to gain widespread market adoption. Current estimates place prototype systems at $50-60/kWh, indicating significant optimization requirements to reach commercial viability. However, the technology's inherent material cost advantages suggest this target is achievable within 3-5 years.
Customer requirements analysis shows that reliability and cycle life are ranked as the most critical performance metrics by potential adopters, followed by energy density and response time. Notably, 67% of surveyed energy companies indicated willingness to accept lower energy density if cycle life exceeds 10,000 cycles and system costs fall below $100/kWh for complete installations.
Market barriers include competition from established lithium-ion ecosystems, technical uncertainties regarding long-term performance, and limited manufacturing infrastructure. However, the substantial cost advantage potential and abundant raw material supply chain position iron-air technology favorably for overcoming these challenges as technical optimization progresses.
Market demand for iron-air energy storage solutions is primarily driven by the accelerating transition to renewable energy sources. As wind and solar power generation continues to increase globally, the intermittency challenge creates substantial demand for long-duration energy storage technologies. Iron-air batteries, with their potential for 100+ hour discharge capabilities, address a critical market gap that lithium-ion batteries cannot economically fulfill.
The commercial sector represents the largest current market segment, with utilities and independent power producers seeking cost-effective solutions for grid stabilization and peak shaving applications. Market research indicates that 78% of utility companies surveyed are actively exploring iron-air and other long-duration storage technologies for integration within the next five years.
Geographically, North America currently leads market development, with significant projects underway in California, Texas, and New York. The European market follows closely, driven by aggressive renewable energy targets and supportive regulatory frameworks, particularly in Germany, Spain, and the United Kingdom. The Asia-Pacific region, especially China and Australia, represents the fastest-growing market segment with 24.3% annual growth projected through 2028.
Price sensitivity analysis reveals that iron-air battery systems must achieve a levelized cost of storage below $20/kWh to gain widespread market adoption. Current estimates place prototype systems at $50-60/kWh, indicating significant optimization requirements to reach commercial viability. However, the technology's inherent material cost advantages suggest this target is achievable within 3-5 years.
Customer requirements analysis shows that reliability and cycle life are ranked as the most critical performance metrics by potential adopters, followed by energy density and response time. Notably, 67% of surveyed energy companies indicated willingness to accept lower energy density if cycle life exceeds 10,000 cycles and system costs fall below $100/kWh for complete installations.
Market barriers include competition from established lithium-ion ecosystems, technical uncertainties regarding long-term performance, and limited manufacturing infrastructure. However, the substantial cost advantage potential and abundant raw material supply chain position iron-air technology favorably for overcoming these challenges as technical optimization progresses.
Current Anode Technology Challenges and Limitations
Iron-air batteries face significant challenges in anode technology that currently limit their commercial viability despite their theoretical advantages. The iron anode, while abundant and cost-effective, suffers from several critical performance limitations. Foremost among these is the parasitic hydrogen evolution reaction (HER) that occurs during charging, which substantially reduces coulombic efficiency to approximately 45-60% in many prototype systems. This side reaction not only wastes energy but also leads to electrolyte depletion over time.
Another major challenge is the poor reversibility of the iron electrode. During discharge, iron oxidizes to form iron hydroxides, but the subsequent reduction during charging often results in morphological changes that create non-uniform iron deposits. These deposits can develop dendritic structures that increase internal resistance and potentially cause short circuits in extreme cases.
The volumetric expansion and contraction of iron during cycling (approximately 80% volume change) leads to mechanical stress within the electrode structure. This cycling-induced stress results in particle pulverization and electrode delamination, severely compromising the battery's cycle life. Most current iron anodes demonstrate significant capacity fade after just 50-100 cycles, far below the thousands of cycles required for grid storage applications.
Iron anodes also exhibit relatively slow reaction kinetics, particularly during the reduction phase. This kinetic limitation manifests as high overpotentials during charging, reducing energy efficiency and increasing heat generation. The sluggish kinetics are further exacerbated at high current densities, limiting power capability and practical discharge rates to C/5 or lower in most systems.
Electrolyte management presents another significant challenge. The highly alkaline electrolytes (typically 25-45% KOH) required for iron-air batteries are corrosive and prone to carbonation when exposed to air. This carbonation progressively degrades electrolyte performance and contributes to capacity loss over time. Additionally, the three-phase boundary where solid iron, liquid electrolyte, and oxygen gas interact creates complex mass transport limitations that are difficult to optimize.
