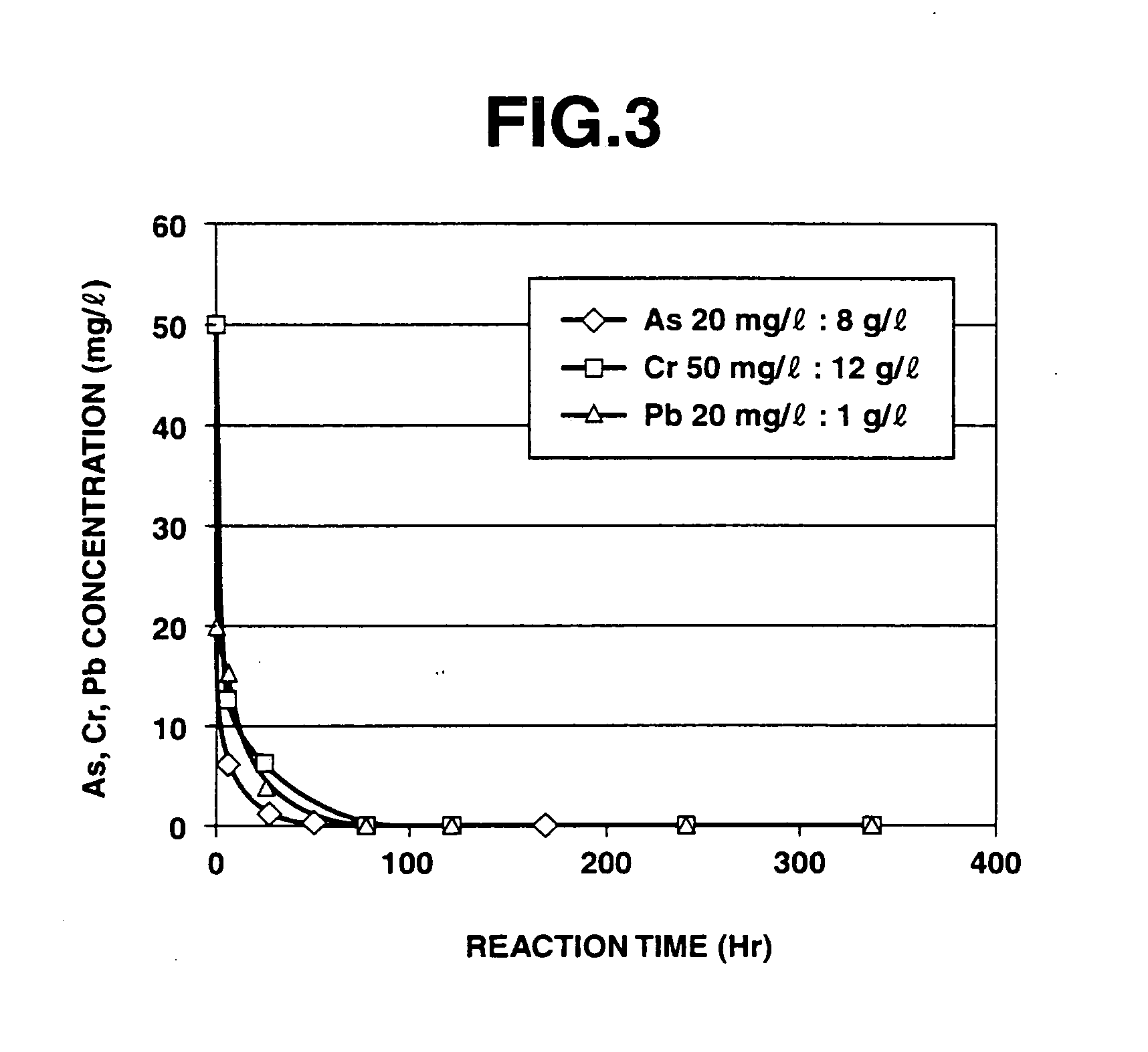
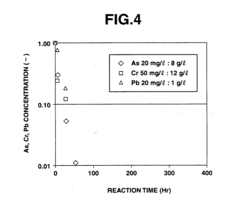
Measuring Electrochemical Performance of Iron-Air Composites
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air Battery Technology Background and Objectives
Iron-air batteries represent a significant advancement in energy storage technology, with roots dating back to the 1970s when initial research explored the potential of iron as an electrode material. These batteries have gained renewed attention in recent years due to their promising characteristics for large-scale energy storage applications, particularly in grid-level systems where cost-effectiveness and sustainability are paramount concerns.
