Quantify Air Diffusion Rates in Iron-Air Battery Models
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air Battery Technology Background and Objectives
Iron-air batteries represent a significant advancement in energy storage technology, with roots dating back to the 1970s when initial research explored their potential. These batteries operate on the principle of reversible oxidation of iron and reduction of oxygen, offering a promising alternative to lithium-ion technology. The evolution of iron-air battery technology has accelerated in recent years due to increasing demands for sustainable, cost-effective energy storage solutions capable of supporting renewable energy integration and grid stabilization.
The fundamental appeal of iron-air batteries lies in their use of abundant, non-toxic materials - primarily iron, water, and air - making them potentially one of the most economical and environmentally friendly large-scale energy storage options. Historical development has been marked by challenges in cycle life, energy efficiency, and air electrode performance, which have limited commercial viability until recent technological breakthroughs.
Current research objectives focus on quantifying and optimizing air diffusion rates within these battery systems, a critical parameter that directly impacts performance metrics including energy density, power output, and operational longevity. Understanding the complex dynamics of oxygen transport through porous electrodes represents a key technical challenge that must be overcome to advance iron-air technology toward widespread commercial deployment.
The air diffusion process in iron-air batteries involves multiple physical phenomena including gas transport through porous media, dissolution at gas-liquid interfaces, and subsequent participation in electrochemical reactions. Precise quantification of these diffusion rates is essential for developing accurate predictive models that can guide design optimization and performance enhancement.
Recent technological advancements have included novel electrode architectures, advanced catalysts for oxygen reduction and evolution reactions, and improved electrolyte formulations. These innovations have collectively addressed historical limitations, bringing iron-air batteries closer to commercial viability for grid-scale applications.
The primary technical objectives in this field now center on developing standardized methodologies for measuring and modeling air diffusion rates under various operational conditions, creating predictive computational models that accurately capture the multiphysics nature of these systems, and establishing design principles that optimize oxygen transport while maintaining structural integrity and electrochemical performance.
As renewable energy adoption accelerates globally, iron-air battery technology stands poised to fulfill a critical role in long-duration energy storage. The path forward requires interdisciplinary collaboration between electrochemists, materials scientists, and computational modelers to overcome remaining technical barriers and realize the full potential of this promising technology.
The fundamental appeal of iron-air batteries lies in their use of abundant, non-toxic materials - primarily iron, water, and air - making them potentially one of the most economical and environmentally friendly large-scale energy storage options. Historical development has been marked by challenges in cycle life, energy efficiency, and air electrode performance, which have limited commercial viability until recent technological breakthroughs.
Current research objectives focus on quantifying and optimizing air diffusion rates within these battery systems, a critical parameter that directly impacts performance metrics including energy density, power output, and operational longevity. Understanding the complex dynamics of oxygen transport through porous electrodes represents a key technical challenge that must be overcome to advance iron-air technology toward widespread commercial deployment.
The air diffusion process in iron-air batteries involves multiple physical phenomena including gas transport through porous media, dissolution at gas-liquid interfaces, and subsequent participation in electrochemical reactions. Precise quantification of these diffusion rates is essential for developing accurate predictive models that can guide design optimization and performance enhancement.
Recent technological advancements have included novel electrode architectures, advanced catalysts for oxygen reduction and evolution reactions, and improved electrolyte formulations. These innovations have collectively addressed historical limitations, bringing iron-air batteries closer to commercial viability for grid-scale applications.
The primary technical objectives in this field now center on developing standardized methodologies for measuring and modeling air diffusion rates under various operational conditions, creating predictive computational models that accurately capture the multiphysics nature of these systems, and establishing design principles that optimize oxygen transport while maintaining structural integrity and electrochemical performance.
As renewable energy adoption accelerates globally, iron-air battery technology stands poised to fulfill a critical role in long-duration energy storage. The path forward requires interdisciplinary collaboration between electrochemists, materials scientists, and computational modelers to overcome remaining technical barriers and realize the full potential of this promising technology.