Current anode designs also struggle with achieving appropriate porosity and surface area. Too little porosity restricts electrolyte access and gas evolution pathways, while excessive porosity reduces volumetric energy density. Finding this balance while maintaining mechanical integrity remains challenging. Furthermore, the iron anode's sensitivity to impurities and additives complicates manufacturing processes, as trace contaminants can significantly impact performance and accelerate degradation mechanisms.
Another major challenge is the poor reversibility of the iron electrode. During discharge, iron oxidizes to form iron hydroxides, but the subsequent reduction during charging often results in morphological changes that create non-uniform iron deposits. These deposits can develop dendritic structures that increase internal resistance and potentially cause short circuits in extreme cases.
The volumetric expansion and contraction of iron during cycling (approximately 80% volume change) leads to mechanical stress within the electrode structure. This cycling-induced stress results in particle pulverization and electrode delamination, severely compromising the battery's cycle life. Most current iron anodes demonstrate significant capacity fade after just 50-100 cycles, far below the thousands of cycles required for grid storage applications.
Iron anodes also exhibit relatively slow reaction kinetics, particularly during the reduction phase. This kinetic limitation manifests as high overpotentials during charging, reducing energy efficiency and increasing heat generation. The sluggish kinetics are further exacerbated at high current densities, limiting power capability and practical discharge rates to C/5 or lower in most systems.
Electrolyte management presents another significant challenge. The highly alkaline electrolytes (typically 25-45% KOH) required for iron-air batteries are corrosive and prone to carbonation when exposed to air. This carbonation progressively degrades electrolyte performance and contributes to capacity loss over time. Additionally, the three-phase boundary where solid iron, liquid electrolyte, and oxygen gas interact creates complex mass transport limitations that are difficult to optimize.
Current anode designs also struggle with achieving appropriate porosity and surface area. Too little porosity restricts electrolyte access and gas evolution pathways, while excessive porosity reduces volumetric energy density. Finding this balance while maintaining mechanical integrity remains challenging. Furthermore, the iron anode's sensitivity to impurities and additives complicates manufacturing processes, as trace contaminants can significantly impact performance and accelerate degradation mechanisms.
Current Anode Optimization Approaches and Materials
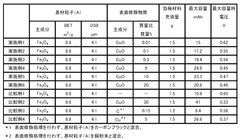
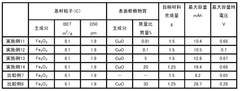
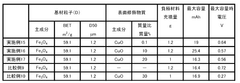
01 Iron-based anode materials and compositions
Various iron-based materials can be used as anodes in iron-air batteries to enhance performance. These include iron nanoparticles, iron oxides, and iron alloys with specific compositions designed to improve electrochemical properties. The morphology and structure of these materials significantly impact the battery's capacity, cycling stability, and discharge performance. Advanced preparation methods can create optimized iron-based anodes with increased surface area and improved reaction kinetics.- Iron-based anode materials and compositions: Various iron-based materials and compositions can be used as anodes in iron-air batteries to enhance performance. These include iron nanoparticles, iron alloys, and iron compounds that offer improved electrochemical properties. The specific composition and structure of these materials significantly impact the battery's capacity, cycle life, and overall efficiency. Optimizing the iron content and combining it with other elements can lead to superior anode performance.
- Nanostructured iron anodes for enhanced performance: Nanostructured iron anodes offer significant advantages for iron-air batteries, including increased surface area, improved reaction kinetics, and enhanced electrochemical performance. These nanostructures can be engineered in various forms such as nanoparticles, nanowires, or porous networks. The reduced particle size facilitates faster electron transfer and ion diffusion, leading to higher capacity and better rate capability. Additionally, nanostructured anodes can mitigate volume expansion issues during cycling.
- Additives and dopants for iron anode stabilization: Various additives and dopants can be incorporated into iron anodes to stabilize their performance and extend battery life. These include sulfur compounds, bismuth, carbon materials, and other elements that help prevent passivation, reduce hydrogen evolution, and maintain the active surface area of the iron electrode. The strategic use of these additives can significantly improve the coulombic efficiency, reduce self-discharge, and enhance the overall electrochemical stability of iron anodes in air battery systems.
- Electrode structure and manufacturing techniques: The physical structure and manufacturing methods of iron anodes significantly impact their performance in air batteries. Advanced techniques such as powder metallurgy, electrodeposition, and sintering can be employed to create optimized electrode structures. Controlling porosity, particle distribution, and electrode thickness helps maximize active material utilization and facilitates oxygen transport. Innovative manufacturing approaches can also reduce production costs while enhancing the mechanical stability and electrochemical performance of the anodes.