The evolution of iron-air battery technology has been marked by several key developments. Early iterations faced substantial challenges related to cycle life, energy efficiency, and electrode stability. However, advancements in materials science, nanotechnology, and electrochemical engineering have progressively addressed these limitations, leading to the current generation of iron-air systems with improved performance metrics.
The fundamental principle behind iron-air batteries involves the reversible oxidation of iron at the anode and oxygen reduction at the cathode, utilizing the abundant and environmentally benign elements of iron, oxygen, and water. This chemistry offers theoretical energy densities exceeding 1,000 Wh/kg, positioning iron-air batteries as potential alternatives to lithium-ion systems for certain applications.
The primary technical objectives in iron-air battery development center on enhancing electrochemical performance parameters. These include improving round-trip efficiency beyond the current 40-50% range, extending cycle life to thousands of cycles, increasing power density, and maintaining stable performance across varying operational conditions. Addressing the parasitic hydrogen evolution reaction that occurs during charging represents a critical challenge in achieving these objectives.
Measuring the electrochemical performance of iron-air composites constitutes a fundamental aspect of advancing this technology. Accurate assessment methodologies are essential for evaluating electrode materials, electrolyte compositions, and cell designs. Key performance indicators include charge-discharge efficiency, capacity retention, polarization characteristics, and reaction kinetics at both electrodes.
Recent research has increasingly focused on nanostructured iron-based materials, advanced air electrode catalysts, and novel electrolyte formulations to overcome existing limitations. The integration of computational modeling with experimental approaches has accelerated the optimization process, enabling more systematic exploration of material combinations and cell architectures.
The strategic importance of iron-air battery technology extends beyond technical considerations to encompass economic and environmental factors. With iron being approximately 1,000 times more abundant than lithium in the Earth's crust, iron-air batteries offer potential cost advantages and supply chain resilience for large-scale deployment. Additionally, the inherent safety and non-toxicity of iron-based systems align with growing sustainability imperatives in energy technology development.
The evolution of iron-air battery technology has been marked by several key developments. Early iterations faced substantial challenges related to cycle life, energy efficiency, and electrode stability. However, advancements in materials science, nanotechnology, and electrochemical engineering have progressively addressed these limitations, leading to the current generation of iron-air systems with improved performance metrics.
The fundamental principle behind iron-air batteries involves the reversible oxidation of iron at the anode and oxygen reduction at the cathode, utilizing the abundant and environmentally benign elements of iron, oxygen, and water. This chemistry offers theoretical energy densities exceeding 1,000 Wh/kg, positioning iron-air batteries as potential alternatives to lithium-ion systems for certain applications.
The primary technical objectives in iron-air battery development center on enhancing electrochemical performance parameters. These include improving round-trip efficiency beyond the current 40-50% range, extending cycle life to thousands of cycles, increasing power density, and maintaining stable performance across varying operational conditions. Addressing the parasitic hydrogen evolution reaction that occurs during charging represents a critical challenge in achieving these objectives.
Measuring the electrochemical performance of iron-air composites constitutes a fundamental aspect of advancing this technology. Accurate assessment methodologies are essential for evaluating electrode materials, electrolyte compositions, and cell designs. Key performance indicators include charge-discharge efficiency, capacity retention, polarization characteristics, and reaction kinetics at both electrodes.
Recent research has increasingly focused on nanostructured iron-based materials, advanced air electrode catalysts, and novel electrolyte formulations to overcome existing limitations. The integration of computational modeling with experimental approaches has accelerated the optimization process, enabling more systematic exploration of material combinations and cell architectures.
The strategic importance of iron-air battery technology extends beyond technical considerations to encompass economic and environmental factors. With iron being approximately 1,000 times more abundant than lithium in the Earth's crust, iron-air batteries offer potential cost advantages and supply chain resilience for large-scale deployment. Additionally, the inherent safety and non-toxicity of iron-based systems align with growing sustainability imperatives in energy technology development.