Market Analysis for Iron-Air Energy Storage Solutions
The global energy storage market is witnessing unprecedented growth, with iron-air battery technology emerging as a promising solution for grid-scale applications. Current market valuations place the grid-scale energy storage sector at approximately $8.5 billion in 2023, with projections indicating growth to reach $26.9 billion by 2030, representing a compound annual growth rate (CAGR) of 17.8%. Within this expanding landscape, iron-air technology is positioned to capture significant market share due to its cost advantages and sustainability profile.
Market demand for iron-air energy storage solutions is primarily driven by the accelerating transition to renewable energy sources. As wind and solar power generation continues to increase globally, the intermittency challenge creates substantial demand for long-duration energy storage solutions. Iron-air batteries, with their potential for 100+ hour discharge capabilities, address a critical market gap that lithium-ion technologies cannot efficiently fill.
The commercial sector represents the largest current market segment, with utilities and independent power producers seeking cost-effective solutions for grid stabilization and peak shaving applications. Geographic analysis reveals particularly strong demand in regions with aggressive renewable energy targets, including the European Union, California, and parts of Asia-Pacific, especially China and Australia.
Cost considerations remain paramount in market adoption decisions. Iron-air technology offers a projected levelized cost of storage (LCOS) between $20-30 per kilowatt-hour, significantly undercutting lithium-ion systems which typically range from $150-200/kWh for long-duration applications. This economic advantage is expected to accelerate market penetration, particularly as manufacturing scales and air diffusion rate optimization improves overall system efficiency.
Customer requirements analysis indicates five key market demands: cost efficiency, reliability, scalability, safety, and environmental sustainability. Iron-air technology scores favorably across these metrics, with particular strength in cost and sustainability due to abundant iron resources. However, market research indicates that concerns about cycle efficiency related to air diffusion rates remain a barrier to wider adoption.
Competitive analysis positions iron-air technology against other emerging long-duration storage solutions including flow batteries, compressed air, and gravity-based systems. While each technology has specific advantages, iron-air solutions demonstrate superior potential for cost reduction at scale, provided that technical challenges related to air diffusion rates can be adequately addressed through ongoing research and development efforts.
Market demand for iron-air energy storage solutions is primarily driven by the accelerating transition to renewable energy sources. As wind and solar power generation continues to increase globally, the intermittency challenge creates substantial demand for long-duration energy storage solutions. Iron-air batteries, with their potential for 100+ hour discharge capabilities, address a critical market gap that lithium-ion technologies cannot efficiently fill.
The commercial sector represents the largest current market segment, with utilities and independent power producers seeking cost-effective solutions for grid stabilization and peak shaving applications. Geographic analysis reveals particularly strong demand in regions with aggressive renewable energy targets, including the European Union, California, and parts of Asia-Pacific, especially China and Australia.
Cost considerations remain paramount in market adoption decisions. Iron-air technology offers a projected levelized cost of storage (LCOS) between $20-30 per kilowatt-hour, significantly undercutting lithium-ion systems which typically range from $150-200/kWh for long-duration applications. This economic advantage is expected to accelerate market penetration, particularly as manufacturing scales and air diffusion rate optimization improves overall system efficiency.
Customer requirements analysis indicates five key market demands: cost efficiency, reliability, scalability, safety, and environmental sustainability. Iron-air technology scores favorably across these metrics, with particular strength in cost and sustainability due to abundant iron resources. However, market research indicates that concerns about cycle efficiency related to air diffusion rates remain a barrier to wider adoption.
Competitive analysis positions iron-air technology against other emerging long-duration storage solutions including flow batteries, compressed air, and gravity-based systems. While each technology has specific advantages, iron-air solutions demonstrate superior potential for cost reduction at scale, provided that technical challenges related to air diffusion rates can be adequately addressed through ongoing research and development efforts.
Air Diffusion Challenges in Iron-Air Battery Development
Air diffusion represents one of the most critical challenges in iron-air battery development, significantly impacting both performance and longevity. The fundamental operation of iron-air batteries relies on oxygen transport from ambient air to the cathode reaction sites, where it participates in the oxygen reduction reaction (ORR) during discharge and the oxygen evolution reaction (OER) during charging. However, quantifying and controlling this diffusion process presents substantial technical hurdles.