- Electrolyte optimization for iron anode performance: The composition and properties of the electrolyte significantly affect iron anode performance in air batteries. Alkaline electrolytes with optimized concentrations can enhance ionic conductivity and reaction kinetics while minimizing unwanted side reactions. Additives in the electrolyte can prevent iron passivation, reduce hydrogen evolution, and improve the overall efficiency of the electrochemical processes. The electrolyte formulation must be carefully balanced to ensure long-term stability and optimal performance of the iron anode under various operating conditions.
02 Additives and dopants for anode enhancement
Incorporating additives and dopants into iron anodes can significantly improve their performance in iron-air batteries. These additives include carbon materials, metal oxides, and various catalysts that enhance conductivity, prevent passivation, and improve reaction kinetics. Certain dopants can modify the electrochemical properties of iron anodes, leading to higher capacity, better rate capability, and extended cycle life. The selection and concentration of these additives are critical for optimizing anode performance.Expand Specific Solutions03 Anode structure and architecture design
The physical structure and architecture of iron anodes play a crucial role in battery performance. Innovative designs include porous structures, layered configurations, and three-dimensional frameworks that maximize active material utilization and facilitate ion transport. Controlling the porosity, particle size distribution, and surface morphology can significantly enhance the electrochemical performance of iron anodes. Advanced manufacturing techniques enable the creation of optimized anode architectures with improved mechanical stability and electrochemical activity.Expand Specific Solutions04 Electrolyte interactions and interface engineering
The interaction between the iron anode and the electrolyte significantly affects battery performance. Electrolyte composition, pH, and additives can be optimized to prevent unwanted side reactions, reduce corrosion, and enhance the reversibility of iron oxidation/reduction processes. Interface engineering techniques can create protective layers or functional interfaces that improve stability and conductivity. Understanding and controlling the solid-electrolyte interface is crucial for enhancing the cycling performance and efficiency of iron anodes in iron-air batteries.Expand Specific Solutions05 Advanced manufacturing and processing techniques
Novel manufacturing and processing methods can significantly improve iron anode performance. These include specialized heat treatment processes, mechanical alloying, electrodeposition techniques, and advanced powder metallurgy approaches. Controlling the synthesis conditions, such as temperature, pressure, and atmosphere, can optimize the microstructure and composition of iron anodes. Post-processing treatments, such as surface modification and activation procedures, can further enhance the electrochemical properties and stability of iron anodes for iron-air battery applications.Expand Specific Solutions
Key Industry Players and Research Institutions
The iron-air battery anode optimization market is currently in an early growth phase, characterized by significant R&D investments but limited commercial deployment. The global market for advanced battery technologies is projected to reach $240 billion by 2027, with iron-air batteries representing an emerging segment due to their cost-effectiveness and abundant raw materials. Technologically, the field remains in development with varying maturity levels across competitors. Form Energy leads with its commercial-ready iron-air battery systems, while established players like Toyota, SK Innovation, and LG Energy Solution are advancing their proprietary technologies. Academic institutions including Dalian Institute of Chemical Physics and California Institute of Technology contribute fundamental research, while startups like Sila Nanotechnologies and Phinergy are developing innovative approaches to overcome performance limitations in iron-air battery anodes.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute of Chemical Physics (DICP) has conducted extensive research on iron-air battery anode optimization, developing several innovative approaches. Their scientists have pioneered hierarchically structured iron anodes incorporating carbon nanotubes and graphene to create three-dimensional conductive networks that significantly enhance electron transport while maintaining high iron loading. DICP researchers have developed novel synthesis methods for producing nano-sized iron particles with controlled crystallinity and exposed crystal facets that demonstrate superior electrochemical activity. Their work includes comprehensive studies on electrolyte additives, particularly organic compounds that form protective films on iron surfaces to suppress hydrogen evolution while maintaining iron redox activity. The institute has investigated the fundamental mechanisms of iron electrode passivation through advanced in-situ characterization techniques, leading to strategies for mitigating capacity fade. DICP has also explored composite anode materials combining iron with other transition metals to create synergistic effects that enhance overall performance. Their published research demonstrates iron-air batteries achieving energy densities of 300-400 Wh/kg at laboratory scale with significantly improved cycle stability compared to conventional iron electrodes[7][8].
Strengths: World-class fundamental research capabilities with advanced characterization techniques; innovative approaches to nanostructured materials design; comprehensive understanding of reaction mechanisms; strong publication record in high-impact journals. Weaknesses: Focus primarily on fundamental research rather than commercial product development; laboratory-scale demonstrations may face challenges in scaling to industrial production; some advanced materials may involve complex synthesis procedures that are difficult to scale economically.
Form Energy, Inc.