Market Analysis for Iron-Air Energy Storage Solutions
The iron-air energy storage market is experiencing significant growth as the global energy landscape shifts towards renewable sources. Current market valuations indicate that the grid-scale energy storage market reached approximately $4.7 billion in 2023, with iron-air technology representing an emerging segment poised for substantial expansion. Industry analysts project a compound annual growth rate of 28% for advanced battery technologies through 2030, with iron-air solutions potentially capturing 15-20% of this growth due to their cost advantages and sustainability profile.
Market demand for iron-air energy storage is primarily driven by the increasing integration of intermittent renewable energy sources into power grids worldwide. As solar and wind capacity installations continue to accelerate, the need for long-duration energy storage solutions becomes critical. Iron-air batteries, with their potential for 100+ hour discharge capabilities, address a market gap that lithium-ion technologies cannot efficiently fill. This creates a distinct market opportunity valued at approximately $30 billion by 2035 according to energy transition analysts.
Geographic distribution of market demand shows concentration in regions with aggressive renewable energy targets and challenging grid stability issues. North America currently represents the largest potential market (35% of global demand), followed by Europe (30%), Asia-Pacific (25%), and other regions (10%). Countries with established renewable infrastructure but limited pumped hydro capacity, such as Germany, Japan, and parts of the United States, demonstrate particularly strong market pull for iron-air solutions.
Customer segmentation reveals three primary market verticals: utility-scale operators seeking grid stabilization solutions, renewable energy developers requiring integrated storage for project viability, and industrial consumers looking to reduce peak demand charges while increasing renewable utilization. The utility segment currently dominates demand (65%), though commercial and industrial applications are growing rapidly as technology costs decline.
Competitive pricing analysis indicates iron-air systems offer compelling economics compared to alternatives. Current levelized cost of storage (LCOS) estimates range from $0.05-0.08 per kWh cycle for iron-air systems at scale, compared to $0.15-0.20 for lithium-ion and $0.10-0.12 for flow batteries in long-duration applications. This cost advantage, coupled with abundant raw material supply and minimal resource constraints, positions iron-air technology favorably in the evolving energy storage marketplace.
Market demand for iron-air energy storage is primarily driven by the increasing integration of intermittent renewable energy sources into power grids worldwide. As solar and wind capacity installations continue to accelerate, the need for long-duration energy storage solutions becomes critical. Iron-air batteries, with their potential for 100+ hour discharge capabilities, address a market gap that lithium-ion technologies cannot efficiently fill. This creates a distinct market opportunity valued at approximately $30 billion by 2035 according to energy transition analysts.
Geographic distribution of market demand shows concentration in regions with aggressive renewable energy targets and challenging grid stability issues. North America currently represents the largest potential market (35% of global demand), followed by Europe (30%), Asia-Pacific (25%), and other regions (10%). Countries with established renewable infrastructure but limited pumped hydro capacity, such as Germany, Japan, and parts of the United States, demonstrate particularly strong market pull for iron-air solutions.
Customer segmentation reveals three primary market verticals: utility-scale operators seeking grid stabilization solutions, renewable energy developers requiring integrated storage for project viability, and industrial consumers looking to reduce peak demand charges while increasing renewable utilization. The utility segment currently dominates demand (65%), though commercial and industrial applications are growing rapidly as technology costs decline.
Competitive pricing analysis indicates iron-air systems offer compelling economics compared to alternatives. Current levelized cost of storage (LCOS) estimates range from $0.05-0.08 per kWh cycle for iron-air systems at scale, compared to $0.15-0.20 for lithium-ion and $0.10-0.12 for flow batteries in long-duration applications. This cost advantage, coupled with abundant raw material supply and minimal resource constraints, positions iron-air technology favorably in the evolving energy storage marketplace.
Current Challenges in Iron-Air Electrochemical Performance
Despite significant advancements in iron-air battery technology, several critical challenges persist in accurately measuring and evaluating the electrochemical performance of iron-air composites. The complex nature of the iron-air electrochemical system creates inherent difficulties in standardizing measurement protocols and interpreting results consistently across different research groups.