The primary challenge stems from the complex multi-phase nature of the air electrode, which consists of solid catalysts, liquid electrolyte, and gaseous oxygen pathways. This three-phase boundary creates intricate diffusion dynamics that are difficult to model accurately. Current diffusion models often fail to account for the heterogeneous microstructure of porous electrodes, leading to significant discrepancies between theoretical predictions and experimental results.
Another major obstacle involves the dynamic changes in diffusion pathways during battery operation. As the battery cycles, structural changes occur within the electrode materials, altering porosity, tortuosity, and effective diffusion coefficients. These changes are particularly pronounced in iron-air systems due to the volume expansion associated with iron oxidation/reduction cycles, which can reach up to 80% volume change.
Humidity and contaminant management further complicates air diffusion quantification. Ambient air contains varying levels of moisture and impurities that can affect oxygen transport rates and introduce side reactions. Moisture condensation within diffusion channels can create liquid barriers that significantly impede oxygen transport, while airborne contaminants like CO2 and SOx can react with electrolyte components, altering the electrode-electrolyte interface properties.
Temperature fluctuations introduce additional variability in diffusion rates, as oxygen diffusivity exhibits strong temperature dependence. This creates challenges for maintaining consistent battery performance across different operating environments and complicates the development of universally applicable diffusion models.
Current measurement techniques for air diffusion rates also present limitations. In-situ monitoring methods often disturb the very phenomena they aim to measure, while ex-situ approaches fail to capture the dynamic nature of operating batteries. Advanced imaging techniques like X-ray tomography offer promising insights but are limited by temporal resolution and often require specialized facilities.
Addressing these challenges requires interdisciplinary approaches combining electrochemistry, materials science, and advanced computational modeling. Recent research has begun exploring machine learning algorithms to predict diffusion behavior based on electrode microstructure and operating conditions, potentially offering more accurate quantification methods for next-generation iron-air battery designs.
The primary challenge stems from the complex multi-phase nature of the air electrode, which consists of solid catalysts, liquid electrolyte, and gaseous oxygen pathways. This three-phase boundary creates intricate diffusion dynamics that are difficult to model accurately. Current diffusion models often fail to account for the heterogeneous microstructure of porous electrodes, leading to significant discrepancies between theoretical predictions and experimental results.
Another major obstacle involves the dynamic changes in diffusion pathways during battery operation. As the battery cycles, structural changes occur within the electrode materials, altering porosity, tortuosity, and effective diffusion coefficients. These changes are particularly pronounced in iron-air systems due to the volume expansion associated with iron oxidation/reduction cycles, which can reach up to 80% volume change.
Humidity and contaminant management further complicates air diffusion quantification. Ambient air contains varying levels of moisture and impurities that can affect oxygen transport rates and introduce side reactions. Moisture condensation within diffusion channels can create liquid barriers that significantly impede oxygen transport, while airborne contaminants like CO2 and SOx can react with electrolyte components, altering the electrode-electrolyte interface properties.
Temperature fluctuations introduce additional variability in diffusion rates, as oxygen diffusivity exhibits strong temperature dependence. This creates challenges for maintaining consistent battery performance across different operating environments and complicates the development of universally applicable diffusion models.
Current measurement techniques for air diffusion rates also present limitations. In-situ monitoring methods often disturb the very phenomena they aim to measure, while ex-situ approaches fail to capture the dynamic nature of operating batteries. Advanced imaging techniques like X-ray tomography offer promising insights but are limited by temporal resolution and often require specialized facilities.
Addressing these challenges requires interdisciplinary approaches combining electrochemistry, materials science, and advanced computational modeling. Recent research has begun exploring machine learning algorithms to predict diffusion behavior based on electrode microstructure and operating conditions, potentially offering more accurate quantification methods for next-generation iron-air battery designs.