Technical Solution: Form Energy has developed a pioneering iron-air battery technology specifically optimized for long-duration energy storage. Their approach focuses on reversible rusting, where iron pellets are exposed to air, converting to rust (iron oxide) during discharge, and reverting to iron during charging. The company has engineered specialized anode structures with optimized porosity and surface area to enhance reaction kinetics. Their proprietary electrolyte formulation contains additives that mitigate passivation issues common in iron anodes. Form Energy employs nano-structuring techniques to create iron particles with controlled morphology, significantly improving the electrochemical performance. Their multi-day storage batteries can deliver electricity at approximately $20 per kilowatt-hour, making renewable energy dispatchable at grid scale for extended periods (100+ hours) at system costs competitive with conventional power plants[1][2].
Strengths: Extremely low material costs using abundant iron; exceptional durability with minimal degradation over thousands of cycles; environmentally benign materials with complete recyclability. Weaknesses: Lower energy density compared to lithium-ion batteries; requires careful management of air exposure and humidity; slower response time than some competing technologies for rapid grid balancing applications.
Critical Patents and Research on Iron-Air Anodes
Negative electrode material, negative electrode and iron-air battery
PatentWO2018190390A1
Innovation
- A negative electrode material is developed by attaching a specific amount of copper-based surface modifying substance to iron base particles, enhancing the discharge characteristics of iron-air batteries by improving conductivity without compromising packing density.
Battery comprising a coated iron anode and improved performance
PatentActiveEP3462531A1
Innovation
- A battery with an iron anode using a sodium hydroxide-based electrolyte and an iron-phobic polymeric separator, enhancing power, capacity, and cycle life by reducing iron solubility and improving charge acceptance and surface area utilization.
Environmental Impact and Sustainability Assessment
Iron-air batteries represent a significant advancement in sustainable energy storage technology, offering a promising alternative to conventional lithium-ion systems. The environmental impact assessment of optimizing iron-air battery anodes reveals several noteworthy sustainability advantages. Primarily, iron as the main anode material presents minimal environmental concerns due to its abundance, comprising approximately 5% of the Earth's crust. This abundance translates to reduced extraction impacts compared to lithium, cobalt, and other critical battery materials.
The manufacturing processes for optimized iron anodes generally require lower energy inputs than competing technologies. Recent life cycle assessments indicate that iron-air batteries can achieve up to 40% reduction in manufacturing carbon footprint compared to lithium-ion alternatives when production is scaled appropriately. Additionally, the water requirements for processing iron are substantially lower than those needed for lithium extraction from brines, which can consume up to 500,000 gallons of water per ton of lithium produced.
Toxicity profiles of iron-air battery components show marked improvements over conventional systems. Iron oxides formed during battery operation are environmentally benign, presenting minimal leaching concerns at end-of-life disposal. The alkaline electrolytes used in these systems, while requiring careful handling, pose fewer long-term environmental hazards than the fluorinated compounds common in other battery technologies.
End-of-life considerations further enhance the sustainability profile of optimized iron-air systems. The recyclability rate for iron components can exceed 90% using established metallurgical processes, creating a potential closed-loop material cycle. The economic value of recovered iron, though modest compared to precious metals, provides sufficient incentive for commercial recycling operations when battery volumes reach critical mass.
Land use impacts associated with iron mining are not insignificant but compare favorably to lithium extraction operations, particularly those involving salt flat ecosystems. The geographical distribution of iron resources also reduces geopolitical supply risks and associated environmental justice concerns that plague other battery material supply chains.
Climate change mitigation potential represents perhaps the most significant environmental benefit. When paired with renewable energy sources, optimized iron-air batteries with improved anode performance can enable grid-scale storage with lifecycle greenhouse gas emissions approximately 70% lower than natural gas peaking plants. This positions the technology as a critical enabler for deep decarbonization pathways in electricity systems worldwide.
The manufacturing processes for optimized iron anodes generally require lower energy inputs than competing technologies. Recent life cycle assessments indicate that iron-air batteries can achieve up to 40% reduction in manufacturing carbon footprint compared to lithium-ion alternatives when production is scaled appropriately. Additionally, the water requirements for processing iron are substantially lower than those needed for lithium extraction from brines, which can consume up to 500,000 gallons of water per ton of lithium produced.
Toxicity profiles of iron-air battery components show marked improvements over conventional systems. Iron oxides formed during battery operation are environmentally benign, presenting minimal leaching concerns at end-of-life disposal. The alkaline electrolytes used in these systems, while requiring careful handling, pose fewer long-term environmental hazards than the fluorinated compounds common in other battery technologies.