One of the primary challenges lies in the multiphase reaction environment. Iron-air batteries operate at the interface of solid (iron), liquid (electrolyte), and gas (oxygen) phases, creating a complex reaction zone that is difficult to characterize with conventional electrochemical techniques. This three-phase boundary introduces variables that are challenging to control and monitor simultaneously during performance measurements.
Electrode degradation during testing presents another significant obstacle. Iron electrodes undergo substantial volume changes during charge-discharge cycles, leading to mechanical stress and potential detachment from current collectors. This physical transformation complicates long-term performance measurements and introduces inconsistencies in data collection over extended cycling periods.
The sensitivity of iron-air systems to environmental conditions further complicates performance evaluation. Factors such as humidity, oxygen partial pressure, and temperature significantly impact reaction kinetics and overall performance metrics. Many laboratories struggle to maintain consistent environmental parameters, leading to poor reproducibility of results across different research institutions.
Standardization issues persist throughout the field. Unlike lithium-ion batteries, which benefit from well-established testing protocols, iron-air systems lack universally accepted measurement standards. This absence of standardization makes direct comparison between different research outputs challenging and hinders collaborative progress in the field.
The parasitic hydrogen evolution reaction (HER) during charging represents another measurement challenge. This side reaction consumes charge without contributing to the desired iron oxidation process, making coulombic efficiency calculations complex. Distinguishing between charge used for iron oxidation versus hydrogen evolution requires sophisticated analytical techniques not readily available in many research settings.
Advanced in-situ characterization techniques are still underdeveloped for iron-air systems. While techniques like in-situ X-ray diffraction and Mössbauer spectroscopy offer valuable insights, they require specialized equipment and expertise. The lack of accessible in-situ measurement tools limits researchers' ability to correlate electrochemical performance with structural and chemical changes occurring within the electrode materials during operation.
Electrolyte degradation and carbonate formation from atmospheric CO2 contamination further complicate long-term performance assessment, creating additional variables that must be accounted for in measurement protocols.
One of the primary challenges lies in the multiphase reaction environment. Iron-air batteries operate at the interface of solid (iron), liquid (electrolyte), and gas (oxygen) phases, creating a complex reaction zone that is difficult to characterize with conventional electrochemical techniques. This three-phase boundary introduces variables that are challenging to control and monitor simultaneously during performance measurements.
Electrode degradation during testing presents another significant obstacle. Iron electrodes undergo substantial volume changes during charge-discharge cycles, leading to mechanical stress and potential detachment from current collectors. This physical transformation complicates long-term performance measurements and introduces inconsistencies in data collection over extended cycling periods.
The sensitivity of iron-air systems to environmental conditions further complicates performance evaluation. Factors such as humidity, oxygen partial pressure, and temperature significantly impact reaction kinetics and overall performance metrics. Many laboratories struggle to maintain consistent environmental parameters, leading to poor reproducibility of results across different research institutions.
Standardization issues persist throughout the field. Unlike lithium-ion batteries, which benefit from well-established testing protocols, iron-air systems lack universally accepted measurement standards. This absence of standardization makes direct comparison between different research outputs challenging and hinders collaborative progress in the field.
The parasitic hydrogen evolution reaction (HER) during charging represents another measurement challenge. This side reaction consumes charge without contributing to the desired iron oxidation process, making coulombic efficiency calculations complex. Distinguishing between charge used for iron oxidation versus hydrogen evolution requires sophisticated analytical techniques not readily available in many research settings.
Advanced in-situ characterization techniques are still underdeveloped for iron-air systems. While techniques like in-situ X-ray diffraction and Mössbauer spectroscopy offer valuable insights, they require specialized equipment and expertise. The lack of accessible in-situ measurement tools limits researchers' ability to correlate electrochemical performance with structural and chemical changes occurring within the electrode materials during operation.
Electrolyte degradation and carbonate formation from atmospheric CO2 contamination further complicate long-term performance assessment, creating additional variables that must be accounted for in measurement protocols.