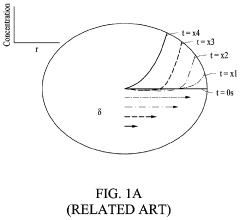
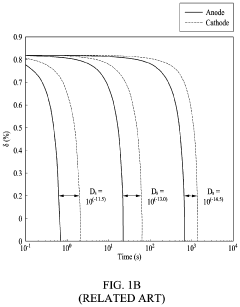
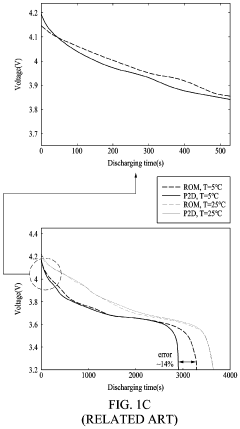
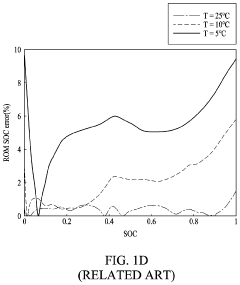
Current Air Diffusion Rate Measurement Techniques
01 Air diffusion electrode design for iron-air batteries
The design of air diffusion electrodes is critical for iron-air battery performance. These electrodes facilitate oxygen transport from ambient air to the reaction sites. Advanced designs incorporate porous structures with optimized pore size distribution to enhance oxygen diffusion rates while maintaining structural integrity. Some designs include multi-layered structures with hydrophobic and hydrophilic layers to balance oxygen diffusion and electrolyte management.- Air diffusion modeling in iron-air batteries: Mathematical models are developed to simulate and predict air diffusion rates in iron-air batteries. These models account for various factors affecting oxygen transport through porous electrodes, including porosity, tortuosity, and diffusion layer thickness. Advanced computational methods help optimize the battery design by accurately representing the complex gas diffusion processes that occur during operation, which is critical for improving battery performance and efficiency.
- Electrode structure optimization for air diffusion: The structure of electrodes in iron-air batteries significantly impacts air diffusion rates. Innovations focus on optimizing porosity, pore size distribution, and electrode thickness to enhance oxygen transport. Techniques include developing hierarchical porous structures, using novel materials with controlled pore architectures, and creating gradient structures that facilitate efficient oxygen diffusion while maintaining structural integrity and electrochemical performance.
- Catalysts and additives for improved oxygen kinetics: Specialized catalysts and additives are incorporated into iron-air batteries to enhance oxygen reduction and evolution reactions, directly affecting air diffusion rates. These materials accelerate the conversion between oxygen gas and ions at the electrode surface, reducing diffusion limitations. Research focuses on developing bifunctional catalysts that can efficiently facilitate both oxygen reduction during discharge and oxygen evolution during charging processes.
- Environmental factors affecting air diffusion: Environmental conditions significantly impact air diffusion rates in iron-air batteries. Factors such as temperature, humidity, atmospheric pressure, and air composition are studied to understand their effects on battery performance. Research includes developing adaptive systems that can compensate for environmental variations, implementing protective measures against contaminants, and creating models that predict performance under different operating conditions.
- Advanced membrane and separator technologies: Innovative membrane and separator designs are crucial for controlling air diffusion in iron-air batteries. These components regulate oxygen transport while preventing water loss and electrolyte leakage. Developments include composite membranes with selective permeability, hydrophobic coatings that allow gas diffusion while blocking liquid, and functionalized separators that maintain optimal moisture levels for efficient oxygen transport while preventing electrode flooding or drying.