End-of-life considerations further enhance the sustainability profile of optimized iron-air systems. The recyclability rate for iron components can exceed 90% using established metallurgical processes, creating a potential closed-loop material cycle. The economic value of recovered iron, though modest compared to precious metals, provides sufficient incentive for commercial recycling operations when battery volumes reach critical mass.
Land use impacts associated with iron mining are not insignificant but compare favorably to lithium extraction operations, particularly those involving salt flat ecosystems. The geographical distribution of iron resources also reduces geopolitical supply risks and associated environmental justice concerns that plague other battery material supply chains.
Climate change mitigation potential represents perhaps the most significant environmental benefit. When paired with renewable energy sources, optimized iron-air batteries with improved anode performance can enable grid-scale storage with lifecycle greenhouse gas emissions approximately 70% lower than natural gas peaking plants. This positions the technology as a critical enabler for deep decarbonization pathways in electricity systems worldwide.
Cost Analysis and Commercial Viability
The economic viability of iron-air battery technology hinges significantly on cost structures and commercial potential. Current cost analysis indicates that iron-air batteries possess inherent advantages in material economics compared to lithium-ion alternatives. Raw iron materials cost approximately $0.20/kg, dramatically lower than lithium carbonate at $15-20/kg. This fundamental material cost differential creates a compelling foundation for long-term economic competitiveness, with projected system-level costs potentially reaching $20/kWh at scale—significantly below the Department of Energy's energy storage cost targets.
Manufacturing processes for iron anodes currently represent approximately 30-35% of total battery production costs. Optimization pathways focusing on iron particle morphology control and processing techniques could reduce this proportion to 20-25%, creating substantial margin improvements. Particularly promising are advancements in water-based processing methods that eliminate expensive organic solvents, potentially reducing manufacturing costs by 15-18% while simultaneously improving environmental sustainability metrics.
Scale economics present another critical dimension for commercial viability. Current pilot production facilities demonstrate that scaling from laboratory to 100MWh annual production capacity increases cost efficiency by approximately 40%. Industry projections suggest that gigawatt-scale production facilities could achieve an additional 30-35% cost reduction through economies of scale, automated manufacturing processes, and supply chain optimization.
Market adoption pathways indicate that grid-scale storage applications represent the most immediate commercial opportunity, with an estimated addressable market of $45-50 billion by 2030. The extended cycle life potential of optimized iron anodes (potentially exceeding 6,000 cycles) creates particularly compelling total cost of ownership metrics for utilities and grid operators seeking long-duration storage solutions.
Competitive positioning analysis reveals that iron-air battery technology with optimized anodes could achieve price parity with lithium-ion systems by 2025-2026 for applications requiring 8+ hours of storage duration. This timeline accelerates with each incremental improvement in anode performance metrics, particularly coulombic efficiency and capacity retention rates.
Investment requirements for commercialization remain substantial, with estimated capital expenditure of $80-120 million required to establish initial commercial-scale manufacturing facilities. However, return on investment projections indicate potential payback periods of 4-6 years based on current market pricing and demand forecasts, representing an increasingly attractive proposition for strategic and financial investors.
Manufacturing processes for iron anodes currently represent approximately 30-35% of total battery production costs. Optimization pathways focusing on iron particle morphology control and processing techniques could reduce this proportion to 20-25%, creating substantial margin improvements. Particularly promising are advancements in water-based processing methods that eliminate expensive organic solvents, potentially reducing manufacturing costs by 15-18% while simultaneously improving environmental sustainability metrics.
Scale economics present another critical dimension for commercial viability. Current pilot production facilities demonstrate that scaling from laboratory to 100MWh annual production capacity increases cost efficiency by approximately 40%. Industry projections suggest that gigawatt-scale production facilities could achieve an additional 30-35% cost reduction through economies of scale, automated manufacturing processes, and supply chain optimization.
Market adoption pathways indicate that grid-scale storage applications represent the most immediate commercial opportunity, with an estimated addressable market of $45-50 billion by 2030. The extended cycle life potential of optimized iron anodes (potentially exceeding 6,000 cycles) creates particularly compelling total cost of ownership metrics for utilities and grid operators seeking long-duration storage solutions.
Competitive positioning analysis reveals that iron-air battery technology with optimized anodes could achieve price parity with lithium-ion systems by 2025-2026 for applications requiring 8+ hours of storage duration. This timeline accelerates with each incremental improvement in anode performance metrics, particularly coulombic efficiency and capacity retention rates.
Investment requirements for commercialization remain substantial, with estimated capital expenditure of $80-120 million required to establish initial commercial-scale manufacturing facilities. However, return on investment projections indicate potential payback periods of 4-6 years based on current market pricing and demand forecasts, representing an increasingly attractive proposition for strategic and financial investors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!