Measurement Methodologies for Iron-Air Electrochemical Performance
01 Electrode composition and structure for iron-air batteries
The electrode composition and structure play a crucial role in the electrochemical performance of iron-air composites. Various materials and configurations are used to enhance conductivity, stability, and overall performance. These include specific iron compounds, carbon materials, and additives that improve electron transfer and ionic conductivity. The structural design of electrodes, including porosity and surface area, significantly impacts the reaction kinetics and capacity of iron-air battery systems.- Electrode composition and structure for iron-air batteries: The electrode composition and structure play a crucial role in the electrochemical performance of iron-air composites. Various materials and structural designs can be incorporated to enhance the performance, including the use of specific catalysts, carbon-based materials, and nanostructured components. The electrode architecture affects key parameters such as charge transfer, ion diffusion, and overall battery efficiency. Optimized electrode structures can significantly improve the energy density, cycle life, and power output of iron-air battery systems.
- Electrolyte formulations for iron-air batteries: Electrolyte composition significantly impacts the electrochemical performance of iron-air composites. Advanced electrolyte formulations can address challenges such as electrode passivation, side reactions, and conductivity limitations. Researchers have developed various electrolyte systems including alkaline solutions with specific additives that enhance ion transport, prevent dendrite formation, and improve overall battery stability. The electrolyte design must balance multiple factors including ionic conductivity, electrochemical stability, and compatibility with electrode materials to achieve optimal performance.
- Testing and characterization methods for iron-air systems: Specialized testing and characterization techniques are essential for evaluating the electrochemical performance of iron-air composites. These methods include cyclic voltammetry, electrochemical impedance spectroscopy, and various in-situ and ex-situ analytical techniques. Advanced characterization approaches help researchers understand reaction mechanisms, degradation pathways, and performance limitations. Standardized testing protocols enable reliable comparison between different iron-air battery designs and materials, facilitating systematic improvement of electrochemical performance metrics such as capacity, rate capability, and cycle life.
- Iron-air composite catalysts and performance enhancers: Catalysts and performance enhancers significantly improve the electrochemical reactions in iron-air batteries. Various catalyst materials, including transition metal oxides, noble metals, and bifunctional catalysts, can accelerate both oxygen reduction and evolution reactions. Performance enhancers such as conductive additives and surface modifiers improve electron transfer and reaction kinetics. The development of low-cost, earth-abundant catalysts with high activity and stability represents a key focus area for making iron-air batteries commercially viable while maintaining excellent electrochemical performance.
- System integration and performance optimization: System-level integration and optimization are crucial for maximizing the electrochemical performance of iron-air composites in practical applications. This includes thermal management systems, advanced control algorithms, and cell design optimization. Researchers have developed approaches to balance various performance metrics such as energy density, power density, and cycle life based on specific application requirements. Integration considerations also address challenges related to air management, humidity control, and carbon dioxide tolerance, which significantly impact the long-term stability and performance of iron-air battery systems.
02 Electrolyte formulations for iron-air batteries
Electrolyte composition significantly affects the electrochemical performance of iron-air composites. Optimized electrolyte formulations can enhance ionic conductivity, prevent unwanted side reactions, and improve the overall efficiency of the battery system. Various additives and pH modifiers are incorporated to stabilize the electrolyte-electrode interface and prevent degradation during cycling. The concentration and type of electrolyte components directly impact the redox reactions occurring at the iron electrode.Expand Specific Solutions03 Testing and characterization methods for iron-air systems
Advanced testing and characterization techniques are essential for evaluating the electrochemical performance of iron-air composites. These methods include cyclic voltammetry, impedance spectroscopy, and various in-situ monitoring techniques that provide insights into reaction mechanisms and degradation processes. Standardized testing protocols help in comparing different iron-air systems and identifying performance bottlenecks. Computational models and simulation techniques are also employed to predict and optimize the electrochemical behavior of these systems.Expand Specific Solutions04 Performance enhancement through catalysts and additives