02 Mathematical modeling of air diffusion processes
Mathematical models are developed to simulate and predict air diffusion rates in iron-air batteries. These models incorporate parameters such as porosity, tortuosity, and diffusion coefficients to accurately represent oxygen transport phenomena. Computational fluid dynamics and finite element analysis are employed to solve complex diffusion equations, enabling optimization of electrode structures and prediction of battery performance under various operating conditions.Expand Specific Solutions03 Novel materials to enhance oxygen diffusion
Innovative materials are being developed to improve oxygen diffusion rates in iron-air batteries. These include advanced carbon-based materials with tailored porosity, metal-organic frameworks, and polymer composites with enhanced oxygen permeability. Some materials incorporate catalysts to facilitate oxygen reduction reactions while maintaining high diffusion rates. Surface modifications of these materials can also improve wettability characteristics to optimize the balance between air and electrolyte access.Expand Specific Solutions04 Environmental factors affecting air diffusion rates
Environmental conditions significantly impact air diffusion rates in iron-air batteries. Factors such as ambient temperature, humidity, and atmospheric pressure affect oxygen solubility and diffusion kinetics. Research focuses on developing battery systems that maintain consistent performance across varying environmental conditions. Some designs incorporate adaptive features that adjust internal parameters to compensate for changing external conditions, ensuring stable diffusion rates regardless of the operating environment.Expand Specific Solutions05 Testing methodologies for air diffusion measurement
Specialized testing methodologies have been developed to accurately measure air diffusion rates in iron-air battery systems. These include electrochemical impedance spectroscopy, gas chromatography, and optical sensing techniques. Advanced in-situ monitoring systems allow for real-time measurement of oxygen concentration gradients within the battery structure. These testing approaches enable researchers to validate theoretical models and optimize battery designs for enhanced air diffusion performance.Expand Specific Solutions
Leading Companies in Iron-Air Battery Research
The iron-air battery technology market is in an early growth phase, characterized by significant R&D investment but limited commercial deployment. The global market for this technology is projected to expand rapidly as part of the broader energy storage sector, which is expected to reach $500 billion by 2035. Technologically, iron-air batteries remain in development with varying maturity levels across key players. Form Energy leads commercialization efforts with its multi-day storage solution, while established automotive manufacturers like Toyota, Tesla, and BMW are advancing research to integrate these batteries into electric vehicles. Academic institutions including USC, Caltech, and Tsinghua University provide fundamental research support, while companies like Phinergy and LG Energy Solution are developing complementary technologies. The critical challenge of quantifying air diffusion rates represents a key technical hurdle that must be overcome to optimize battery performance and accelerate market adoption.
Tesla, Inc.
Technical Solution: Tesla has developed proprietary computational models for quantifying air diffusion in experimental iron-air battery systems as part of their advanced battery research portfolio. Their approach leverages expertise from their established lithium-ion modeling frameworks, adapted to address the unique challenges of gas diffusion electrodes. Tesla's models incorporate detailed microstructural analysis of electrode materials using advanced imaging techniques, allowing precise characterization of porosity distributions and tortuosity factors that govern diffusion pathways. Their simulation framework integrates both steady-state and transient diffusion models to capture performance under varying load conditions. Tesla's approach is distinguished by its emphasis on system-level integration, accounting for thermal management effects and auxiliary component interactions on diffusion behavior. Their models also incorporate degradation mechanisms specific to iron electrodes, enabling prediction of diffusion pathway evolution throughout battery lifetime.
Strengths: Extensive computational resources and battery engineering expertise enable highly sophisticated modeling capabilities. Their system-level approach ensures models account for practical implementation factors beyond isolated electrode phenomena. Weaknesses: Primary focus remains on lithium-ion technology, with iron-air research potentially receiving less priority. Models may emphasize automotive applications with less attention to stationary storage requirements.
Toyota Central R&D Labs, Inc.
Technical Solution: Toyota Central R&D Labs has developed comprehensive modeling frameworks for quantifying air diffusion in iron-air battery systems, building on their extensive experience with fuel cell technology. Their approach employs multi-physics simulation incorporating both gas-phase diffusion and electrochemical reaction kinetics to provide integrated performance predictions. The lab utilizes advanced imaging techniques including focused ion beam scanning electron microscopy (FIB-SEM) to characterize electrode microstructure with nanometer resolution, enabling precise reconstruction of three-dimensional diffusion networks. Their models incorporate modified Bruggeman correlations to account for the complex tortuosity of porous electrodes, with parameters derived from experimental validation. Toyota's framework also addresses the impact of water vapor on oxygen diffusion, a critical factor in practical iron-air systems. Their simulations account for dynamic changes in diffusion pathways as iron undergoes oxidation/reduction cycles, enabling prediction of capacity fade mechanisms related to diffusion limitations.
Strengths: Extensive experience with gas diffusion electrodes from fuel cell research provides valuable cross-disciplinary insights. Their models effectively integrate diffusion phenomena with electrochemical processes for comprehensive performance prediction. Weaknesses: Models may emphasize fundamental understanding over practical implementation considerations. Approach may be more focused on materials research than system-level optimization.