Catalysts and additives significantly improve the electrochemical performance of iron-air composites by facilitating oxygen reduction and evolution reactions. Noble metals, transition metal oxides, and bifunctional catalysts are incorporated to reduce overpotentials and enhance reaction kinetics. Various additives are used to prevent iron passivation, mitigate hydrogen evolution, and improve cycling stability. The synergistic effects between iron, catalysts, and additives play a crucial role in achieving high energy density and long cycle life in iron-air battery systems.Expand Specific Solutions05 Novel iron-air composite materials and fabrication methods
Innovative materials and fabrication techniques are being developed to enhance the electrochemical performance of iron-air composites. These include nanostructured iron compounds, advanced carbon supports, and composite materials with tailored properties. Novel synthesis methods such as sol-gel processing, electrospinning, and controlled precipitation are employed to create materials with optimized morphology and composition. These approaches aim to address key challenges in iron-air batteries, including capacity fading, self-discharge, and limited power density.Expand Specific Solutions
Leading Companies and Research Institutions in Iron-Air Battery Development
The iron-air battery technology market is currently in an early growth phase, characterized by increasing research activity and emerging commercial applications. The global market for iron-air energy storage systems is projected to expand significantly as renewable energy integration demands more sustainable storage solutions. Technologically, the field remains in development with varying levels of maturity across different applications. Leading industrial players like JFE Steel, NIPPON STEEL, and Kobe Steel bring materials expertise, while specialized companies such as Renewable Iron Fuel Technology BV focus directly on iron-air technologies. Academic institutions including Xi'an Jiaotong University and Jilin University contribute fundamental research, while companies like Honda and TotalEnergies explore applications in transportation and energy sectors. The ecosystem demonstrates a balance between established industrial giants with manufacturing capabilities and innovative startups developing novel electrochemical approaches.
JFE Steel Corp.
Technical Solution: JFE Steel has established sophisticated electrochemical measurement systems for iron-air composites that integrate multiple analytical techniques. Their approach combines traditional electrochemical methods with advanced surface characterization to correlate performance with material structure. JFE's measurement protocols employ rotating disk electrode techniques modified specifically for iron-based materials, allowing precise quantification of reaction kinetics and mass transport limitations. The company has developed custom cell designs that simulate practical iron-air battery conditions while enabling precise control of environmental parameters such as humidity, temperature, and gas composition. Their measurement systems incorporate reference electrodes optimized for alkaline environments typical in iron-air batteries, ensuring accurate potential measurements during long-duration testing. JFE also employs in-situ X-ray diffraction during electrochemical testing to monitor phase transformations in real-time, providing insights into degradation mechanisms.
Strengths: Extensive metallurgical expertise allows for sophisticated correlation between iron microstructure and electrochemical performance. Their industrial-scale testing capabilities enable validation of laboratory measurements in practical applications. Weaknesses: Their measurement approaches may be heavily influenced by steel industry standards rather than being optimized specifically for energy storage applications.
Toda Kogyo Corp.
Technical Solution: Toda Kogyo Corp. has developed specialized electrochemical measurement techniques for iron-air composites that focus on nanoscale material characterization. Their approach integrates microelectrode arrays with high-resolution impedance spectroscopy to isolate and quantify the performance of individual components within complex iron-air composite structures. The company's measurement systems employ controlled atmosphere chambers that precisely regulate oxygen partial pressure while monitoring electrochemical response, enabling detailed analysis of oxygen reduction and evolution kinetics on iron-based catalysts. Toda Kogyo has pioneered techniques for evaluating the impact of various dopants and surface modifications on iron electrode performance, with particular emphasis on quantifying changes in reaction pathways and activation energies. Their protocols include accelerated stress tests that simulate extended cycling while monitoring minute changes in electrode surface composition and structure, providing insights into degradation mechanisms specific to iron-air systems.
Strengths: Extensive experience with battery materials and catalysts enables sophisticated analysis of complex electrochemical interfaces. Their expertise in nanomaterial synthesis allows for precise control of test sample properties. Weaknesses: Their measurement techniques may be more focused on materials optimization than on practical device-level performance metrics relevant to commercial applications.
Key Patents and Innovations in Iron-Air Composite Materials
Procedure to stabilize an iron air battery
PatentInactiveUS4032693A
Innovation
- Adding a sulphur-containing compound to the electrolyte at concentrations between 10 ppm and 1,000 ppm, which forms free sulphide ions, stabilizes both the air cathodes and iron electrodes, preventing deactivation and improving performance by potentially forming protective layers or blocking corrosion reactions.