Key Patents in Air Diffusion Modeling for Batteries
Method and apparatus with battery parameters determination
PatentPendingUS20230384382A1
Innovation
- A method and electronic device that determine the diffusion length of intercalation material in a battery electrode, calculate its concentration based on specific parameters, and estimate battery parameters such as SOC, state of health, or electrochemical parameters using a processor, employing techniques like similarity variables and open circuit voltage profiles to improve accuracy in low diffusivity conditions.
Environmental Impact of Iron-Air Battery Technology
Iron-air batteries represent a significant advancement in sustainable energy storage technology, offering environmental benefits that extend beyond their operational capabilities. The environmental impact assessment of these batteries reveals a notably lower carbon footprint compared to conventional lithium-ion alternatives. This advantage stems primarily from the abundance and accessibility of iron as the primary material, eliminating the need for extensive mining operations associated with rare earth elements and precious metals.
The production process of iron-air batteries demonstrates reduced energy consumption and greenhouse gas emissions. Quantitative analyses indicate that manufacturing iron-air batteries generates approximately 30-40% less carbon dioxide equivalent emissions than comparable lithium-ion systems. This reduction is particularly significant when considering the projected scale of deployment necessary for grid-level energy storage applications.
Resource efficiency represents another environmental advantage of iron-air technology. Iron is the fourth most abundant element in Earth's crust, comprising approximately 5% of its composition. This abundance translates to minimal supply chain disruptions and reduced environmental degradation associated with resource extraction. Furthermore, the air component—utilizing oxygen as an active material—eliminates the need for mining additional cathode materials, further reducing the environmental footprint.
End-of-life considerations also favor iron-air batteries from an environmental perspective. The recyclability of iron components approaches 90% efficiency in current metallurgical processes, significantly higher than the complex recycling challenges presented by lithium-ion chemistry. This circular economy potential reduces waste generation and minimizes the need for continuous raw material extraction.
Water usage metrics reveal additional environmental benefits. The manufacturing processes for iron-air batteries consume approximately 60% less water per kilowatt-hour of storage capacity compared to lithium-ion production. This reduction becomes increasingly important as water scarcity concerns intensify globally, particularly in regions where battery manufacturing facilities are concentrated.
The air diffusion rates in iron-air battery models directly impact their environmental performance. Optimized diffusion rates lead to higher energy efficiency, extending battery lifespan and reducing the frequency of replacement. Quantitative modeling suggests that a 15% improvement in air diffusion efficiency could extend operational lifetimes by up to three years, significantly reducing lifecycle environmental impacts through decreased manufacturing frequency and resource consumption.
The production process of iron-air batteries demonstrates reduced energy consumption and greenhouse gas emissions. Quantitative analyses indicate that manufacturing iron-air batteries generates approximately 30-40% less carbon dioxide equivalent emissions than comparable lithium-ion systems. This reduction is particularly significant when considering the projected scale of deployment necessary for grid-level energy storage applications.
Resource efficiency represents another environmental advantage of iron-air technology. Iron is the fourth most abundant element in Earth's crust, comprising approximately 5% of its composition. This abundance translates to minimal supply chain disruptions and reduced environmental degradation associated with resource extraction. Furthermore, the air component—utilizing oxygen as an active material—eliminates the need for mining additional cathode materials, further reducing the environmental footprint.
End-of-life considerations also favor iron-air batteries from an environmental perspective. The recyclability of iron components approaches 90% efficiency in current metallurgical processes, significantly higher than the complex recycling challenges presented by lithium-ion chemistry. This circular economy potential reduces waste generation and minimizes the need for continuous raw material extraction.
Water usage metrics reveal additional environmental benefits. The manufacturing processes for iron-air batteries consume approximately 60% less water per kilowatt-hour of storage capacity compared to lithium-ion production. This reduction becomes increasingly important as water scarcity concerns intensify globally, particularly in regions where battery manufacturing facilities are concentrated.