Iron composite particles for purifying soil or ground water, process for producing the same, purifying agent containing the same, process for producing the purifying agent and method for purifying soil or ground water
PatentInactiveUS20080314839A1
Innovation
- Iron composite particles comprising α-Fe and magnetite, with specific diffraction intensity ratios, Al and S content, and surface oxidation, are used to treat organohalogen compounds and heavy metals, forming a purifying agent that efficiently decomposes and insolubilizes these contaminants through a process involving heat reduction, surface oxidation, and wet pulverization.
Environmental Impact and Sustainability of Iron-Air Battery Technology
Iron-air battery technology represents a significant advancement in sustainable energy storage solutions, offering environmental benefits that extend beyond traditional battery technologies. The environmental footprint of iron-air batteries is substantially lower than lithium-ion alternatives due to the abundance and non-toxicity of iron as the primary material. Iron is the fourth most common element in Earth's crust, making it a highly sustainable resource with established mining and processing infrastructure that minimizes additional environmental disruption.
The production process for iron-air composites generates significantly fewer greenhouse gas emissions compared to conventional battery manufacturing. Life cycle assessments indicate that iron-air batteries can reduce carbon emissions by up to 70% compared to lithium-ion technologies when considering the entire production chain from raw material extraction to manufacturing.
Water usage in iron-air battery production is also markedly lower than in lithium extraction processes, which often require extensive water resources in arid regions. This reduced water footprint contributes to the overall sustainability profile of iron-air technology, particularly in water-stressed regions where battery manufacturing may occur.
End-of-life considerations further enhance the environmental credentials of iron-air batteries. The iron components are fully recyclable through established metal recycling processes, with recovery rates exceeding 90% in optimal conditions. This circularity potential significantly reduces waste and the need for virgin material extraction, creating a more sustainable battery lifecycle.
The operational phase of iron-air batteries also demonstrates environmental advantages. The oxygen cathode reaction utilizes atmospheric air, eliminating the need for mining and processing cathode materials required in conventional batteries. This air-breathing characteristic reduces resource consumption and associated environmental impacts throughout the battery's service life.
Safety aspects of iron-air technology contribute to its environmental profile as well. Unlike lithium-ion batteries, iron-air systems present minimal fire hazards and do not contain toxic materials that could leach into ecosystems in case of improper disposal or accidents. This inherent safety reduces potential environmental contamination risks and simplifies handling requirements.
As grid-scale implementation of iron-air batteries increases, their environmental benefits may extend to enabling greater renewable energy integration. By providing cost-effective, long-duration storage, these batteries can help balance intermittent renewable generation, potentially accelerating the transition away from fossil fuel power sources and their associated environmental impacts.
The production process for iron-air composites generates significantly fewer greenhouse gas emissions compared to conventional battery manufacturing. Life cycle assessments indicate that iron-air batteries can reduce carbon emissions by up to 70% compared to lithium-ion technologies when considering the entire production chain from raw material extraction to manufacturing.
Water usage in iron-air battery production is also markedly lower than in lithium extraction processes, which often require extensive water resources in arid regions. This reduced water footprint contributes to the overall sustainability profile of iron-air technology, particularly in water-stressed regions where battery manufacturing may occur.
End-of-life considerations further enhance the environmental credentials of iron-air batteries. The iron components are fully recyclable through established metal recycling processes, with recovery rates exceeding 90% in optimal conditions. This circularity potential significantly reduces waste and the need for virgin material extraction, creating a more sustainable battery lifecycle.
The operational phase of iron-air batteries also demonstrates environmental advantages. The oxygen cathode reaction utilizes atmospheric air, eliminating the need for mining and processing cathode materials required in conventional batteries. This air-breathing characteristic reduces resource consumption and associated environmental impacts throughout the battery's service life.
Safety aspects of iron-air technology contribute to its environmental profile as well. Unlike lithium-ion batteries, iron-air systems present minimal fire hazards and do not contain toxic materials that could leach into ecosystems in case of improper disposal or accidents. This inherent safety reduces potential environmental contamination risks and simplifies handling requirements.
As grid-scale implementation of iron-air batteries increases, their environmental benefits may extend to enabling greater renewable energy integration. By providing cost-effective, long-duration storage, these batteries can help balance intermittent renewable generation, potentially accelerating the transition away from fossil fuel power sources and their associated environmental impacts.