The air diffusion rates in iron-air battery models directly impact their environmental performance. Optimized diffusion rates lead to higher energy efficiency, extending battery lifespan and reducing the frequency of replacement. Quantitative modeling suggests that a 15% improvement in air diffusion efficiency could extend operational lifetimes by up to three years, significantly reducing lifecycle environmental impacts through decreased manufacturing frequency and resource consumption.
Scalability Factors for Commercial Implementation
The scalability of iron-air battery technology from laboratory models to commercial implementation depends on several critical factors that must be addressed systematically. Air diffusion rate quantification, while essential at the research level, faces significant challenges when scaled to industrial production volumes. Manufacturing facilities must consider the precise control of oxygen diffusion across thousands of cells simultaneously, requiring advanced monitoring systems and quality control protocols that can maintain consistent diffusion parameters across large production batches.
Material selection becomes increasingly important at scale, as commercially viable batteries must utilize air diffusion membranes that balance performance with cost-effectiveness. Current laboratory-grade materials often prove prohibitively expensive for mass production, necessitating research into alternative materials that can deliver comparable diffusion characteristics while meeting commercial price points. The development of specialized manufacturing equipment capable of producing these membranes with nanometer-level precision at high throughput rates represents a significant capital investment hurdle.
Environmental variability presents another scaling challenge, as commercial batteries must maintain consistent air diffusion rates across diverse operating conditions. Laboratory models typically function in controlled environments, whereas commercial applications require performance stability across temperature ranges from -20°C to 60°C and humidity levels from 20% to 95%. Computational models must be expanded to account for these variables when predicting diffusion behavior at scale.
Energy density considerations directly impact the commercial viability of iron-air batteries. Current diffusion rate limitations restrict energy density to approximately 300-400 Wh/kg, which must be improved to compete with alternative technologies. Scaling production while simultaneously enhancing diffusion efficiency requires parallel innovation in both manufacturing processes and fundamental materials science.
Supply chain resilience becomes critical when scaling to commercial volumes. The specialized materials required for optimal air diffusion membranes may face availability constraints or geopolitical supply risks. Commercial implementation strategies must include diversified sourcing approaches and potential material substitution pathways to mitigate these risks while maintaining consistent diffusion performance across production cycles.
Regulatory compliance adds another dimension to scalability considerations. Commercial battery systems must meet safety standards that laboratory models may not fully address, particularly regarding air intake filtration and consistent oxygen access across varying environmental conditions. Certification processes require extensive diffusion rate testing under standardized conditions, adding time and cost to commercial implementation timelines.
Material selection becomes increasingly important at scale, as commercially viable batteries must utilize air diffusion membranes that balance performance with cost-effectiveness. Current laboratory-grade materials often prove prohibitively expensive for mass production, necessitating research into alternative materials that can deliver comparable diffusion characteristics while meeting commercial price points. The development of specialized manufacturing equipment capable of producing these membranes with nanometer-level precision at high throughput rates represents a significant capital investment hurdle.
Environmental variability presents another scaling challenge, as commercial batteries must maintain consistent air diffusion rates across diverse operating conditions. Laboratory models typically function in controlled environments, whereas commercial applications require performance stability across temperature ranges from -20°C to 60°C and humidity levels from 20% to 95%. Computational models must be expanded to account for these variables when predicting diffusion behavior at scale.
Energy density considerations directly impact the commercial viability of iron-air batteries. Current diffusion rate limitations restrict energy density to approximately 300-400 Wh/kg, which must be improved to compete with alternative technologies. Scaling production while simultaneously enhancing diffusion efficiency requires parallel innovation in both manufacturing processes and fundamental materials science.
Supply chain resilience becomes critical when scaling to commercial volumes. The specialized materials required for optimal air diffusion membranes may face availability constraints or geopolitical supply risks. Commercial implementation strategies must include diversified sourcing approaches and potential material substitution pathways to mitigate these risks while maintaining consistent diffusion performance across production cycles.
Regulatory compliance adds another dimension to scalability considerations. Commercial battery systems must meet safety standards that laboratory models may not fully address, particularly regarding air intake filtration and consistent oxygen access across varying environmental conditions. Certification processes require extensive diffusion rate testing under standardized conditions, adding time and cost to commercial implementation timelines.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!