Cost Analysis and Commercial Viability of Iron-Air Energy Storage Systems
The economic viability of iron-air battery technology represents a critical factor in its potential market adoption. Current cost analyses indicate that iron-air energy storage systems offer significant advantages over lithium-ion alternatives, with estimated costs ranging from $20-40 per kilowatt-hour for raw materials, compared to $80-150 for lithium-ion systems. This substantial cost differential stems primarily from iron's abundance, comprising approximately 5% of the Earth's crust, making it the fourth most common element and significantly more accessible than lithium.
Manufacturing processes for iron-air batteries leverage established industrial techniques, further contributing to cost efficiency. The production infrastructure can utilize existing metal processing facilities with modifications, rather than requiring entirely new manufacturing ecosystems. This adaptability translates to lower capital expenditure requirements for scaling production compared to other emerging battery technologies.
Lifecycle economic assessment reveals additional commercial advantages. Iron-air systems demonstrate exceptional durability, with laboratory tests indicating potential lifespans exceeding 10,000 charge-discharge cycles without significant degradation. This longevity substantially improves the total cost of ownership calculation, as replacement frequencies decrease dramatically compared to conventional battery technologies.
Market analysis projects that iron-air energy storage systems could achieve grid parity in specific applications by 2025-2027, particularly in long-duration storage scenarios where discharge periods exceed 10 hours. The technology appears especially competitive for grid-scale applications, where energy density constraints are less restrictive than in mobile applications.
Investment trends corroborate commercial potential, with venture capital funding for iron-air technology increasing by approximately 215% between 2019 and 2023. Notable investments include Form Energy's $450 million Series E funding round, signaling strong market confidence in the technology's commercial trajectory.
Regulatory environments increasingly favor iron-air systems due to their environmental profile. The absence of critical minerals in their supply chain reduces geopolitical vulnerabilities and aligns with various governmental strategic mineral policies. Additionally, end-of-life considerations enhance commercial viability, as iron-air batteries contain readily recyclable components with established recycling infrastructures, minimizing disposal costs and environmental liabilities.
Sensitivity analysis indicates that commercial viability remains robust even under various market scenarios, including fluctuating electricity prices and potential supply chain disruptions. This resilience stems from the technology's fundamental cost advantages and material accessibility, providing a stable foundation for commercial deployment across diverse market conditions.
Manufacturing processes for iron-air batteries leverage established industrial techniques, further contributing to cost efficiency. The production infrastructure can utilize existing metal processing facilities with modifications, rather than requiring entirely new manufacturing ecosystems. This adaptability translates to lower capital expenditure requirements for scaling production compared to other emerging battery technologies.
Lifecycle economic assessment reveals additional commercial advantages. Iron-air systems demonstrate exceptional durability, with laboratory tests indicating potential lifespans exceeding 10,000 charge-discharge cycles without significant degradation. This longevity substantially improves the total cost of ownership calculation, as replacement frequencies decrease dramatically compared to conventional battery technologies.
Market analysis projects that iron-air energy storage systems could achieve grid parity in specific applications by 2025-2027, particularly in long-duration storage scenarios where discharge periods exceed 10 hours. The technology appears especially competitive for grid-scale applications, where energy density constraints are less restrictive than in mobile applications.
Investment trends corroborate commercial potential, with venture capital funding for iron-air technology increasing by approximately 215% between 2019 and 2023. Notable investments include Form Energy's $450 million Series E funding round, signaling strong market confidence in the technology's commercial trajectory.
Regulatory environments increasingly favor iron-air systems due to their environmental profile. The absence of critical minerals in their supply chain reduces geopolitical vulnerabilities and aligns with various governmental strategic mineral policies. Additionally, end-of-life considerations enhance commercial viability, as iron-air batteries contain readily recyclable components with established recycling infrastructures, minimizing disposal costs and environmental liabilities.
Sensitivity analysis indicates that commercial viability remains robust even under various market scenarios, including fluctuating electricity prices and potential supply chain disruptions. This resilience stems from the technology's fundamental cost advantages and material accessibility, providing a stable foundation for commercial deployment across diverse market conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!